Abstract
We analyzed the viral mRNA's present in fibroblast nonproducer clones transformed by avian erythroblastosis virus. Two size classes of mRNA (28 to 30S and 22 to 24S) were identified by solution hybridization with both complementary DNA strong stop and complementary DNA made against the unique sequences of avian erythroblastosis virus. Based upon the kinetics of hybridization with complementary DNA made against the unique sequences of avian erythroblastosis virus, we estimated that there were 400 to 500 copies of the 28 to 30S RNA per cell and 200 to 250 copies of the 22 to 24S RNA per cell. Both RNA species were packaged in the virion. In vitro translation of the 28 to 30S virion RNA yielded a 75,000-dalton protein which was the 75,000-dalton gag-related polyprotein found in avian erythroblastosis virus-transformed cells. In vitro translation of the 22 to 24S virion RNA yielded two proteins (46,000 and 48,000 daltons). This indicates that there may be two genes in avian erythroblastosis virus, one coding for the 75,000-dalton gag-related polyprotein and the second coding for the 46,000- or 48,000-dalton protein or both.
Full text
PDF

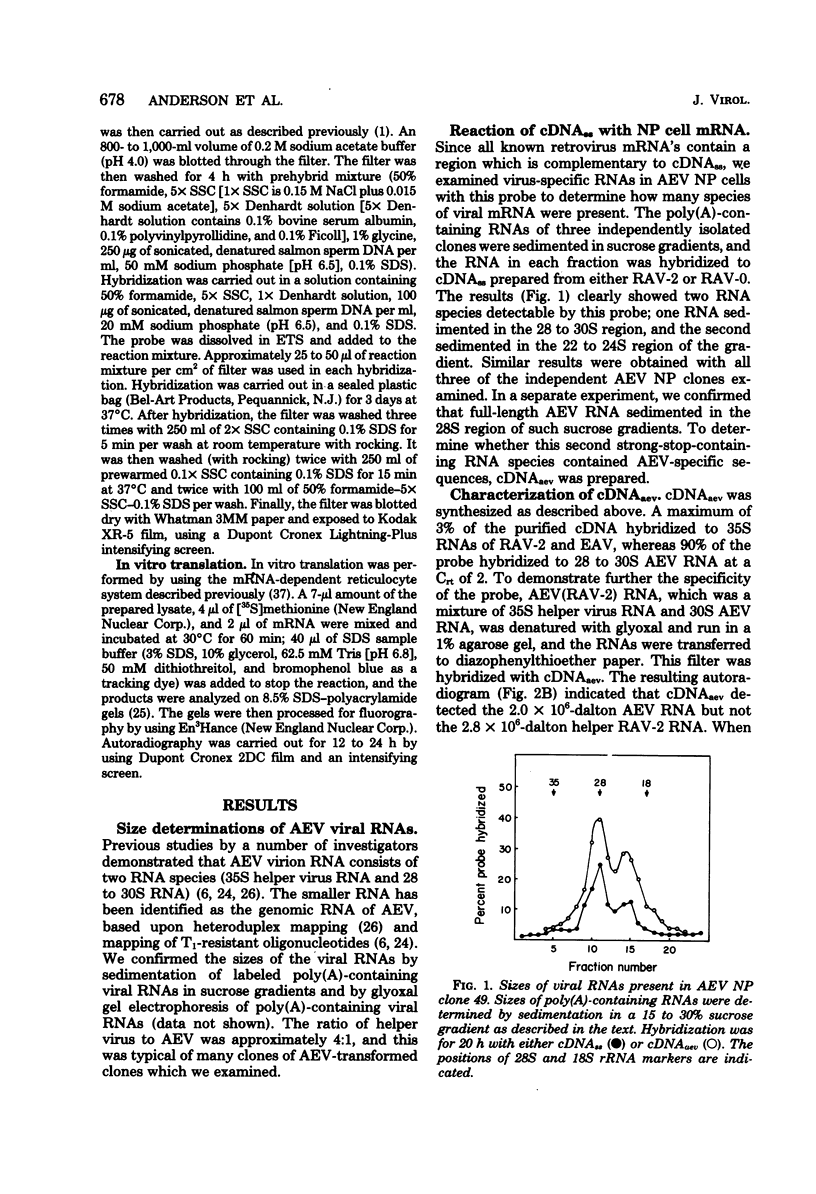





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Claviez M., Jockusch B. M., Graf T. Differential expression of Rous Sarcoma virus-specific transformation parameters in enucleated cells. Cell. 1978 Aug;14(4):843–856. doi: 10.1016/0092-8674(78)90340-9. [DOI] [PubMed] [Google Scholar]
- Beug H., Graf T. Transformation parameters of chicken embryo fibroblasts infected with the ts34 mutant of avian erythroblastosis virus. Virology. 1980 Jan 30;100(2):348–356. doi: 10.1016/0042-6822(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Structure and specific sequences of avian erythroblastosis virus RNA: evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1633–1637. doi: 10.1073/pnas.76.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R., Schwarz R. T., Schmidt M. F. Phenotypes of Rous sarcoma virus-transformed fibroblasts: an argument for a multifunctional Src gene product. Med Microbiol Immunol. 1977;164(1-3):155–165. doi: 10.1007/BF02121311. [DOI] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Fink D., Beug H., Royer-Pokora B. Oncornavirus-induced sarcoma formation obscured by rapid development of lethal leukemia. Cancer Res. 1977 Jan;37(1):59–63. [PubMed] [Google Scholar]
- Graf T. In vitro transformation of chicken bone marrow cells with avian erythroblastosis virus. Z Naturforsch C. 1975 Nov-Dec;30(6):847–849. doi: 10.1515/znc-1975-11-1232. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Gross J. L., Rifkin D. B. The effect of avian retroviruses on limb bud chondrogenesis in vitro. Cell. 1979 Nov;18(3):707–718. doi: 10.1016/0092-8674(79)90125-9. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Braverman S. B., Astrin S. M. Transcriptional products and DNA structure of endogenous avian proviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1111–1121. doi: 10.1101/sqb.1980.044.01.120. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Kamahora T., Sugiyama H., Nomoto A., Yoshida M., Toyshima K. RNA specific for the transforming component of avian erythroblastosis virus strain R. Virology. 1979 Jul 15;96(1):291–294. doi: 10.1016/0042-6822(79)90196-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Hu S. S., Vogt P. K. Avian erythroblastosis virus: transformation-specific sequences form a contiguous segment of 3.25 kb located in the middle of the 6-kb genome. Virology. 1979 Sep;97(2):366–377. doi: 10.1016/0042-6822(79)90347-7. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Neil J. C., Vogt P. K. Cell-free translation of avian erythroblastosis virus RNA yields two specific and distinct proteins with molecular weights of 75,000 and 40,000. Virology. 1980 Jan 30;100(2):475–483. doi: 10.1016/0042-6822(80)90537-1. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Gazzolo L., Moscovici M. G. Focus assay and defectiveness of avian myeloblastosis virus. Virology. 1975 Nov;68(1):173–181. doi: 10.1016/0042-6822(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Oker-Blom N., Hortling L., Kallio A., Nurmiaho E. L., Westermarck H. OK 10 virus, an avian retrovirus resembling the acute leukaemia viruses. J Gen Virol. 1978 Sep;40(3):623–633. doi: 10.1099/0022-1317-40-3-623. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Marciani D. J., Papas T. S. Cell-free synthesis of the precursor polypeptide for avian myeloblastosis virus DNA polymerase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4951–4954. doi: 10.1073/pnas.74.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Harvey R., Smith A. E. The size of Rous sarcoma virus mRNAs active in cell-free translation. Nature. 1977 Aug 4;268(5619):416–420. doi: 10.1038/268416a0. [DOI] [PubMed] [Google Scholar]
- Pawson T., Martin G. S. Cell-free translation of avian erythroblastosis virus RNA. J Virol. 1980 Apr;34(1):280–284. doi: 10.1128/jvi.34.1.280-284.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Mellon P., Duesberg P. H., Martin G. S. env Gene of Rous sarcoma virus: identification of the gene product by cell-free translation. J Virol. 1980 Mar;33(3):993–1003. doi: 10.1128/jvi.33.3.993-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Brugge J. S., Erikson R. L. Identification of a polypeptide encoded by the avian sarcoma virus src gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1567–1571. doi: 10.1073/pnas.75.3.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Anderson S. M., Riemen M. W., Hanafusa H. gag-Related polypeptides encoded by replication-defective avian oncoviruses. J Virol. 1979 Dec;32(3):749–761. doi: 10.1128/jvi.32.3.749-761.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Royer-Pokora B., Grieser S., Beug H., Graf T. Mutant avian erythroblastosis virus with restricted target cell specificity. Nature. 1979 Dec 13;282(5740):750–752. doi: 10.1038/282750a0. [DOI] [PubMed] [Google Scholar]
- Stéhelin D., Graf T. Avian myelocytomatosis and erythroblastosis viruses lack the transforming gene src of avian sarcoma viruses. Cell. 1978 Apr;13(4):745–750. doi: 10.1016/0092-8674(78)90224-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Jay G., Pastan I. Unusual features in the nucleotide sequence of a cDNA clone derived from the common region of avian sarcoma virus messenger RNA. Proc Natl Acad Sci U S A. 1980 Jan;77(1):176–180. doi: 10.1073/pnas.77.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Toyoshima K. In vitro translation of avian erythroblastosis virus RNA: identification of two major polypeptides. Virology. 1980 Jan 30;100(2):484–487. doi: 10.1016/0042-6822(80)90538-3. [DOI] [PubMed] [Google Scholar]
- von der Helm K., Duesberg P. H. Translation of Rous sarcoma virus RNA in a cell-free system from ascites Krebs II cells. Proc Natl Acad Sci U S A. 1975 Feb;72(2):614–618. doi: 10.1073/pnas.72.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]