Abstract
Central cannabinoid receptors are thought to mediate neural oscillations and are localized to brain regions implicated in auditory P50 sensory gating, including the hippocampus and neocortex. The current study therefore examined if neural oscillations evoked by the paired clicks (S1, S2) are associated with impaired P50 gating reported in cannabis users. Seventeen heavy cannabis users and 16 cannabis naïve controls participated. Analyses included P50 amplitudes, and time x frequency analyses examining event-related spectral perturbations (ERSP) and inter-trial coherence (ITC). In agreement with prior studies, cannabis users exhibited reduced P50 gating. The ERSP analysis yielded attenuated high frequency activity in the beta range (13-29 Hz) post-S1 and in the gamma range (30-50 Hz) post-S2 in the cannabis group, compared to the control group. Attenuated ITC was also observed in the cannabis group in the post-S2 theta band (4-7 Hz). Greater levels of cannabis use were positively associated with high P50 ratios and negatively with post-S2 ERSP gamma power. These findings suggest that heavy cannabis use is associated with aberrant beta and gamma activity in the dual-click procedure, which corroborates recent work demonstrating disruption of beta/gamma by cannabinoid receptor (CB1) agonists in a rat analogue of this procedure and highlights the translational potential of the dual-click procedure.
Keywords: P50, Marijuana, auditory gating, beta, gamma, time-frequency
Introduction
The dual-click “sensory gating” procedure is widely used as in index of sensory registration and suppression (e.g., Braff, Swerdlow, & Geyer, 1995). In the typical dual-click procedure, amplitude of the P50 event-related brain potential (ERP) to the second paired click (S2) is attenuated relative to the P50 amplitude to the first click (S1; 500 ms inter-stimulus interval). While the P50 to S1 is thought to assess the capacity of the central nervous system to register salient stimuli, the relative decrement of the P50 amplitude to S2 (compared to S1) is thought to be associated with suppression or “inhibitory gating” of redundant and irrelevant stimuli (Boutros, Belger, Campbell, D’Souza, & Krystal, 1999; Braff, Swerdlow, & Geyer, 1995). Both human (Freedman, Adler, & Waldo, 1987) and non-human studies (N40; Boutros and Kwan, 1998) suggest that the reduction of S2 amplitude may reflect recurrent inhibitory mechanisms activated by the neural response to the first click. This gating effect is typically indexed using an amplitude ratio (S2/S1) or difference score (S1 – S2) (Smith, Boutros, & Schwarzkopf, 1994). Clinically, sensory gating disturbances are associated with posttraumatic stress disorder (Neylan et al., 1999), bipolar disorder (Olincy and Martin, 2005), schizotypal personality disorder (Cadenhead et al., 2000), and, most notably, schizophrenia (e.g., Patterson et al., 2008).
While the exact mechanisms of P50 sensory gating remain unclear, three brain regions have been consistently implicated in the P50 ERP to the dual clicks: the hippocampus, the superior temporal gyrus, and the prefrontal cortex. The hippocampus has been implicated in rat sensory gating models (N40; Boutros, Bonnet, Millana, & Liu, 1997; Boutros and Kwan, 1998; Bickford-Wimer et al., 1990; Freedman et al., 1996) and has been demonstrated by intracranial hippocampal recordings (Boutros et al., 2005; Grunwald et al., 2003) to be engaged in humans during the dual-click procedure, though not related to the P50 response itself. Recordings made directly from the human hippocampus during the dual-click procedure do not show prominent activity during the mid-latency, including P50, time window; instead, neural activity occurs at approximately 200 ms post S1 and persists through S2 onset (Boutros et al., 2005).
The superior temporal gyrus and medial frontal cortex have been identified as generators of the P50 ERP using electrophysiological source localization (Korzyukov et al., 2007; Weisser et al., 2001), MEG (Hanlon et al., 2005), fMRI (Mayer et al., 2008), and intracranial recordings (Grunwald et al., 2003; Kurthen et al., 2007). Data from these studies have been used to posit that frontal cortex mechanisms activated by the first click inhibit responsiveness of the auditory cortex to the second click– resulting in the relative attenuation of the S2 P50 ERP (Grunwald et al., 2003; Mayer et al., 2008). The involvement of these cortical regions in mid-latency ERPs suggests that they mediate preattentive sensory gating, whereas it has been speculated that the hippocampus may mediate gating of longer latency, perhaps, attentional processes (Grunwald et al., 2003).
The consistently reported involvement of human frontal and temporal cortices and possible involvement of hippocampus in sensory gating is notable because in primates these structures contain comparable densities of the CB1 cannabinoid receptors (Eggan & Lewis, 2007). In both the hippocampus and neocortex, CB1 receptors are mostly found on cholecystokinin expressing GABAergic interneurons and mediate depolarization-induced suppression of inhibition (Trettel, Fortin, & Levine, 2004). In rat and primate neocortex, however, limited evidence shows CB1 receptors may also be expressed on glutamatergic neurons (Ong and Mackie, 1999; Hill et al., 2007). The fact that structures containing high densities of CB1 receptors have been implicated as possible neural substrates of the auditory response to paired stimuli raises the possibility that the endogenous cannabinoid system may mediate hippocampal and neocortical processes related to sensory gating.
Because the primary psychoactive constituent of cannabis, delta-9-tetra-hydrocannabinol (THC), is a CB1 receptor agonist (Iversen, 2003), a growing number of studies have sought to examine if P50 gating is disrupted in heavy cannabis users. Indeed, three studies have reported that cannabis users exhibit higher P50 gating ratios (worse gating) compared to controls (Patrick et al., 1999; Patrick and Struve, 2000; Rentzsch et al., 2007). Impaired P50 gating in the cannabis users seems to be driven primarily by increased P50 amplitude to the testing click (S2) (Patrick et al., 1999; Patrick and Struve, 2000; Rentzsch et al., 20071). Traditionally, increased S2 P50 amplitude has been interpreted to indicate the failure of an inhibitory mechanism activated by the processing of S1 (Braff, Swerdlow, & Geyer, 1995). Because abstinence criteria have ranged from 24 hours (Patrick and Struve, 2000) to 28 days (Rentzsch et al., 2007) across these studies, yet the reported P50 ratio deficits are similar, the observed gating abnormality seems to be a robust correlate of heavy cannabis use. In addition, each of these studies reported that increased cannabis use (e.g., number of joints per week and the number of years smoking) was associated with higher (worse) P50 gating ratios. Despite these intriguing findings, analyses from these studies were limited to traditional N40-P50 trough-peak measures and did not assess neural oscillatory activity in frequencies known to either comprise the P50 ERP or manifest pronounced gating effects in the dual-click procedure, such as low frequency activity (e.g., 0-20 Hz; see Johannesen et al., 2005; Clementz et al., 1997; Blumenfeld & Clementz, 2001) and higher frequency ranges such as beta (Hong et al., 2004) and gamma band activity (see Blumenfeld & Clementz, 2001; Brockhaus-Dumke, Mueller, Faigle & Klosterkoetter, 2008; Clementz et al., 1997; Johannesen et al., 2005).
In humans, neural oscillatory activity in the above mentioned frequency domains have been associated with different functional roles in information processing. For instance, neural oscillations in the gamma frequency range (about 20/30-50 Hz) emerge after exposure to a discrete stimulus and are thought to be an index of stimulus registration (Blumenfeld & Clementz, 2001; Karakas & Basar, 1998). Beta oscillations are typically observed following gamma activity and have been associated with perceptual binding (von Stein, Rappelsberger, Sarnthein, & Petsche, 1999) and novelty detection (Whittington, Traub, Faulkner, Stanford, & Jefferys, 1997), and may be capable of transmitting/integrating information across more distal, higher-order, brain regions than gamma (Kopell, Ermentrout, Whittington, & Traub, 2000). Some research has separated broad beta activity into low beta (beta1; 12-20 Hz) and high beta (beta2; 21-30 Hz), and suggest that beta1 oscillations are sensitive to novelty (Haenschel, Baldeweg, Croft, Whittington, and Gruzelier, 2000). Once a stimulus has been registered, supplementary attentional mechanisms further encode the stimulus as reflected by event-related neural oscillations in low frequency bands (e.g., Blumenfeld & Clementz, 2001). Specifically, theta and alpha have been associated with selective attention processes and working memory, respectively (see Başar, Başar-Eroğlu, Karakaş, & Schürmann, 1999). Thus, examining distinct information processing components of the auditory evoked brain response by parsing separable oscillatory frequency bands can inform the nature of the task-activated neural mechanisms related to stimulus registration and higher-order stimulus processing.
The relationship between neural oscillatory abnormalities and cannabis use is an important issue to address because emerging evidence suggests that the endogenous cannabinoid system is a mediator of hippocampal oscillations in the low frequency theta (4-7 Hz) range (Reich, Karson, Karnup, Jones, & Alger, 2005) and higher beta/gamma frequency range (e.g., 12-50 Hz; Wilson and Nicoll, 2002; Hájos et al., 2000, see also Soltesz and Staley, 2006). In addition, assessment of neocortical cells in vitro suggests that cannabinoids mediate cortical oscillations (Bacci, Huguenard, & Prince, 2004). A study by Hajós, Hoffman, and Kocsis (2008) examined the performance of rats in the dual-click procedure following administration of the CB1 agonist CP-55940. The CB1 agonist impaired N40 sensory gating and was associated with desynchronization of beta/gamma and theta range hippocampal oscillations following the paired clicks. Prefrontal cortical recordings yielded a similar attenuation of beta/gamma and theta power after drug administration during free movement. Both of these effects were reversed by the CB1 antagonist AM-251. Thus, the identification of oscillatory abnormalities in the hippocampus and neocortex that were induced by disruption of the cannabinoid system strongly supports investigation of such processes in humans with a disturbed cannabinoid system, such as long-term cannabis users (Villares, 2007).
The primary aim of the current study was to extend the translational potential of the P50 cannabis literature by elucidating neural oscillations that may accompany P50 sensory gating disturbances in heavy cannabis users (Patrick et al., 1999; Patrick and Struve, 2000; Rentzsch et al., 2007). Traditional methods of indexing information processing mechanisms by measuring peak and trough of the time-domain grand average ERP conceal changes in frequency-specific neural bands that may be associated with perturbed information processing mechanisms (Delorme and Makeig, 2004). Thus, assessment of frequency-specific induced and evoked neural oscillations may provide a clearer picture of information processing abnormalities observed in heavy cannabis users with the dual-click procedure, as well as clarify the relationship between mid-latency ERPs and frequency-specific oscillations. Toward this end, a Fourier-based time-frequency analysis was conducted on each trial in addition to a traditional time-domain assessment of the P50 auditory ERP. The time-frequency procedure generated an event-related spectral perturbation (ERSP) composed of induced and evoked oscillatory power across time and an inter-trial coherence (ITC) measure yielding the degree of between-trial phase variability across time. It is important to emphasize that the ERSP is sensitive to amplitude/magnitude of the response, while ITC is sensitive to the phase of the response but not its amplitude. Based on prior human and rat research (Bacci et al., 2004; Hajós et al., 2008), it was hypothesized that (1) the heavy cannabis users would exhibit higher P50 gating ratios compared to the cannabis naïve control group. (2) Because CB1 receptors are thought to mediate the maintenance of hippocampal and neocortical rhythms in the theta, beta, and gamma range, it was also predicted that cannabis use would be associated with attenuated neural oscillations in these frequencies. Because the current study assessed cortical activity, it was expected that these group differences would occur in the first 200 ms post-stimulus, where these P50 ERP component frequencies typically exhibit their greatest power (e.g., Johannesen et al., 2005; Blumenfeld and Clementz, 2001). Finally, (3) consistent with the prior research we expected that rates of cannabis use would be positively associated with impaired gating.
Methods
Participants
Seventeen heavy cannabis users (group CB; mean age = 20.53, SD = 2.48) and 16 healthy cannabis-naïve controls (group HN; mean age = 22.19, SD = 3.82) were assessed. The study was approved by the human subjects institutional review board. Participants were recruited from the local university community, provided written informed consent, and were paid for their participation. Demographic information and rates of cannabis use are shown in Table 1. The inclusion criteria were as follows. For the cannabis group: 1 ≥ joints per week for the past month (24 hour abstinence prior to testing to control acute effects while permitting possible long-term effects of drug use); positive urine toxicology screen for only THC given just prior to testing; no other illicit substance use during the past three months; and no DSM-IV diagnosis of Axis I or II disorders except cannabis abuse or dependence. For the control group: no history of illicit substance use; a negative urine toxicology screen for all drugs tested; and no history of psychiatric illness (Axis I). For all participants: no reported hearing problems, neurological disease, learning disability, or head injury resulting in loss of consciousness. Participants were excluded if they reported consumption of more than 2 alcoholic drinks on average per day (1 per day for females) during the past month.
Table 1.
Demographic and drug use histories for the cannabis group and control participants.
| Variable | Cannabis Group (n = 17) |
Control Group (n = 16) |
Value* | p * |
|---|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | |||
| Age (Years) | 20.53 (2.48) | 22.19 (3.82) | F = 2.22 | 0.15 |
| Years of Education | 14.62 (1.41) | 15.13 (1.50) | F = 1.00 | 0.32 |
| WAIS (Picture Completion) ** | 11.76 (2.79) | 12.06 (3.26) | F = 0.08 | 0.78 |
| WAIS (Digit Symbol) ** | 11.77 (2.80) | 13.19 (3.49) | F = 1.68 | 0.20 |
| WAIS (Similarities) ** | 13.71 (2.54) | 12.25 (2.65) | F = 2.60 | 0.12 |
| WAIS (Digit Span) ** | 12.41 (2.74) | 10.94 (2.89) | F = 2.27 | 0.14 |
| PAS | 2.59 (2.15) | 1.50 (1.51) | F = 2.80 | 0.10 |
| SPQ | 14.47 (12.42) | 10.44 (6.27) | F = 1.36 | 0.25 |
| Mean Cannabis (Joints) Per Week (Past Month) | 6.50 (6.05) | 0(0) | ||
| Cannabis Use Past Month | 42.81 (39.02) | 0(0) | ||
| Cannabis Use Past 6 Months | 204.06 (181.33) | 0(0) | ||
| Age of First Cannabis Use | 15.63 (1.92) | 0(0) | ||
| Total Years of Cannabis Use | 4.18 (1.60) | 0(0) | ||
| Mean Alcoholic Drinks Per Week (Past Month) | 4.97 (3.43) | 2.46 (4.18) | F = 3.15 | 0.09 |
| Mean Number of Cigarettes Per Day (Past Month) | 1.63 (3.95) | 0.14 (0.53) | F = 3.39 | 0.08 |
| N(%) | N(%) | |||
| Male | 13 (76.5%) | 7 (43.8%) | ||
| Female | 4 (23.5%) | 9 (56.3%) | ||
| Diagnosis of Cannabis Abuse | 7 (30%) | 0(0%) | ||
| Diagnosis of Cananbis Dependence | 4 (23%) | 0(0%) |
One-way ANOVA for the cannabis group versus control participants
Age-scaled WAIS scores
Clinical Interviews, Questionnaires, and Drug Use Assessment
The Structured Clinical Interview for DSM-IV Axis I and II Disorders (SCID I and SCID II) and subscales of the Wechsler Adult Intelligence Scale III (Picture completion, Digit Symbol, Similarities, and Digit Span) were administered to rule out psychiatric conditions and characterize general neuropsychological function (see Table 1). The clinical interview was used to ascertain current and past diagnoses for alcohol and substance abuse and dependence. Measures of frequency, quantity, and density of cannabis consumption were determined via the interview.
Urine screens (Q10-1, Proxam) were administered immediately preceding all testing. The kit screened for cannabis (11-nor-Δ-9-tetrahydrocannabinol-9-COOH ; 50 ng/mL sensitivity), opiates, amphetamines, cocaine, MDMA (ecstasy), tricyclic antidepressants, phencyclidine, benzodiazepines, methamphetamines, and barbituates.
Experimental Stimuli and Procedure
The experimental design consisted of 102 trials of paired click stimuli (500 ms SOA, 81 dB SPL, 3 ms white noise bursts; binaural). To facilitate similar levels of engagement and alertness during the testing session, participants were asked to press a button to additional infrequent paired tones (2000 Hz, 500 ms SOA, 81 dB SPL, 3 ms duration; binaural) that randomly occurred 18 times during the procedure, thus yielding a total of 120 trials. Because it was important that the data be free of preparatory activity, the participants were specifically told not to prepare to respond to the infrequent paired tones and that the speed of their response was irrelevant. Recorded activity to the infrequent clicks was not examined. Foam ear inserts (E-A-RLINK, Aearo Company Auditory Systems, Indianapolis, IN) were used to present the auditory stimuli. All paired-trials were separated with a 9 ± 3 s inter-trial interval (i.e., S1 to S1). During the experiment, participants were seated up-right in a recliner within a sound-attenuated, dimly lit, and electrically shielded booth. The procedure lasted about 18 minutes.
Data Acquisition and Processing
EEG activity at FCz (referenced to the nose) and vertical electrooculogram (EOG) activity was recorded with 10 mm disc gold electrodes (MedAssociated, St. Albans, VT) filled with EC2 electrode cream (Astro-Med inc., RI). The vertical EOG was recorded using two electrodes placed 1 inch above and below the right eye. A ground electrode was placed on the forehead. The skin was cleaned with NuPrep prior to electrode application. All electrode impedances were below 10 kΩ. Electrophysiological data were continuously recorded at a sampling rate of 2.5 kHz with a Sensorium EPA-6 bioamplifier. The electroencephalographic data (analog highpass filter = 0.02 Hz, 12 dB/octave; lowpass filter = 300 Hz, 8th order elliptic; gain = 10,000 for EEG) and electrooculargraphic data (analog highpass filter = 1 Hz, 12 dB/octave; lowpass filter = 300 Hz, 8th order elliptic; gain = 5000) were acquired using the software Neuroscan (v.4.1, El Paso, TX).
P50 ERP
To assess the P50 ERP the following steps were executed using Neuroscan software: recorded EEG was segmented into 2000 ms epochs (500 ms pre-stimulus and 1500 ms post-S1 onset); -50 to 0 ms baseline amplitude correction at S1; VEOG artifact rejection excluded epochs with voltage exceeding ± 75 μV; EEG artifact rejection excluded epochs when FCz voltage exceeded ± 75 μV (this relatively conservative rejection criteria retained about 80% of the trials across groups); visual inspection of remaining trials; 0.02 – 50 Hz bandpass filter (24 dB/octave) was applied; epochs were cut to -50 to 1000 ms and then averaged. N40 (greatest negative peak between 35 and 50 ms) and P50 ERP peaks (greatest positive peak between 50 and 85 ms) were automatically selected by an algorithm and then checked by an experimenter (CRE), who was blind to group membership. An additional criterion was that the P50 peak and trough latencies were within 10 ms of the response to the first click (Brenner et al., 2004). P50 amplitude was determined as the P50-N40 amplitude difference. In accord with prior research, individuals with non-discernable S1 P50 amplitudes (e.g., less than 1 microvolt) were considered non-responders (2 controls, 1 cannabis user) and excluded from P50 analyses (but not the time x frequency analyses). If there was no positive ERP deflection recorded at S2 (i.e., complete P50 suppression), an amplitude of .01 microvolts was entered so that the gating ratio could be derived (1 control).
Time x Frequency Analysis
EEG power across time was assessed using the EEGLab toolbox for Matlab software (Delorme and Makeig, 2004). A baseline normalized event-related spectral perturbation (ERSP) was obtained separately for each group by applying a Fast Fourier Transform (FFT) using a 128 ms sliding window on single trial data. The 500 ms interval prior to stimulus onset (S1) was used as the baseline for computing the ERSP. After 500 ms padding, a frequency resolution of 2.44 Hz was obtained and the time resolution was 6.51 ms. The ERSP figures are shown in Figure 2.
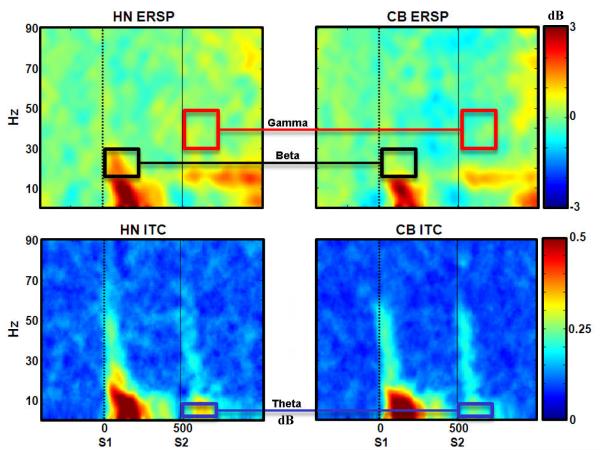
Figure 2.
Visualization of the ERSP (top) and ITC (bottom) data between groups. Dotted white lines indicate each click stimuli. ERSP color values are in dB while ITC color values in are phase-locked correlations where 1 represents perfect phase synchrony across trials and 0 represent the lack of synchrony. Boxes indicate regions where groups statistically differed.
Following generation of the ERSP, the intertrial coherence (ITC) was computed to determine whether possible abnormalities in ERSP power were due to impairment in phase variability across trials. While the ERSP is sensitive to magnitude/amplitude of the response, the ITC is a measure of phase synchronization of EEG activity across trials at particular temporal intervals and frequencies and is less sensitive to amplitude (Delorme and Makeig, 2004; O’Donnell et al., 2004). To compute ITC, the complex output of the baseline normalized ERSP was divided by its complex norm (absolute value), which was then averaged across trials. The complex norm of this averaged value results in the ITC for different time and frequency points. ITC values range from 0 (absence of synchronization) to 1 (perfect synchronization, or phase reproducibility across trials at a given latency). The ITC figures are depicted in Figure 2. The following frequency domains were assessed: theta (4-7 Hz), alpha (8-12 Hz), broad beta (13-29 Hz), low beta (13-20 Hz), high beta (21-29 Hz), and gamma (30-50 Hz).
Statistical Analysis
An ANOVA was employed using the between-subject factor of cannabis use for the dependent variable P50 ratio. A 2 (group) by 5 (time: average power in five 40 ms windows following each click) repeated-measures ANOVA was used to examine changes in frequency power and ITC across time separately for each click. For analysis of frequency changes between clicks an ANOVA analysis was conducted across all subjects. For the repeated-measures ANOVAs, the effect sizes are reported in the form of partial eta2 (PES): small effect sizes are less than .06; moderate effect sizes range from .06 to .14; large effect sizes are greater than .14 (Cohen, 1973). All tests were two-tailed, and significance was established at p < 0.05. For pairwise comparisons, Cohen’s d estimates of effect size are reported where values of 0.2, 0.5, and 0.8 are considered small, medium, and large effects sizes, respectively (Cohen, 1992).
Results
P50 Gating
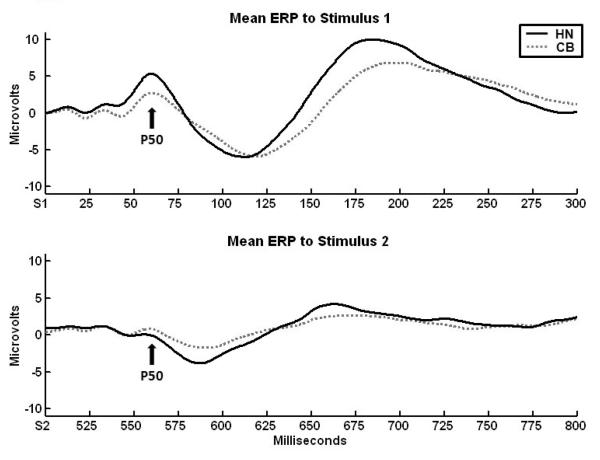
The grand average P50 waveforms for each group are shown in Figure 1. Although the groups did not statistically differ in their P50 amplitudes to S1 (HN mean = 4.89, SD = 4.29; CB mean = 3.98, SD = 2.67), F (1,28) = .49, p = .49, d = .25, or S2 (HN mean = .96, SD = 1.69; CB mean = 1.53, SD = 1.21), F(1,28) = 1.14, p = .294, d = .39, the P50 ratio (S2/S1) was significantly larger in the CB group (mean = .42, SD = .33) compared to the HN control group (mean = .11, SD = .12), F(1,28) = 10.63, p = .003, d = 1.25. As with prior studies of P50 gating in cannabis users, only the P50 ratio measure, not the S1-S2 difference score (F(1,28) = 2.51, p = .13 , d = .59), differentiated the groups.
Figure 1.
Mean ERP to S1 and S2 between groups illustrating the attenuation of the P50. The S2/S1 ratio significantly distinguished between the HN and CB group (p < .01), the CB group xhibiting a larger ratio.
Event-Related Spectral Perturbation
Consistent with the prediction, (see Figure 2), ERSP power was most prominent in the first 200 ms post click. The data were therefore assessed within just the first 200 ms (5 time windows of 40 ms each). Because Table 2 shows the primary descriptives, main effects, and interactions for all frequencies, only the findings achieving statistical significance will be fully presented in the text. In addition, the effects of time will also not be discussed here because a main effect of time would be expected (see Figure 3) in an event-related response with the greatest magnitude of difference typically occurring closer to the stimuli (unless otherwise noted). All time x group interactions were non-significant (see Table 2).
Table 2.
ANOVA Results for Time × Frequency Analysis of ITC and ERSP Data
| ITC | S1 Click | S2 Click | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean(SE) | Group(G) | Time(T) | GxT Int. | Mean(SE) | Group(G) | Time(T) | GxT Int. | |
| Gamma | HN=.17(.01);CB=.15(.01) | F=l.43 | F=28.59** | F=.43 | HN=.13(.01);CB=.13(.01) | F=.13 | F=20.33** | F=.72 |
| Beta | HN=.25(.02);CB=.19(.02) | F=3.52 | F=19.81** | F=1.08 | HN=.14(.01);CB=.14(.01) | F=.O8 | F=8.72** | F=l.09 |
| Beta 2 | HN=.22(.02);CB=17(.02) | F=2.53 | F=24.78** | F=.79 | HN=.14(.01);CB=.14(.01) | F=.05 | F=.8.17** | F=.69 |
| Beta 1 | HN=.29(.03);CB=.22(.03) | F=3.17 | F=.6.52** | F=.70 | HN=.15(.01);CB=.14(.01) | F=.05 | F=2.70* | F=.75 |
| Alpha | HN=.42(.04);CB=.38(.04) | F=.51 | F=14.66** | F=.15 | HN=.20(.02);CB=.16(.02) | F=2.76 | F=4.45** | F=.56 |
| Theta | HN=A6(.04);CB=.47(.03) | F=.06 | F=81.81** | F=.04 | HN=.28(.02);CB=.21(.02) | F=4.63** | F=6.47** | F=1.29 |
| ERSP | S1 Click | S2 Click | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean(SE) | Group(G) | Time(T) | GxT Int. | Mean(SE) | Group(G) | Time(T) | GxT Int. | |
| Gamma | HN=.00(.14);CB=−.14(.12) | F=.59 | F=3.31** | F=07 | HN=.19(.14);CB=−.28(.12) | F=6.61** | F=.91 | F=1.16 |
| Beta | HN=1.12(.21);CB= 58(.19) | F=3.67 | F=6.70** | F=.68 | HN=. 99(.27);CB=.98(.25) | F=.00 | F=2.66* | F=.19 |
| Beta 2 | HN=.63(.18);CB=.09(.16) | F=5.06* | F=7.18** | F=.91 | HN=.20(.16);CB=−.18(.15) | F=2.97 | F=.49 | F=.10 |
| Beta 1 | HN=1.12(.21);CB=58(.19) | F=3.67 | F=6.70** | F=.68 | HN=.99(.27);CB=.98(.25) | F=.00 | F=2.66* | F=.19 |
| Alpha | HN=2.00(.34);CB=1.51 (.31) | F=1.14 | F=31.13** | F=.21 | HN=.45(.33);CB=.53(.30) | F=.03 | F=9.44* | F=l.43 |
| Theta | HN=2. 19(.36);CB=1.56(.32) | F=1.78 | F=58.69** | F=.05 | HN=.02(40);CB=−.16(.36) | F=.10 | F=11.55** | F=.08 |
p<.05,
p<0l
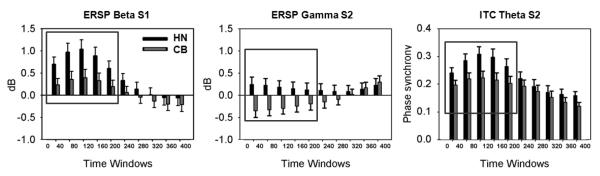
Figure 3.
Illustration of the time x frequency analysis across a 400 ms post-stimulus period. Thus, each window corresponds to the mean activity (power for ERSP or phase-locking value for ITC) in a 40 ms time window within a given frequency domain. The red boxes indicate the windows submitted to the statistical analysis. For the ERSP, there was a significant main effect of group for the first 5 windows post-stimulus for broad band beta following S1 (p < .05) and for gamma following S2 (p < .05). For the ITC theta, there was a significant main effect of group (p < .05), with cannabis using participants exhibiting less phase-locked theta post-S2.
Click Effects Across Groups
Examination of frequency power between clicks (collapsed across groups) in the broad beta domain yielded a click x time interaction (F(4,112) = 5.05, p = .016, PES = .15), which reflected a larger beta component 100-150 ms post-S1 compared to the distribution of activity in the time bins post-S2. This effect appeared to be carried by the high beta2 domain in which a larger S1 response was reflected in a main effect of click (S1>S2; F(1,28) = 5.04, p = .033, PES = .15) as well as in the click x time interaction (F(4,112) = 7.82, p = .002, PES = .22). The interaction represented S1 activity maximizing by 40-80 ms ((t(28) = 2.87, p = .008) and returning to comparable S2 levels in the 120-160ms window (t(28) = 1.77, p = .086). The alpha activity was also greater post-S1 as evidenced by a main effect of click (S1>S2; F(1,28) = 18.56, p = .001, PES = .40) and a click x time interaction (F(4,112) = 5.63, p = .015, PES = .17. The interaction corresponded to increasing S1 activity, compared to S2 activity across the window with the most prominent click difference occurring in the 120-160ms window (t(28) = 3.88, p = .001). For the theta band, there was a main effect of click (S1>S2; F(1,28) = 51.57, p = .001, PES = .65) and a click x time interaction (F(4,112) = 37.93, p = .001, PES = .58), with comparable theta activity for S1 and S2 in the 0-40 ms bin with increasing S1 activity maximizing in the 160-200ms window (t(28) = 8.56, p = .001). Attenuated power was observed across all frequencies at S2 compared to S1, with the exception of the gamma band, indicating a pronounced gating effect across frequencies (see Figure 2 for a visual illustration of click effects, and Table 2 for means and standard deviations).
Group Differences by Click
For frequency data following S1, a significant main effect of group was found across the 5 time windows of the broad beta frequency indicating lower levels in cannabis users (see Figure 3), F(1,27) = 5.28, p = .03, PES = .16 (HN mean = .84, SE = .17; CB mean = .30, SE = .16). This pattern was evident in the high beta2 frequency domain at S1, F(1,27) = 5.06, p = .03, PES = .16 (HN mean = .63, SE = .18; CB mean = .09, SE = .16), but not in the lower beta1 domain. For gamma activity following S2, there was also a significant main effect of group (see Figure 3), F(1,27) = 6.61, p = .02, PES = .20, with the CB group exhibiting markedly less gamma power, compared to controls (HN mean = .19, SE = .14; CB mean = -.28, SE = .12).
To summarize, analysis of the ERSP showed a striking gating effect across all frequency domains except the gamma band. The group x time analyses demonstrated attenuated high beta2 activity after S1 and attenuated gamma activity following S2.
Intertrial Coherence
The magnitude of phase-locked activity (i.e., ITC) was also evaluated in 5 time segments, each 40 ms in duration, across the 200 ms post-click window between groups. In the interest of space only significant group findings will be noted here (see Table 2 for full statistics).
Click Effects Across Groups
Examination of phase-locking between clicks (across subjects) in the gamma domain yielded a main effect of click (S1>S2; F(1,28) = 13.44, p = .001, PES = .32), with greater gamma phase-locking post-S1. Analysis of broad beta activity yielded a main effect of click (S1>S2; F(1,28) = 27.04, p = .001, PES = .49) and an click x time interaction (F(4,112) = 6.16, p = .008, PES = .18), with the greatest S1 broad beta phase-locking difference occurring in the 40-80 ms window and approaching reconvergence by 200 ms (p < .01). Greater beta phase-locking after S1 was also reflected in a main effect of click for low beta1 activity (F(1,28) = 26.90, p = .001, PES = .49) and a main effect of click (S1>S2; F(1,28) = 11.15, p = .002, PES = .29). High beta2 activity also showed a main effect of click (S1>S2; F(1,28) = 11.147, p = .001, PES = .28), as well as a click x time interaction (F(1,28) = 4.90, p = .02, PES = .15) with the greatest click difference occurring at 80-120ms (t(28) = 3.67, p = .001) and approaching reconversion in the 200 ms window. Alpha phase-locking was similarly reduced at S2 as indicated by a main effect of click (S1>S2; F(1,28) = 61.54, p = .001, PES = .69) and a significant interaction (F(4,112) = 8.20, p = .003, PES = .23) with the click difference maximizing in the 160-200ms window. For the theta band, there was a main effect of click (S1>S2; F(1,28) = 38.79, p = .001, PES = .58) and interaction (F(4,112) = 50.47, p = .001, PES = .64). Theta ITC click differences increased throughout the window and maximized in the 160-200ms time bin (t(28) = 7.56, p = .001). Taken together, phase-locked activity was attenuated across all frequencies post-S2.
Group Differences by Click
A main effect of group was observed in theta phase-locking following S2, F(1,27) = 4.63, p = .04, PES = .146, with the cannabis group showing a greater attenuation of theta coherence (see Figure 3).
To summarize the ITC analyses, attenuated phase-locking was found across all frequency domains following S2. However, the cannabis group only exhibited reduced theta after S2, compared to controls.
Pearson Correlations
P50 gating and frequency analysis
The 41-80 ms window (which is the window during which the P50 occurs) was utilized for assessing the relationship between S1 and S2 P50 amplitudes and the ERSP and ITC activity. To correct for multiple comparisons between the two clicks, the six frequency domains, and the two frequency measures (i.e., ITC and ERSP), a Bonferroni adjustment was employed yielding a significance criterion of p < .002 (.05/24).
S1 P50 amplitude was positively correlated with several mean S1 ITC beta frequency values including broadband beta, r(29) = .66, p = .0001, as well as low, r(29) = .58, p = .001, and high beta, r(29) = .60, p = .0006. S2 P50 amplitude was positively correlated with S2 ITC broadband beta, r(29) = .71, p = .00001, and high beta, r(29) = .74, p = .000005. With regard to mean ERSP data, S1 P50 amplitude was significantly correlated with broad beta, r(29) = .61, p = .0005, and high beta, r(29) = .60, p = .0006, but not the low beta frequency. Interestingly, S1 P50 amplitude also correlated with S1 ERSP alpha power, r(29) = .72, p = .00001. However, S2 P50 amplitudes were not significantly associated with any of the ERSP frequency measures in the 41-80 mean window following S2.
In sum, whereas the P50 amplitudes at both S1 and S2 were positively associated with post-click phase-locked beta activity, only variability in post-S1 beta and alpha spectral power was positively associated with S1 amplitude.
Cannabis use
To examine the prediction that cannabis use would be associated with indices of sensory gating impairments, correlations between the number of cannabis joints reportedly smoked in the previous 6 months and the ERP amplitudes, ITC values, and ERSP values were assessed.
There was a positive relationship between the reported number of joints smoked in the prior 6 months and the P50 gating ratio, r(16) = .50, p = .05, suggesting that increased estimates of long-term cannabis use were associated with poor P50 gating. While phase-locking values did not significantly correlate with cannabis use, decreased mean ERSP gamma power in the post-S2 200 ms window was correlated significantly with increased cannabis use in the prior 6 months, r(16) = -.528, p = .036.
Discussion
Consistent with previous reports, heavy cannabis users exhibited impaired P50 sensory gating in the dual-click procedure compared to cannabis naïve participants. When groups were collapsed, all ERSP and ITC activity was markedly attenuated after S2, with the only exception being gamma power. Analysis of inter-trial coherence indicated that cannabis users showed attenuated phase-locked theta activity following S2 in the 200 ms following stimulus onset. Analysis of the ERSP indicated that cannabis users exhibited a time-specific reduction in frequency power immediately following S1 in high-frequency bands, including gamma, broad beta, and beta2, and following S2 in just the gamma frequency. Increased rates of cannabis use were associated with reduced post-S2 ERSP gamma power and, in agreement with prior studies, associated with high P50 ratios (Patrick et al., 1999; Patrick and Struve, 2000; Rentzsch et al., 2007).
Finding reduced oscillatory power in the beta range in the cannabis group post-S1 is consistent with human studies demonstrating that cannabis use is associated with attenuated beta power elicited to auditory (Skosnik et al., 2006a) and visual (Skosnik et al., 2006b) steady state stimulation. These data also corroborate two very recent animal studies. Dissanayake et al. (2008) reported that CB1 agonist administration (WIN55,212-22) disrupted auditory gating in rats as measured by local field potentials in the dentate gyrus, CA3, and medial prefrontal cortex; these effects were reversed by the CB1 antagonist rimonabant. Similarly, a study by Hajós and colleagues (2008) reported disruption of sensory gating (rat N40) in hippocampal areas CA3 and entorhinal cortex following administration of the CB1 agonist CP-55940 that was reversed by the CB1 antagonist AM-251. Moreover, this group expanded traditional ERP measures of gating and identified reduced power of high beta/gamma and theta oscillations during the paired-click presentations by the agonist that were also reversed by the antagonist (frequency measures were analyzed over the entire testing period). Likewise, high beta/gamma power and phase-locking in the theta band were attenuated in the EEG of cannabis users in the current study. Given previous evidence linking synchronized theta activity to selective attention processes (see Başar, Başar-Eroğlu, Karakaş, & Schürmann, 1999) it may be speculated that reduced phase-locked theta post-S2 reflects aberrant attentional processes in the cannabis group.
The observation that exposure to cannabinoids in humans and animals seems to impair the generation of neural activity in specific frequencies following exposure to auditory stimuli is consistent with data from Robbe and colleagues (2006) who reported that THC and CP-55940 administration, at doses comparable to human recreational use, markedly decrease the temporal coordination of hippocampal gamma and theta activity. While the relationship between hippocampal and cortical oscillations is currently unclear, these data reflect a substantial role of the cannabinoid system in the generation of neural oscillations and suggest that these mechanisms may be indexed by local field potentials and cortical electrodes during the dual-click paradigm.
One possible explanation for finding high beta and gamma oscillatory abnormalities in the cannabis group (and CB1 agonist treated rats) is based on the localization of CB1 receptors to cholecystokinin positive GABA-A interneurons in the neocortex (Hill et al., 2007) and hippocampus (Hájos et al., 2000). GABA-A interneurons are known to mediate cortical gamma bursts in vitro (Cunningham et al., 2004) and hippocampal rhythmic oscillations in vitro, including beta and gamma oscillations (Haenschel, Baldweg, Croft, Whittington, and Gruzelier, 2000). A genetic liability to beta frequency abnormalities has also been linked with GABA-A receptor genes (Porjesz et al., 2002). However, accumulating evidence suggests that cholecystokinin/CB1 GABA-A interneurons may fine tune gamma oscillations initiated by fast spiking gap-junction connected neuron populations (c.f., Cunningham et al., 2004; Hájos et al., 2000). It is therefore feasible that information processing mechanisms indexed by high frequency beta/gamma activity, such as stimulus registration (Karakas and Basar, 1998) and perceptual binding (von Stein et al., 1999), are impaired by cannabinoid system abnormalities induced by heavy cannabis use or CB1 agonist administration. Although, the endocannabinoid system has been documented to mediate hippocampal theta oscillations (Reich et al., 2005), which are posited to index selective attention (Başar-Eroğlu and Başar, 1991), the relationship between hippocampal theta activity and cortical EEG remains unclear and requires further study.
The identification of strong correlations between S1 and S2 P50 amplitudes and ITC of beta responses and a strong correlation between S1 P50 and the ERSP of beta and alpha frequencies suggests that while the P50 amplitude to S1 is associated with both power and phase-locked activity, the S2 P50 may be principally associated with phase-locked beta oscillations. The fact that none of the gamma measures were associated with P50 amplitudes in this study is not surprising given recent evidence that beta oscillations are more strongly associated with P50 amplitude gating than gamma oscillations (Hong et al., 2008a).
The present findings may be of relevance to the sensory gating literature in schizophrenia (e.g., Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; Patterson et al., 2008) given growing evidence of cannabinoid dysfunction in the disorder (e.g., Emrich et al., 1997; Ujike and Morita, 2004). For instance, in addition to poor sensory gating (greater P50 ratio) (Patterson et al., 2008), schizophrenia is associated with greater cerebral spinal fluid concentrations of anandamide (Giuffrida et al., 2004) and decreased expression of CB1 messenger RNA in dorsolateral prefrontal cortex, compared to controls (Eggan, Hashimoto, & Lewis, 2008). These data may be interpreted to support the model that increased availability of CB1 agonists (e.g., anandamide) in humans is compensated for by downregulating and/or desensitizing CB1 receptors (Villares, 2007; Edwards and Skosnik, in press). In terms of the clinical implications of the current data, the results provide further support that cannabis use is associated with gating disturbances similar to those found in schizophrenia (e.g., Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; Patterson et al., 2008).
Despite the interesting findings and implications of the present study, a number of limitations should be considered. First, the possibility exists that the sample size was insufficient to detect other meaningful differences between the groups. Second, although translational links between the current results and those reported based on studies of rat hippocampal regions have been presented, the relationship between human scalp recorded EEG and human and rat hippocampal activity is unclear and requires further study. Third, although consistent with all previous studies of cannabis users, the current study found that only the P50 S2/S1 gating ratio differed between the cannabis users and naïve controls, not the P50 S1-S2 difference measure. While the P50 S1-S2 difference score has been shown to be more psychometrically reliable than the ratio derivation (Smith et al., 1996), these two indices may assess different neural processes (Freedman et al., 1983, Smith et al., 1996). Finally, it is unclear whether the reported effects are due to the chronic use of cannabis (i.e. CB1 downregulation/desensitization; Villares, 2007), residual THC (Pope, Gruber, & Yurgelun-Todd, 2001), or withdrawal effects (Budney, Hughes, Moore, & Vandrey, 2004). However, the current sample of cannabis users was assessed after 24 hrs of abstinence, which is long enough for most THC clearance, and likely too short of a time period to induce significant withdrawal. Taken together, CB1 downregulation may be the more proximal and salient explanation of the observed effect compared with the possible subtle effects of residual cannabinoids or cannabis withdrawal. Future studies utilizing the P50 paradigm during direct THC administration in humans could address this issue.
In conclusion, these data replicate and extend previous P50 studies in heavy cannabis users by making contributions to human and animal literature. In terms of the human P50 literature, these data support a role for high frequency neural oscillatory activity in mediating differential brain responses to S1 and S2 in the dual-click procedure and suggest that heavy recreational cannabis use may disrupt these mechanisms.
These results also contribute to translational research by demonstrating that humans chronically exposed to CB1 agonists exhibit phase-locking and power abnormalities suggestive of impaired temporal coordination of neural activity, as has been analogously reported in non-human in vivo studies (see Hájos et al., 2008). These data are consistent with the hypothesis that chronic use of cannabis causes downregulation and/or desensitization of CB1 receptors in auditory and hippocampal cortices, which may impair the capacity of GABAergic interneurons in these areas to maintain the theta, alpha, and beta oscillations recently associated with the magnitude of sensory gating (Hong et al., 2008a,b). Examining whether aberrant neuronal oscillations in cannabis users are related to those observed in cannabinoid-treated non-human animals during the dual-click procedure would be an important next step in strengthening the translational potential of this literature.
Acknowledgements
We wish to thank Jennifer Vollmer and Emily Cahill for help running participants. This work was funded by a grant from NIDA (1 R03 DA019630-01; 1 R21 DA023097-01A1) and a NARSAD Young Investigator Award received by Patrick D. Skosnik. This work was also supported by a NIDA (T32 DA024628) training grant awarded to the Program in Neuroscience at Indiana University which provided fellowship funding for Chad R. Edwards.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/bne.
Rentzsch et al. (2007) did not report if S2 amplitudes were significantly different between the control and cannabis groups. However, using the reported means (controls: 2.1, cannabis: 1.1), standard deviations (1.5, 0.8, respectively) and group size (11, 18, respectively), an unpaired t-test analysis yielded a significantly larger P50 S2 amplitude for the cannabis group (t (27) = 2.35, p < .05).
References
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakaş S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta, and delta oscillations in the EEG? Neuroscience Letters. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Başar E. A compound P300-40 Hz response of the cat hippocampus. International Journal of Neuroscience. 1991;60:227–237. doi: 10.3109/00207459109167035. [DOI] [PubMed] [Google Scholar]
- Bickford-Wimer PC, Nagamoto H, Johnson R, Adler LE, Egan M, Rose GM, Freedman R. Auditory sensory gating in hippocampal neurons: a model system in the rat. Biological Psychiatry. 1990;27:183–1192. doi: 10.1016/0006-3223(90)90648-l. [DOI] [PubMed] [Google Scholar]
- Blumenfeld LD, Clementz BA. Response to the first stimulus determines reduced auditory evoked response suppression in schizophrenia: single trials analysis using MEG. Clinical Neurophysiology. 2001;112:1650–1659. doi: 10.1016/s1388-2457(01)00604-6. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Belger A, Campbell D, D’Souza C, Krystal J. Comparison of four components of sensory gating in schizophrenia and normal subjects: A preliminary report. Psychiatry Research. 1999;88:119–130. doi: 10.1016/s0165-1781(99)00074-8. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Bonnet KA, Millana R, Liu JA. A parametric study of the N40 auditory evoked response in rats - component definition. Biological Psychiatry. 1997;42:1051–1059. doi: 10.1016/s0006-3223(97)00161-3. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Kwan SW. Test-retest reliability of the rat N40 auditory evoked response: preliminary data. Psychiatry Research. 1998;81:269–276. doi: 10.1016/s0165-1781(98)00096-1. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Gating and habituation deficits in the schizophrenia disorders. Clinical Neuroscience. 1995;3(2):131–139. [PubMed] [Google Scholar]
- Brenner CA, Edwards CR, Carroll CA, Kieffaber PD, Hetrick WP. P50 and acoustic startle gating are not related in healthy participants. Psychophysiology. 2008;41:702–708. doi: 10.1111/j.1469-8986.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophrenia Research. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the Validity and Significance of Cannabis Withdrawal Syndrome. American Journal of Psychiatry. 2004;161(11):1967. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory Gating Deficits Assessed by the P50 Event-Related Potential in Subjects With Schizotypal Personality Disorder. American Journal of Psychiatry. 2000;157(1):55. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Geyer MA, Braff DL. P50 Suppression Among Schizophrenia and Normal Comparison Subjects: A Methodological Analysis. Biological Psychiatry. 1997;41:1035–1044. doi: 10.1016/S0006-3223(96)00208-9. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FEN, Vogt A, Monyer H, Buhl EB, Traub RD. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Eta-Squared and Partial Eta-Squared in Communication Science. Human Communication Research. 1973;28:473–490. [Google Scholar]
- Cohen J. Quantitative methods in psychology: A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dissanayake DWN, Zachariou M, Marsden CA, Mason R. Auditory gating in rat hippocampus and medial prefrontal cortex: Effect of the cannabinoid agonist WIN55,212-2. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.08.039. doi: 10.1016/j.neuropharm.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Edwards CR, Skosnik PD. Cerebellar-dependent learning as a neurobehavioral index of the cannabinoid system. Critical Reviews in Neurobiology. doi: 10.1615/critrevneurobiol.v19.i1.30. (in press) [DOI] [PubMed] [Google Scholar]
- Eggan SM, Hashimoto T, Lewis DA. Reduced Cortical Cannabinoid 1 Receptor Messenger RNA and Protein Expression in Schizophrenia. Archives of General Psychiatry. 2008;65(7):772. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical Distribution of the Cannabinoid CB1 Receptor in the Primate Neocortex: A Regional and Laminar Analysis. Cerebral Cortex. 2007;17:175. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Emrich HM, Leweke MF, Schneider U. Towards a cannabinoid hypothesis of schizophrenia: cognitive impairments due to dysregulation of the endogenous cannabinoid system. Pharmacology Biochemistry and Behavior. 1997;56:803–807. doi: 10.1016/s0091-3057(96)00426-1. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Waldo M. Gating of the Auditory Evoked Potential in Children and Adults. Psychophysiology. 1987;24:223–227. doi: 10.1111/j.1469-8986.1987.tb00282.x. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Ross RG, Waldo MC, Stevens KE, Adler LE, Leonard S. The genetics of sensory gating deficits in schizophrenia. Current Psychiatry Reports. 2003;5:155–161. doi: 10.1007/s11920-003-0032-2. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkötter, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernández G, Schaller C, Elger CE. Neuronal substrates of sensory gating within the human brain. Biological Psychiatry. 2003;53:511–519. doi: 10.1016/s0006-3223(02)01673-6. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophrenia Research. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Baldeweg T, Croft RJ, Whittington M, Gruzelier J. Gamma and beta frequency oscillations in response to novel auditory stimuli: a comparison of human electroencephalogram (EEG) data with in vitro models. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7645–7650. doi: 10.1073/pnas.120162397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. European Journal of Neuroscience. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- Hájos M, Hoffman WE, Kocsis B. Activation of cannabinoid-1 receptors disrupts sensory gating and neuronal oscillation: relevance to schizophrenia. Biological Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.12.005. in press. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, De Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Science. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. Journal of Neurophysiology. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW. Gamma/beta oscillation and sensory gating deficit in schizophrenia. NeuroReport. 2004;15:155–159. doi: 10.1097/00001756-200401190-00030. [DOI] [PubMed] [Google Scholar]
- Hong LE, Buchanan RW, Thaker GK, Shepard PD, Summerfelt A. Beta (~16 Hz) frequency neural oscillations mediate auditory sensory gating in humans. Psychophysiology. 2008a;45:197–204. doi: 10.1111/j.1469-8986.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, Thaker GK. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Archives of General Psychiatry. 2008b;65:1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophrenia Research. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Karakas S, Basar E. Early gamma response is sensory in origin: a conclusion based on cross-comparison of results from multiple experimental paradigms. International Journal of Psychophysiology. 1998;31:13–31. doi: 10.1016/s0167-8760(98)00030-0. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Hanlon FM, Franco AR, Teshiba TM, Thoma RJ, Clark VP, et al. The neural networks underlying auditory sensory gating. NeuroImage. doi: 10.1016/j.neuroimage.2008.08.025. In Press, Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon KA, Gerhardt GA, Bickford PC, Austin K, Rose GM, Woodward DJ, et al. Multiple single units and population responses during inhibitory gating of hippocampal auditory response in freely-moving rats. Brain Research. 1999;825(1-2):75–85. doi: 10.1016/s0006-8993(99)01187-7. [DOI] [PubMed] [Google Scholar]
- Neylan TC, Fletcher DJ, Lenoci M, McCallin K, Weiss DS, Schoenfeld FB, et al. Sensory gating in chronic posttraumatic stress disorder: reduced auditory p50 suppression in combat veterans. Biological Psychiatry. 1999;46(12):1656–1664. doi: 10.1016/s0006-3223(99)00047-5. [DOI] [PubMed] [Google Scholar]
- O’Donnell B, Hetrick W, Vohs J, Krishnan GP, Carrol CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished Suppression of the P50 Auditory Evoked Potential in Bipolar Disorder Subjects With a History of Psychosis. American Journal of Psychiatry. 2005;162(1):43. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience. 1999;92:1177–1191. doi: 10.1016/s0306-4522(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Patrick G, Straumanis JJ, Struve FA, Fitz-Gerald MJ, Leavitt J, Manno JE. Reduced P50 auditory gating response in psychiatrically normal chronic marihuana users: a pilot study. Biological Psychiatry. 1999;45:1307–1312. doi: 10.1016/s0006-3223(98)00155-3. [DOI] [PubMed] [Google Scholar]
- Patrick G, Struve FA. Reduction of auditory P50 gating response in marihuana users: further supporting data. Clinical Electroencephalography. 2000;31:88–93. doi: 10.1177/155005940003100207. [DOI] [PubMed] [Google Scholar]
- Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, Potkin S, Bunney WE., Jr. P50 sensory gating ratios in schizophrenics and controls: A review and data analysis. Psychiatry Research. 2008;158:226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Yurgelun-Todd D. Residual neuropsychologic effects of cannabis. Current Psychiatry Reports. 2001;3(6):507–512. doi: 10.1007/s11920-001-0045-7. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, et al. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Karson MA, Karnup SV, Jones LM, Alger BE. Regulation of IPSP theta rhythm by muscarinic receptors and endocannabinoids in hippocampus. Journal of Neurophysiology. 2005;94:4290–4299. doi: 10.1152/jn.00480.2005. [DOI] [PubMed] [Google Scholar]
- Rentzsch J, Penzhom A, Kernbichler K, Plöckl D, Gómez-Carrillo de Casto A, Gallinat J, Jockers-Scherübl MC. Differential impact of heavy cannabis use on sensory gating in schizophrenia patients and otherwise healthy controls. Experimental Neurology. 2007;205:241–249. doi: 10.1016/j.expneurol.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nature Neuroscience. 2006;9:1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Aydt EE, Kuhlenshmidt HA, O’Donnell BF. Psychophysiological evidence of altered neural synchronization in cannabis use: relationship to schizotypy. American Journal of Psychiatry. 2006a;163:1798–1805. doi: 10.1176/ajp.2006.163.10.1798. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan GP, Vohs JL, O’Donnell BF. The effect of cannabis use and gender on the visual steady state evoked potential. Clinical Neurophysiology. 2006b;117:144–156. doi: 10.1016/j.clinph.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Staley K. High times for memory: cannabis disrupts temporal coordination among hippocampal neurons. Nature Neuroscience. 2006;9:1461–1463. doi: 10.1038/nn1206-1461. [DOI] [PubMed] [Google Scholar]
- Trettel J, Fortin DA, Levine ES. Endocannabinoid signaling selectively targets perisomatic inhibitory inputs to pyramidal neurons in juvenile mouse neocortex. Journal of Physiology. 2004;556:95–107. doi: 10.1113/jphysiol.2003.058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein A, Rappelsberger P, Sarnthein J, Petsche H. Synchronization between temporal and parietal cortex during multimodal object processing in man. Cerebral Cortex. 1999;9:137–150. doi: 10.1093/cercor/9.2.137. [DOI] [PubMed] [Google Scholar]
- Weisser R, Weisbrod M, Roehrig M, Rupp A, Schroeder J, Scherg M. Is frontal lobe involved in the generation of auditory evoked P50? NeuroReport. 2001;12:3303–3307. doi: 10.1097/00001756-200110290-00031. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Faulkner HJ, Stanford IM, Jefferys JGR. Recurrent excitatory postsynaptic potentials induced by synchronized fast cortical oscillations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12198–12203. doi: 10.1073/pnas.94.22.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Ujike H, Takaki M, Nakata K, Tanaka Y, Takeda T, Kodama M, Fujiwara Y, Sakai A, Kuroda S. CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia. Molecular Psychiatry. 2002;7:515–518. doi: 10.1038/sj.mp.4001029. [DOI] [PubMed] [Google Scholar]
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]