Background: HDAC5 regulation during acute and chronic β-adrenergic receptor (β-AR) stimulation and its cross-talk with Gq signaling remains unclear in adult myocytes.
Results: Acute β-AR activation induces nuclear import of HDAC5 and blocks Gq-driven export.
Conclusion: Acute β-AR activation protects against Gq-induced hypertrophic signaling.
Significance: Acute β-AR regulation is crucial during fight or flight response as a key physiological switch before allowing transcriptional activation.
Keywords: Cardiac Hypertrophy, Heart Failure, Histone Deacetylase, Signal Transduction, Transcription Repressor, Adult Cardiac Myocytes, beta-Adrenergic Activation, Hypertrophic Signaling
Abstract
During hemodynamic stress, catecholamines and neurohumoral stimuli may induce co-activation of Gq-coupled receptors and β-adrenergic receptors (β-AR), leading to cardiac remodeling. Dynamic regulation of histone deacetylase 5 (HDAC5), a transcriptional repressor, is crucial during stress signaling due to its role in epigenetic control of fetal gene markers. Little is known about its regulation during acute and chronic β-AR stimulation and its cross-interaction with Gq signaling in adult cardiac myocytes. Here, we evaluate the potential cross-talk between Gq-driven and β-AR mediated signaling at the level of nucleocytoplasmic shuttling of HDAC5. We show the translocation of GFP-tagged wild type HDAC5 or mutants (S279A and S279D) in response to β-AR or Gq agonists. Isoproterenol (ISO) or PKA activation results in strong nuclear accumulation of HDAC5 in contrast to nuclear export driven by Ca2+-calmodulin protein kinase II and protein kinase D. Moreover, nuclear accumulation of HDAC5 under acute ISO/PKA signaling is dependent on phosphorylation of Ser-279 and can block subsequent Gq-mediated nuclear HDAC5 export. Intriguingly, the attenuation of Gq-induced export is abolished after chronic PKA activation, yet nuclear HDAC5 remains elevated. Last, the effect of chronic β-AR signaling on HDAC5 translocation was examined in adult myocytes from a rabbit model of heart failure, where ISO-induced nuclear import is ablated, but Gq-agonist mediated export is preserved. Acute β-AR/PKA activation protects against hypertrophic signaling by delaying Gq-mediated transcriptional activation. This serves as a key physiological control switch before allowing genetic reprogramming via HDAC5 nuclear export during more severe stress, such as heart failure.
Introduction
Histone deacetylases (HDACs)2 are transcriptional repressors crucial to the regulation of gene transcription and key transducers of extracellular stimuli that impact genetic programming (1, 2). As epigenetic regulators, HDACs interact with a multitude of transcriptional factors to regulate gene transcription or repression and thus have important implications in disease and development (3, 4). HDACs remove acetyl groups from the histone tail of its target proteins to suppress transcriptional activation by occluding chromatin access to transcription machinery. The HDACs antagonize the action of histone acetyl transferases, which promotes transcription via acetylation of histones to create a more relaxed chromatin structure (2, 5, 6). With 18 identified mammalian isoforms to date, the HDAC superfamily is divided into four distinct classes (classes I, II, III, and IV) with a relatively conserved catalytic domain (3, 7–9). Compared with other subtypes, class II HDACs (4–7, 9, 10) exhibit tissue specificity with predominant expression in skeletal muscles, heart, brain, and T-cells. Class I and III HDACs are more ubiquitously expressed in other cell types (10, 11).
Like other class IIa members, HDAC5 contains a catalytic deacetylase domain in the C-terminal half between a nuclear localization sequence at the N terminus and a nuclear export sequence near the C terminus. The intrinsic nuclear localization sequence and nuclear export sequence determine the intracellular localization of HDAC5 and can be targets for modifications, such as phosphorylation. Many in vivo studies have examined the functional role of HDAC5 in the heart and its consequent stress-induced exacerbated cardiac hypertrophy in knock-out mice with a loss-of-function phenotype (3, 12, 13), highlighting the importance of its cellular localization and function in regulating cardiac physiology and pathology. Specifically, fundamental regulation of HDAC5 is crucial to cardiac remodeling and hypertrophy at the cellular level, where activation of the “fetal gene program” is initiated by relieving transcriptional inhibition to activate myocyte enhancer factor 2 (MEF2) controlled genes (14–18).
Compelling evidence has established that HDAC5 nucleocytoplasmic shuttling is highly regulated by its phosphorylation at serines 259 and 498 to initiate phosphorylation-dependent nuclear export via binding to 14-3-3 chaperone proteins and unmasking the nuclear export sequence signal (15, 18–22). Accordingly, we and others have reported that neurohumoral stress stimuli coupled to activation of G-protein receptors can trigger downstream target kinases, such as CaMKII and PKD, to phosphorylate HDAC5 at these sites and mediate nuclear export in cardiac myocytes (23–27). Dynamic shuttling of HDAC5 contributes critically to hypertrophic signaling in the heart, yet the mechanism by which trafficking is regulated remains unclear, especially during activation of multiple stress stimuli.
Several class II HDAC kinases mediate derepression of MEF2 dependent genes via nuclear export of HDAC5. There is little information about the cross-interaction of neurohumoral signaling with β-AR activation, a major pathway involved in sympathetic stimulation that can improve cardiac function acutely but can also trigger pathological cardiac remodeling and heart failure upon chronic activation (28, 29). There are several studies investigating the role of PKA activation on hypertrophic signaling, but the pathway is unclear. For example, PKA has been reported to facilitate PKD activation and mobilize the consequent hypertrophic signaling due to their common association with scaffolding protein, A-kinase anchoring protein (AKAP)-Lbc, in COS7 cells and neonatal rat ventricular myocytes (30–32). Alternatively, PKA has recently been shown to modulate MEF2 regulated gene expression by promoting proteolysis of HDAC4 and phosphorylating HDAC5 directly (33–35), exerting an anti-hypertrophic effect against stress stimuli in HEK cells, COS7 cells, and neonatal rat ventricular myocytes. These biochemical and enzymatic experiments involved the use of immortalized cell lines and neonatal myocytes, which provide very different endogenous cellular environments from adult ventricular myocytes. Thus, the role of PKA activation on hypertrophic signaling remains unclear in adult cardiac myocytes.
In this study, we investigate the effects of β-AR signaling and PKA activation on regulation of nucleocytoplasmic HDAC5 shuttling and its cross-interaction with Gq-driven hypertrophic signaling in adult ventricular myocytes. We focus on contrasting the effects of acute versus chronic β-AR stimulation because chronic β-AR stimulation also triggers maladaptive changes leading to hypertrophy and is associated with decreased cardiac function (28, 29). Acute ISO and forskolin treatments promote strong nuclear HDAC5 accumulation, in contrast to nuclear export observed during Gq agonist stimulation. Thus, acute PKA activation protects against Gq-driven HDAC5 nuclear export and hypertrophic signaling. Prolonged forskolin treatment results in further enhancement of nuclear HDAC5 signal yet permits the subsequent Gq-mediated HDAC5 nuclear export. Myocytes from a rabbit model of heart failure exhibit a more cytosolic HDAC5 distribution with an absence of ISO-induced HDAC5 nuclear accumulation, demonstrating blunted β-AR sensitivity in heart failure cells and reduced gene repression control.
In summary, our study shows for the first time in adult cardiac myocytes that β-AR influences HDAC5 nuclear shuttling at the level of gene transcriptional control and that β-AR signaling interacts with the Gq-driven hypertrophic pathway. Importantly, β-AR-driven HDAC5 nuclear import dominates over Gq-mediated effects during acute stimulation to prevent transcriptional activation. This anti-hypertrophic effect is relieved during chronic β-AR stimulation to allow Gq-driven transcriptional activation as an adaptive mechanism to long term stress.
EXPERIMENTAL PROCEDURES
Adenoviral Generation
WT HDAC5, S279A, S279D, and S259A/S498A variants were subcloned into a pShuttle-cytomegalovirus (CMV) vector by restriction enzyme digestion and ligation. Using the AdEasy adenoviral vector system (Agilent), HDAC5-GFP adenoviral DNA was generated and prepared by Wizard Plus Miniprep DNA kit (Promega). Human embryonic kidney (HEK) cells plated at 90% confluence on 6-cm culture dishes were transfected with GFP-tagged HDAC5 adenoviral DNA (10 μg) using Polyfect transfection reagent (Qiagen) in 2 ml of growth medium supplemented with 3% penicillin/streptomycin and 2% fetal bovine serum (CellGro). After 2 weeks of plaque formation, lysed cells were harvested and centrifuged at 2,000 rpm for 3 min. The supernatant was then transferred onto 10-cm culture dishes plated with HEK cells at 90% confluence. Upon completion of cell lysis, adenovirus DNA was further amplified by transferring to 30 10-cm dishes. Cells were harvested, lysed by freeze-thaw cycles (3 times), and purified using a cesium chloride gradient method. The mature adenovirus particles were then extracted and dialyzed in a Slide-A-Lyzer dialysis cassette (Thermo Scientific) in 1× PBS at 4 °C overnight. The next day, the purified adenovirus was aliquoted and stored at −80 °C.
Cell Isolation and Culturing
We isolated ventricular myocytes from adult New Zealand White rabbits (Charles River Laboratories) as described previously (36). The rabbits were male with an average age of 3.5 months and an average weight of 2.2 kg. We isolated ventricular myocytes from male rats (Charles River Laboratories) with an average age of 3 months and an average weight of 275 g. The rat myocyte isolation protocol was described previously (37). The rat myocytes were used mainly for the Western blot experiment with phospho-Ser-498 HDAC5 rabbit polyclonal antibody to minimize nonspecific signal. All animal procedures were performed in accordance with Institutional Animal Care and Use Committee regulations at the University of California, Davis. Isolated myocytes were allowed to settle by gravity and resuspended in PC-1 medium (Lonza) supplemented with 5% penicillin/streptomycin. 2-well glass coverslips were coated with laminin (Invitrogen) and allowed to air-dry prior to plating. 2 ml of cell resuspension was added to each well of the coverslip and incubated at 37 °C for 45 min for attachment. Cells were gently washed and incubated with fresh PC-1 medium immediately before adenoviral infection with GFP-tagged constructs of WT HDAC5, S259A/S498A, S279A, and S279D at 37 °C overnight (multiplicity of infection at 10–100).
Confocal Imaging
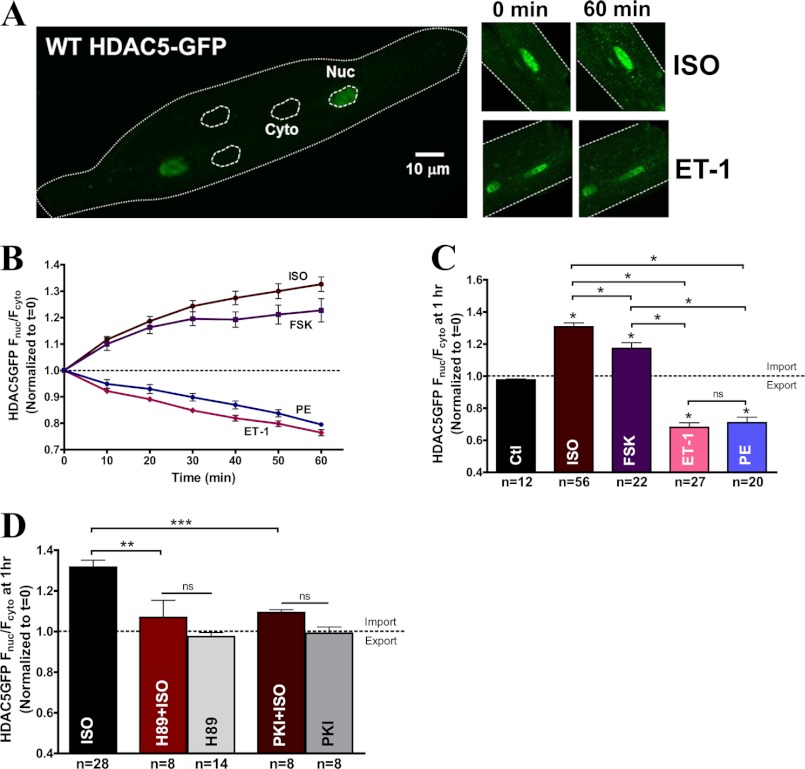
Coverslip was placed on an inverted microscope (LSM5 Pascal or Olympus, Zeiss) equipped with a ×40 water immersion object lens. An argon laser (3–5%) was used to excite GFP at 488 nm, and the emission wavelength was collected with a long pass filter above 505 nm. PC-1 medium was replaced with normal tyrode solution containing 1.8 mm Ca2+ before each experiment. Cells were imaged before and after treatment with agonists, including ET-1 (100 nm), PE (20 μm), ISO (100 nm), and forskolin (10 μm) for 1 h. National Institutes of Health ImageJ software was used for image analysis. The nuclear edge was traced to create a region of interest, and the average fluorescence intensity within the nucleus was divided by the average of three randomly selected regions of interest of equal size in the cytoplasm to compare the nuclear versus cytosolic signal (Fig. 1A). Each Fnuc/Fcyto ratio was normalized to the initial time point after agonist treatment for comparison between different groups. A Fnuc/Fcyto ratio larger than 1 indicates net nuclear accumulation of HDAC5, whereas a ratio smaller than 1 indicates nuclear export.
FIGURE 1.
β-AR signaling via PKA induced HDAC5 nuclear accumulation in contrast to Gq-mediated export. A, representative confocal images of HDAC5-GFP expression in rabbit adult ventricular myocytes at base line, pre- and post-treatment with ISO (100 nm) or ET-1 (100 nm) after 1 h. B, HDAC5-GFP translocation data expressed as normalized Fnuc/Fcyto in response to ISO (100 nm), forskolin (10 μm), ET-1 (100 nm), and PE (20 μm) over the course of 60 min. C, the bar graph summarizes HDAC5-GFP translocation at 1 h post-treatment with the indicated agonists. *, p < 0.05. D, HDAC5-GFP translocation induced by ISO (100 nm), PKA inhibitors, H89 (10 μm), or PKI (36 μm) treatment alone or with a 20-min preincubation with H89 or PKI followed by ISO stimulation. **, p < 0.001; ***, p < 0.0001. ns, not significant. Error bars, S.E.
Heart Failure Animal Model
Heart failure was induced in adult New Zealand White rabbit as described previously (38). Briefly, HF was induced by two sequential survival surgeries, first inducing aortic insufficiency and followed with aortic constriction after 2–8 weeks of postoperative monitoring. Each rabbit was monitored with an echocardiogram to determine the severity of the HF status (typically 4–6 months) and scheduled for isolation procedure accordingly.
Co-immunoprecipitation (Co-IP)
Isolated adult rabbit myocytes were infected with GFP-tagged WT or mutant constructs of HDAC5 and cultured overnight in PC-1 medium (Lonza) (multiplicity of infection = 10–100). After 24 h, myocytes were treated with or without ISO (100 nm) for 20 min prior to the addition of ET-1 (100 nm) for 1 h. Cells were scraped and pelleted at 2,000 rpm for 3 min, and supernatant was removed. Control and treated pellets were frozen in liquid nitrogen and then were lysed with IP buffer (pH 7.4, containing 25 mm Tris-HCl, 150 mm NaCl,1 mm EDTA, 2 mm EGTA, 1% Nonidet P-40, 1 ml of phosphatase inhibitor mixture set III (Calbiochem), 1 ml of protease inhibitor mixture set III (Calbiochem)). Cell lysates were resuspended and incubated on ice for 30 min. Cell debris was pelleted using a hand centrifuge for 1 min, and supernatant was collected to determine protein concentration using a BCA kit (Thermo Scientific). 200 μg of protein from each sample was incubated end over end with GFP antibody conjugated with agarose beads (sc-9996 AC, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in 3 ml of IP buffer at 4 °C overnight. The next day, samples were centrifuged at 2,000 rpm for 3 min and washed three times with washing buffer (pH 7.4, containing 25 mm Tris-HCl, 150 mm NaCl,1 mm EDTA, 2 mm EGTA, 1 ml of phosphatase inhibitor mixture set III (Calbiochem), 1 ml of protease inhibitor mixture set III (Calbiochem)). IP pellets were collected for probing levels of 14-3-3 binding to WT HDAC5-GFP or S279A-GFP using immunoblots.
Western Blotting
Adult rabbit or rat ventricular myocyte samples with Laemmli sample buffer were heated at 95 °C for 5 min prior to loading onto SDS-PAGE gels (Bio-Rad) and subject to electrophoresis at 90 V for 2 h. Proteins were transferred onto nitrocellulose membrane overnight at 20 V for 12 h. The next day, nitrocellulose membranes were blocked with 8% milk in Tris-buffered saline solution (TBS) with 0.2% Tween followed by incubation with primary antibody overnight at 4 °C. The primary antibodies used for immunoblotting were diluted in TBST as follows: rabbit GFP antibody (1:10,000) from Abcam, rabbit pan-14-3-3 antibody (1:1000) from Santa Cruz Biotechnology, Inc. (sc-629), rabbit HDAC5 total (1:2000) from Signal Antibody Technology, rabbit phospho-HDAC5 Ser-498 (1:2000) from Signal Antibody Technology, and mouse GAPDH antibody (1:30,000) from Abcam. The next day, membranes were washed five times with TBS/TBST and incubated with secondary goat anti-rabbit or anti-mouse antibody (1:2,000, plus 0.2% milk) (Thermo Scientific) for 2 h at room temperature. After five washes, membranes were developed using Super Signal West Dura (Thermo Scientific) and digitally captured with a LAS-4000 image reader. Densitometry analysis was performed using National Institutes of Health ImageJ software.
Luciferase Assay
Isolated rabbit ventricular myocytes were infected with MEF2 luciferase reporter and cultured at 37 °C overnight. The next day, cells were treated with ET-1, PE, ISO, or forskolin for 2 h. Some myocytes were also pretreated with ISO or forskolin for 20 min prior to treatment with ET-1 or PE for 2 h. The cells were collected and pelleted at 2,000 rpm for 2 min, and supernatant was aspirated. The Dual-Luciferase reporter assay (catalogue no. E1910, Promega) was used for this assay. All reagents were prepared according to the manufacturer's instructions. 100 μl of 1× passive lysis buffer (Promega) was added to each tube to resuspend the pellet by vortexing. The cell lysates were transferred to 1.5 ml of Eppendorf tubes and centrifuged at 25,000 rpm for 5 min. To begin the luciferase assay, 20 μl of passive lysis buffer lysate/well of each sample was added to the 96-well white costar plate with opaque bottom. (Each sample was prepared as duplicates for the luciferase assay.) 40 μl of both luciferase substrate with buffer and Stop & Glo substrate with buffer were required for each sample. Total amounts of both reagents were calculated and prepared in separate Falcon tubes covered with aluminum foil. The 96-well plate, luciferase reagent, and Renilla reagent were all placed in the Veritas luminometer. The assay was performed using Veritas software to measure firefly luciferase activity followed by Renilla luciferase activity. Cardiac myocytes do not possess endogenous Renilla reporter; thus, the Renilla luciferase readouts were used and subtracted as background. All treatments were normalized to the amount of protein content and control (untreated sample) for each experiment.
Statistical Analysis
Pooled data are represented as means ± S.E. We performed Student's t test or one-way analysis of variance with Bonferroni's post hoc test when applicable, and p < 0.05 was considered statistically significant.
RESULTS
β-AR Signaling via PKA-induced HDAC5 Nuclear Accumulation in Contrast to Gq-mediated Signaling
To study the intracellular translocation of HDAC5 in isolated adult rabbit ventricular myocytes, we used a GFP-tagged HDAC5 construct displaying predominant nuclear expression (Fig. 1A). We compared the ET-1-mediated nuclear export of HDAC5 and ISO-induced nuclear accumulation of HDAC5 at 1 h. To confirm that our GFP-tagged WT HDAC5 localization was truly nuclear, infected adult myocytes expressing WT HDAC5-GFP were stained with a red DNA-binding dye, DRAQ5, and imaged simultaneously for green and red fluorescence to identify the nucleus (supplemental Fig. 1). At base line, the mean Fnuc/Fcyto ratio in myocytes was 3.76 ± 0.14, but the time-dependent effects are typically normalized to the time 0 value before treatment. Fig. 1B shows that the β-AR agonist, ISO, induced a strong accumulation of HDAC5-GFP in the nucleus (Fnuc/Fcyto =1.33 ± 0.03) in contrast to the nuclear depletion mediated by Gq agonists, ET-1 (Fnuc/Fcyto = 0.76 ± 0.01) or PE (Fnuc/Fcyto = 0.79 ± 0.01) at 1 h (Fig. 1B). Because PKA is a known downstream effector of β-AR signaling, we tested the hypothesis that the effect of HDAC5 nuclear accumulation is mainly triggered by PKA. We used forskolin, an adenyl cyclase activator, to activate PKA, which resulted in increased nuclear HDAC5 accumulation as was observed with ISO treatment but to a slightly lesser degree (Fnuc/Fcyto = 1.23 ± 0.04). As a control, we tested whether 100 nm ISO and 10 μm forskolin produced comparable PKA activation, using a fluorescence resonance energy transfer (FRET)-based activity reporter of A kinase (AKAR3) (39, 40). Both agonists produced robust and similar increases in PKA activation in the myocytes (supplemental Fig. 2).
Fig. 1C summarizes the results of HDAC5-GFP translocation at 1 h post-treatment in response to various stimuli. ISO and forskolin increased nuclear accumulation of HDAC5 by 31 ± 2.5% and 17 ± 3.5%, respectively, whereas ET-1 and PE treatments resulted in comparable nuclear export of HDAC5 (Fnuc/Fcyto = 0.68 ± 0.03 for ET-1; Fnuc/Fcyto = 0.71 ± 0.04 for PE). Furthermore, 20-min preinhibition of PKA with H89 (10 μm) or PKI (36 μm) effectively blocked the PKA-mediated nuclear accumulation under ISO treatment (Fig. 1D). ISO-induced nuclear accumulation of HDAC5 was reduced by 81.1 ± 6.3% with H89 pretreatment (p < 0.05) and by 82.6 ± 2.1% with PKI (p < 0.05) compared with ISO treatment alone. Control experiments with H89 and PKI alone did not alter base-line HDAC5 translocation. Reduction of ISO-induced HDAC5 accumulation in the nucleus by these two PKA inhibitors confirms that PKA is the major kinase involved in β-AR signaling-induced HDAC5 nuclear import.
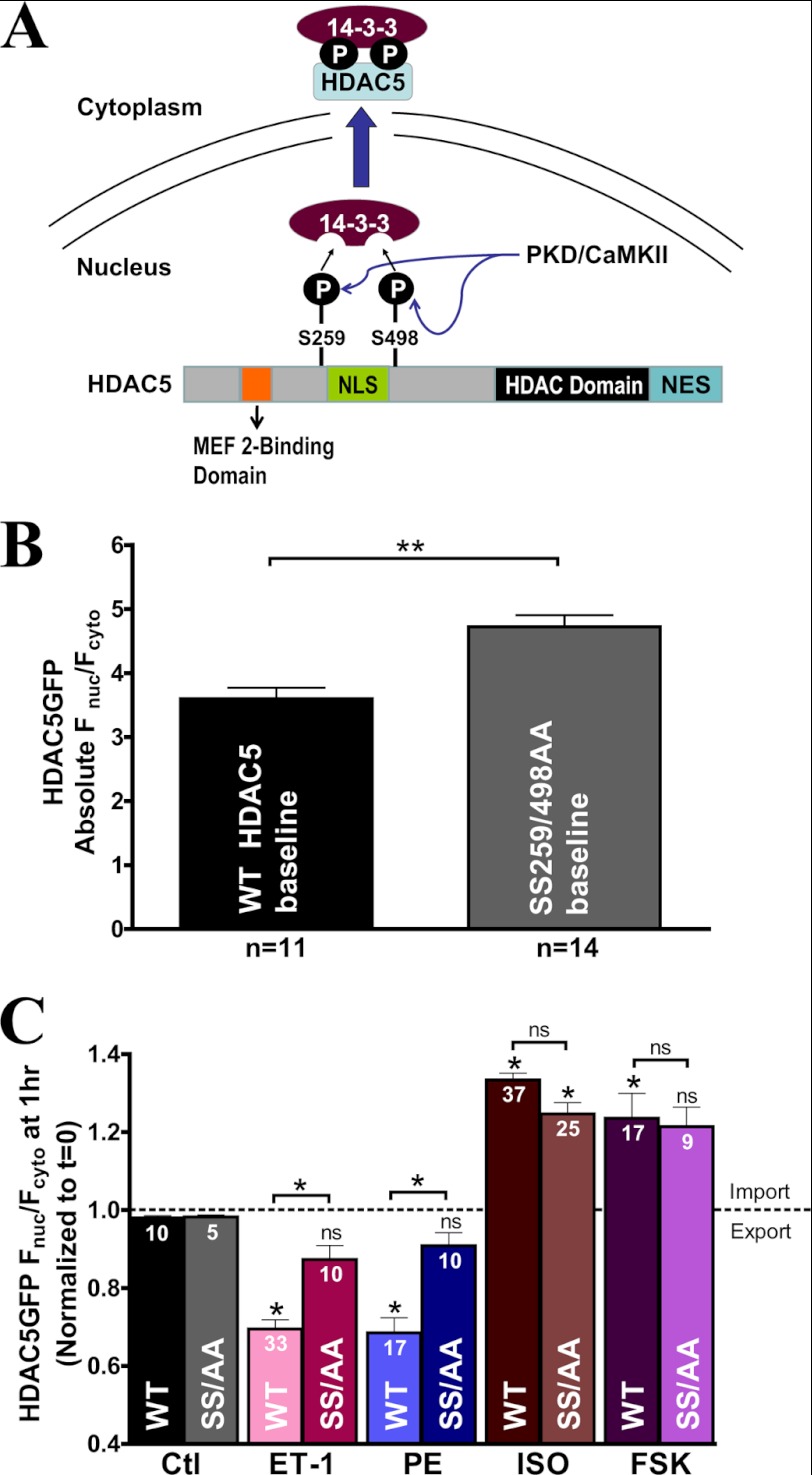
14-3-3 Chaperone Binding Sites Are Crucial for Gq-mediated but Not for β-AR- or PKA-induced HDAC5 Translocation
Several studies have established that serines 259 and 498 of HDAC5 are phosphorylated by CaMKII or PKD to trigger phosphorylation-dependent nuclear export during hypertrophic signaling (24, 25, 27). Phosphorylation at these two residues is important for binding with chaperone protein, 14-3-3, which initiates nuclear export (Fig. 2A) (15, 19–22). We tested whether these two serine residues are also involved in nuclear accumulation of HDAC5 induced by β-AR signaling or PKA activation using a GFP-tagged HDAC5 mutant with alanine substitutions (S259A/S498A). Base-line nuclear localization of S259A/S498A mutant was 31 ± 8% higher compared with the WT HDAC5 (Fig. 2B). This higher basal nuclear expression was probably due to the total absence of serine phosphorylation of the S259A/S498A mutant by CaMKII or PKD, disrupting basal HDAC5 shuttling between the nucleus and the cytosol. This observation implicates that physiologically, there may be some finite level of base-line phosphorylation at these sites.
FIGURE 2.
14-3-3 chaperone binding sites are crucial for Gq-mediated but not for β-AR- or PKA-induced HDAC5 translocation. A, schematic diagram of HDAC5 domains and its phosphorylation sites by CaMKII and PKD for 14-3-3-dependent nuclear export mechanism. B, comparison of absolute base-line localization of WT HDAC5 versus S259A/S498A mutant (SS259/498AA) overexpression in adult cardiac myocytes. C, bar graph comparison of GFP-tagged HDAC5 S259A/S498A (SS/AA) mutant translocation versus WT HDAC5 at 1 h post-treatment with the indicated agonists. *, p < 0.05; **, p < 0.001; ns, not significant. Error bars, S.E.
The non-phosphorylatable mutant S259A/S498A significantly reduced HDAC5 nuclear export induced by ET-1 or PE compared with WT (Fig. 2C). Conversely, the S259A/S498A mutant did not perturb ISO or forskolin-induced nuclear import, suggesting that the PKA-induced nuclear accumulation effect of HDAC5 must occur at site(s) different from Ser-259 and Ser-498. Taken together, the 14-3-3 chaperone binding sites are crucial for the Gq-mediated nuclear export mechanism but are not important for the β-AR- or PKA-induced HDAC5 accumulation in the nucleus.
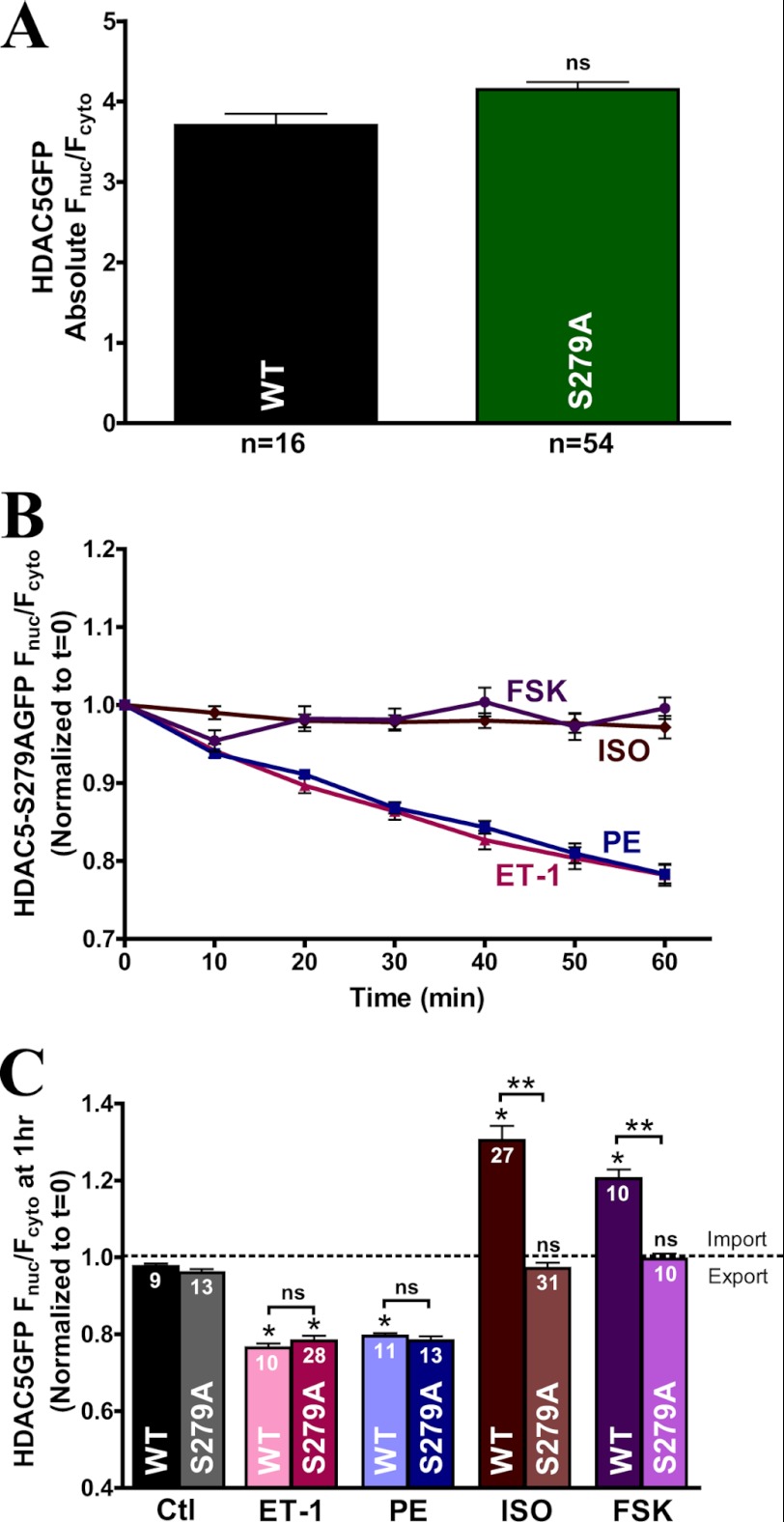
Ser-279 Phosphorylation by β-AR and PKA Stimulation Induces HDAC5 Nuclear Accumulation
Recently, Ser-279 within the nuclear localization sequence region of HDAC5 was identified to be directly phosphorylated by PKA (34). To test whether β-AR or PKA activation mediates its inhibitory effects on HDAC5 nuclear export predominantly via Ser-279 phosphorylation, we designed an adenoviral construct of GFP-tagged HDAC5 mutant with alanine substitution at Ser-279 (S279A). Base-line expression of the GFP-tagged HDAC5 S279A mutant showed similar nuclear targeting compared with WT (Fig. 3A), indicating that base-line nucleocytoplasmic shuttling of S279A remained intact and that basal phosphorylation at Ser-279 might be negligible due to low basal PKA activity. This result was significantly different from the basal expression observed with S259A/S498A mutant, where basal shuttling was disturbed by preventing the phosphorylation at Ser-259 and Ser-498 sites, thus favoring nuclear retention of HDAC5 (Fig. 2B).
FIGURE 3.
S279A mutant completely ablates the β-AR- or PKA-mediated HDAC5 nuclear import. A, comparison of absolute base-line Fnuc/Fcyto of HDAC5 between WT and the S279A variant in adult cardiac myocytes. B, response of GFP-tagged HDAC5 S279A translocation in response to ISO (100 nm), forskolin, ET-1 (100 nm), and PE (20 μm) over 60 min. C, bar graph summary of GFP-tagged HDAC5 S279A mutant at 1 h post-treatment with the indicated agonists in comparison with WT HDAC5. *, p < 0.05. ns, not significant. Error bars, S.E.
ISO and forskolin-induced nuclear import were completely ablated in the S279A mutant, whereas nuclear export remained intact under ET-1 and PE stimulation over 1 h (Fig. 3B). Grouped data summarize S279A translocation at 1 h post-treatment, where ET-1- or PE-mediated nuclear export was comparable with WT, but ISO- or forskolin- induced nuclear accumulation was completely abolished (Fig. 3C). These data suggest that Ser-279 serves as a major site for PKA phosphorylation during β-AR signaling-induced HDAC5 nuclear import in adult cardiac myocytes.
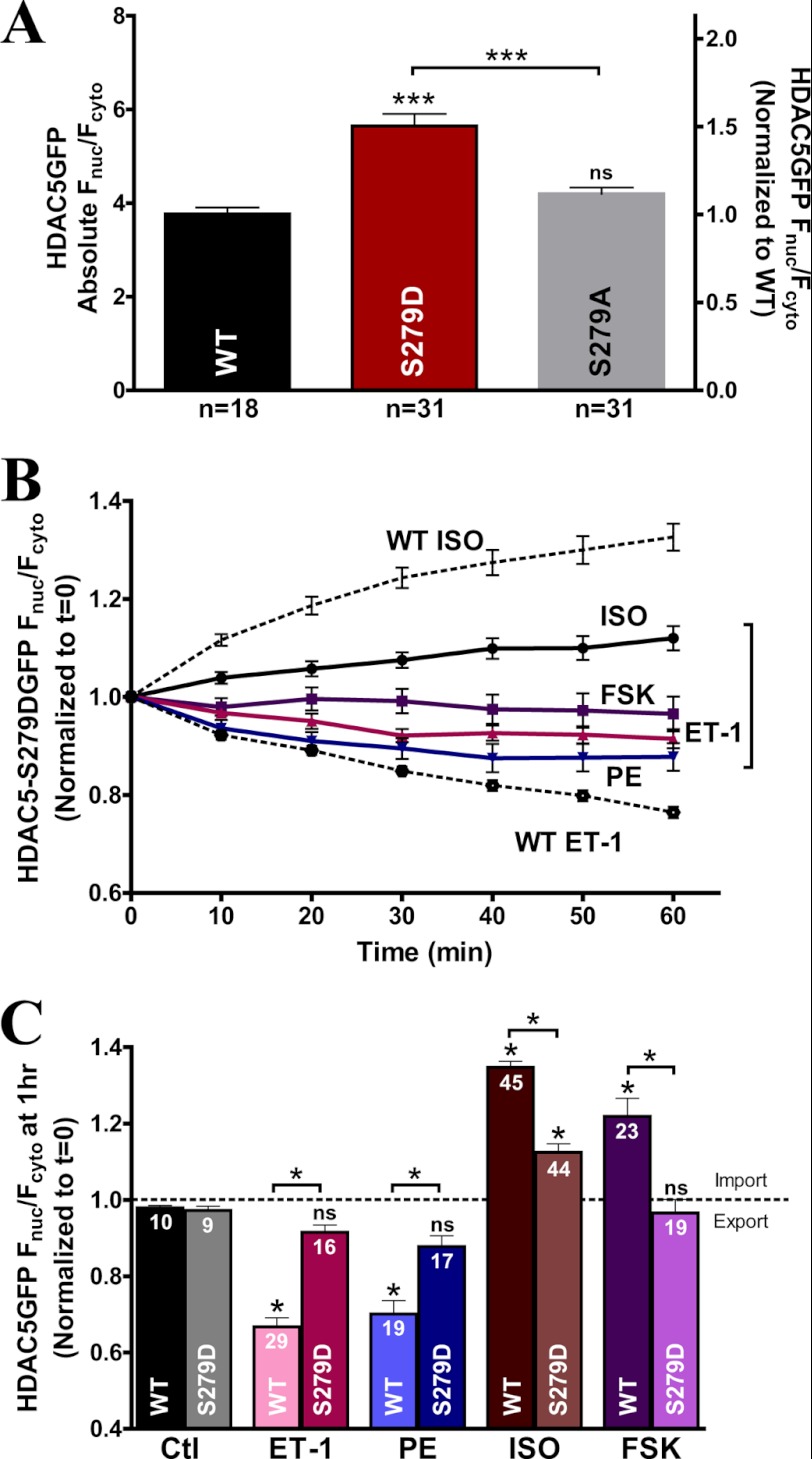
Ser-279 Phosphomimetic Mutant Inhibits HDAC5 Translocation
To test the hypothesis that phosphorylation at Ser-279 prevents nuclear export induced by ET-1 or PE, we designed a phosphomimetic HDAC5 mutant with an aspartic acid substitution at Ser-279 (S279D). Notably, the GFP-tagged S279D phosphomimetic HDAC5 resulted in a 49.9 ± 8.7% increase in basal Fnuc/Fcyto ratio compared with WT (Fig. 4A). This observation validates the S279D as an effective phosphomimetic mutant at base line, producing a similar Fnuc/Fcyto ratio to the maximal ISO- or forskolin-induced nuclear accumulation of WT HDAC5 (Fig. 1B).
FIGURE 4.
S279D phosphomimetic mutant blunts the Gq-mediated nuclear export and β-AR- and PKA-induced HDAC5 nuclear import. A, comparison of absolute base-line Fnuc/Fcyto of HDAC5 between WT, S279D, and S279A variants. B, GFP-tagged S279D mutant translocation in response to ISO (100 nm), forskolin (10 μm), ET-1 (100 nm), and PE (20 μm) over 60 min. C, bar graph summary of HDAC5 S279D mutant at 1 h post-treatment with the indicated agonists compared with WT. *, p < 0.05. ns, not significant. Error bars, S.E.
The GFP-tagged S279D phosphomimetic mutant also completely prevented ET-1- or PE-triggered nuclear export, suggesting that phosphorylation at the Ser-279 site reduces the amount of nuclear export induced by Gq agonists (Fig. 4B). Moreover, forskolin had no further effect on S279D mutant HDAC5 translocation, indicating that the phosphomimetic mutant was able to completely abolish the PKA effects on HDAC5 nuclear accumulation (Fig. 4, B and C). Intriguingly, we still observed slight nuclear import of HDAC5 (∼10%) upon ISO stimulation compared with forskolin. This minor increase suggests that there could be additional PKA-independent effects on HDAC5 translocation during β-AR stimulation by ISO. In summary, the phosphomimetic mutant significantly reduced the nuclear export induced by ET-1 or PE (Fig. 4C) and the HDAC5 nuclear import induced by ISO and PKA. Therefore, β-AR or PKA mediates HDAC5 nuclear accumulation predominantly via phosphorylation of Ser-279, and Ser-279 phosphorylation of HDAC5 effectively attenuates Gq-induced nuclear export.
Acute β-AR or PKA Activation Blocks Gq-mediated HDAC5 Nuclear Export and Favors Nuclear Import
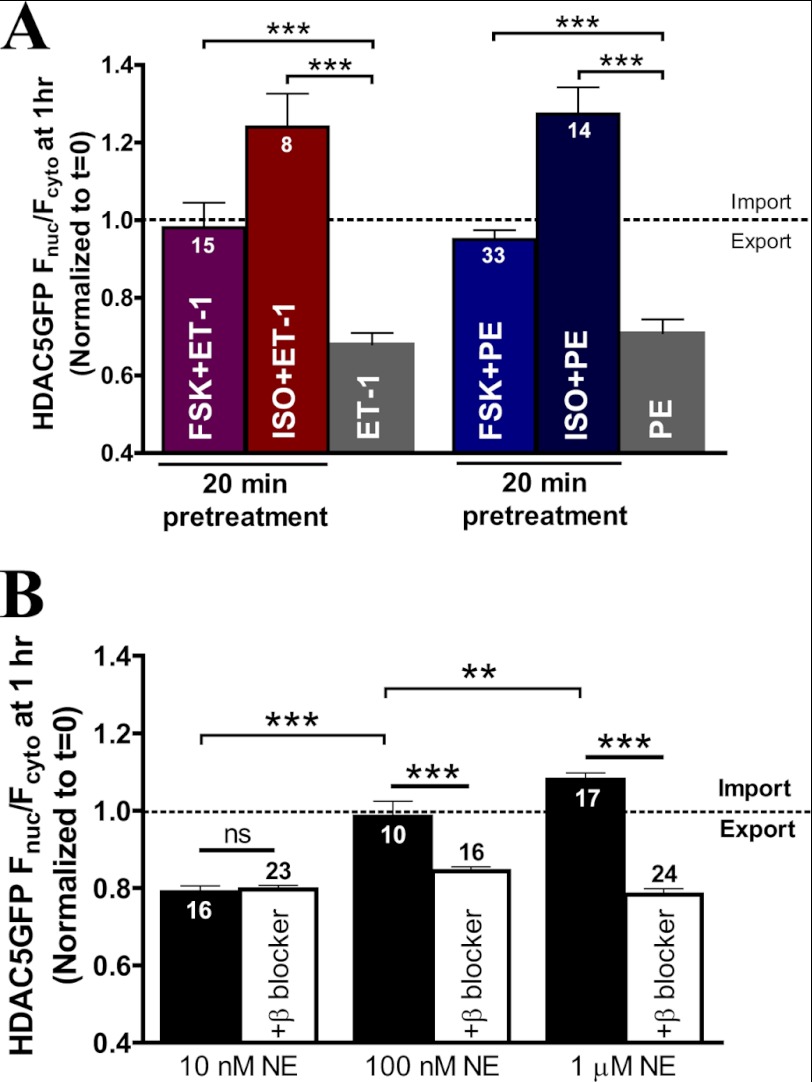
Because PKA drives HDAC5 import via Ser-279 phosphorylation, whereas Gq activation drives HDAC5 nuclear export via Ser-259 and Ser-498 phosphorylation, we next tested which direction dominates HDAC5 translocation upon co-activation. Based on our HDAC5 mutant studies, we hypothesized that the PKA effect would predominate in driving HDAC5 localization. To test our hypothesis, we examined the effects of β-AR signaling on Gq-mediated HDAC5 translocation between the nucleus and the cytosol. Acute PKA prestimulation by forskolin for 20 min abolished the nuclear export of HDAC5 induced by ET-1 or PE treatment (Fig. 5A). Acute β-AR activation with ISO followed by Gq agonists also effectively blocked the HDAC5 nuclear export induced by ET-1 or PE. Additionally, ET-1 and PE induced more nuclear accumulation in the presence of ISO, suggesting that β-AR may have a PKA-independent effect that favors HDAC5 nuclear localization (versus forskolin). Acute prestimulation with ISO or forskolin completely prevented the subsequent Gq agonist-mediated effects on HDAC5 shuttling, indicating that acute β-AR stimulation may be cardioprotective via PKA-mediated suppression of Gq-driven HDAC5 nuclear export.
FIGURE 5.
Acute β-AR or PKA prestimulation blocks Gq-mediated HDAC5 nuclear export. A, comparison of normalized HDAC5-GFP Fnuc/Fcyto at 1 h post-treatment with ET-1 (100 nm) or PE (20 μm) alone or with a 20-min preincubation of forskolin (10 μm) or ISO (100 nm). ***, p < 0.0001. B, HDAC5 translocation in response to dose-dependent NE with or without preincubation of the β-blocker, propranolol (500 nm). **, p < 0.001. ns, not significant. Error bars, S.E.
Norepinephrine (NE) is released during sympathetic stimulation and can activate both α-AR and β-AR activation. To examine the cross-talk of the β-AR- and Gq-signaling pathway in the context of physiological stimulation, we evaluated the effect of HDAC5 translocation in response to NE. Fig. 5B shows that low NE concentration (10 nm) triggered nuclear export of HDAC5 (similar to PE), whereas increasing NE to 1 μm favored HDAC5 nuclear import (similar to ISO). To further dissect the role of α-AR versus β-AR activation, we performed parallel experiments pretreating with the β-blocker, propranolol, which revealed HDAC5 nuclear export at all three NE concentrations. Thus, the α-AR activation pathway appeared to be predominant at low [NE] (10 nm), resulting in HDAC5 nuclear export. Conversely, 1 μm NE induced HDAC5 nuclear import via a β-AR pathway, which was abolished by propranolol pretreatment. Notably, the intermediate [NE] (100 nm) resulted in relatively unaltered HDAC5 translocation but produced nuclear export after propranolol pretreatment, similar to the PE (α-AR agonist) effect. This indicates that 100 nm NE activates both adrenergic receptor types.
The NE data support our results in Fig. 5A (i.e. acute β-AR and PKA activation dominates over the Gq-driven hypertrophic signaling to favor HDAC5 nuclear import). Moreover, the effects of the physiological sympathetic transmitter NE on net HDAC5 depend on the local [NE].
Mechanism of β-AR or PKA Attenuation on Gq-driven HDAC5 Nuclear Export and MEF2 Transcriptional Activity
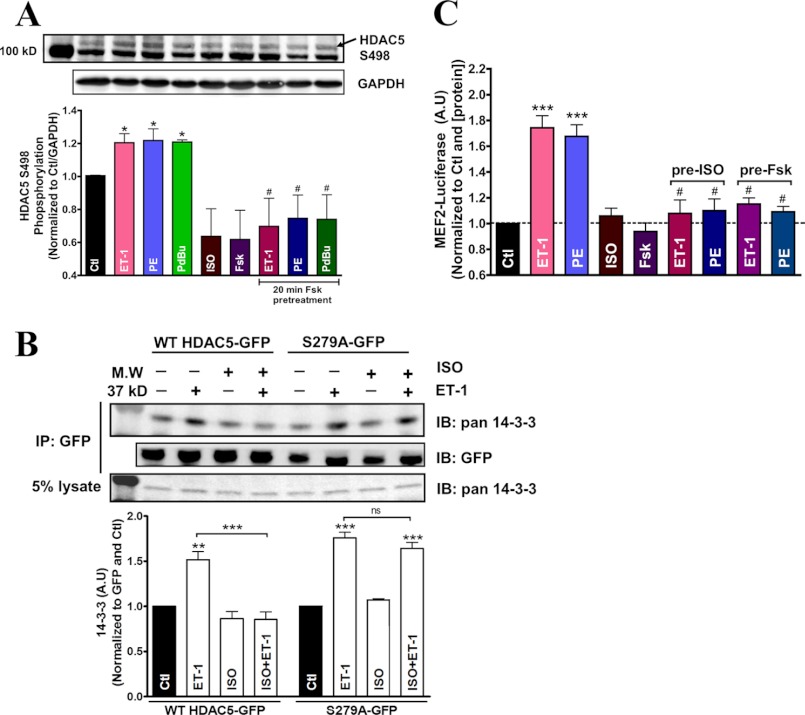
To further test whether β-AR stimulation or PKA activation can prevent or limit HDAC5 Ser-498 phosphorylation in response to Gq activation, we probed for Ser-498 phosphorylation of endogenous HDAC5 in adult rat myocytes. Our results showed that hypertrophic agonists ET-1, PE, and phorbol 12,13-dibutyrate all enhanced Ser-498 phosphorylation compared with the untreated control (Fig. 6A). Conversely, HDAC5 Ser-498 phosphorylation was reduced under ISO or forskolin stimulation, and 20-min pretreatment of forskolin effectively abolished the endogenous HDAC5 Ser-498 phosphorylation induced by ET-1, PE, and phorbol 12,13-dibutyrate. Additionally, co-immunoprecipitation with GFP antibody enables us to measure 14-3-3 binding with HDAC5-GFP during β-AR/PKA and Gq activation (Fig. 6B). ET-1 enhanced the 14-3-3 associated with WT HDAC5-GFP, but this effect was abolished by 20 min of ISO pretreatment. Notably, ISO treatment alone slightly reduced 14-3-3 association with HDAC5-GFP, in agreement with the higher nuclear localization seen in this condition. In contrast, when the PKA-resistant S279A HDAC5-GFP was used, ISO did not perturb basal 14-3-3 association and failed to prevent ET-1-induced 14-3-3 binding.
FIGURE 6.
Mechanism of acute β-AR or PKA attenuation on Gq-driven HDAC5 nuclear export and MEF2 transcriptional activity. A, representative immunoblot of endogenous HDAC5 Ser-498 phosphorylation levels in adult rat myocytes treated with the indicated agonists alone for 1 h or with preincubation of forskolin (10 μm) for 20 min at 37 °C. *, p < 0.05 (n = 3). B, co-immunoprecipitation detecting endogenous 14-3-3 binding to WT HDAC5-GFP or S279A-GFP in adult rabbit myocytes treated with the indicated drugs. C, MEF2 dependent luciferase assay measuring transcriptional activity in adult myocytes. ***, p < 0.0001 (n = 5). Significance with Gq agonist treatment alone and with ISO or forskolin pretreatment. A.U., arbitrary units. IB, immunoblot; Error bars, S.E.
We also examined the effects of β-AR/PKA on MEF2 dependent transcriptional activity in adult cardiac myocytes to test whether the β-AR/PKA effects on HDAC5 translocation resulted in transcriptional regulation. Treatment with ET-1 or PE promoted MEF2 luciferase activity, whereas ISO and forskolin failed to alter basal luciferase activity (Fig. 6C). Preincubation with ISO or forskolin, however, effectively abolished the Gq agonist-induced increase in MEF2 activity. This finding parallels our confocal studies, in which β-AR/PKA preactivation blocks subsequent Gq-driven HDAC5 nuclear export.
The data suggest that ISO or forskolin treatment diminishes basal Ser-498 phosphorylation of endogenous HDAC5, an indicator of nuclear export shuttling. Moreover, acute PKA activation reduces Ser-498 phosphorylation of HDAC5 during hypertrophic agonist stimulation, thereby promoting HDAC5 nuclear retention. Co-immunoprecipitation data confirm that ET-1 enhanced WT HDAC5 interaction with 14-3-3, and that this interaction was prevented by preincubation with ISO. The HDAC5 S279A mutant, which lacks the PKA phosphorylation site, does not exhibit the ISO-inhibitory effect on ET-1-induced 14-3-3 binding. MEF2 dependent luciferase activity is promoted by Gq agonist, but this transcriptional activation can be effectively abolished with pretreatment with ISO or forskolin. Therefore, β-AR and PKA activation can modulate Gq-mediated HDAC5 translocation via Ser-279 phosphorylation, thus reducing phosphorylation at Ser-498 and precluding binding to 14-3-3 chaperone. Consequently, the Gq-driven MEF2 transcriptional activity can be blocked with acute β-AR/PKA activation, indicating that HDAC5 nuclear export is necessary for relieving the HDAC5 repression on MEF2 dependent transcriptional control in adult cardiac myocytes.
Chronic Forskolin Exposure Enhances HDAC5 Nuclear Accumulation
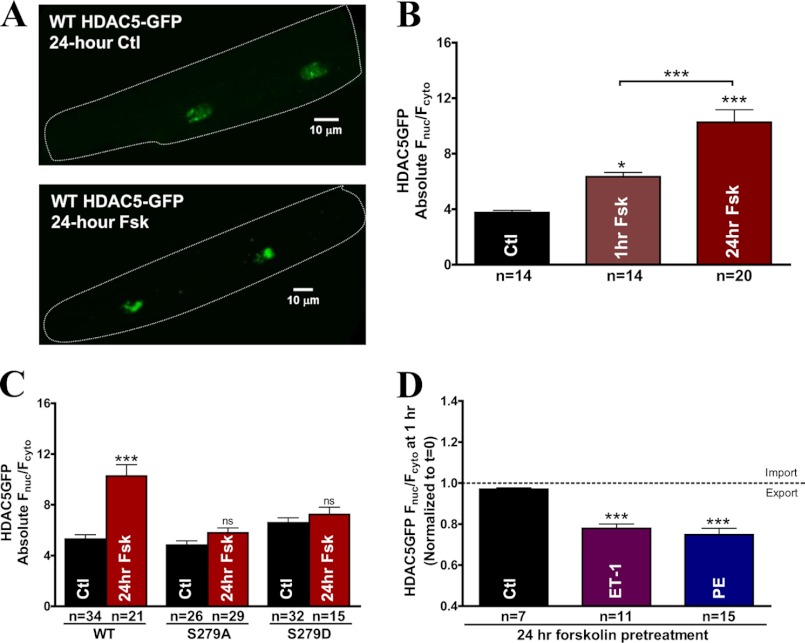
Chronic β-AR activation is associated with hypertrophy and cardiac remodeling, whereas we observe that acute β-AR or PKA stimulation prevents the hypertrophic agonist-induced HDAC5 shuttling. To further study the mechanism of acute versus chronic PKA signaling, we measured the effects of 24-h forskolin exposure on base-line HDAC5 nucleocytoplasmic shuttling. Confocal images of HDAC5-GFP expression after 24-h treatment with forskolin are illustrated in Fig. 7A. Prolonged forskolin exposure increased the nuclear accumulation of HDAC5 1.6-fold (Fnuc/Fcyto = 10.27 ± 0.89 versus Fnuc/Fcyto = 6.33 ± 0.31, p < 0.0001 after 1 h of forskolin exposure) (Fig. 7B). Our acute studies suggest that ISO- and PKA-induced HDAC5 nuclear import is mediated predominantly via Ser-279 phosphorylation. To test whether HDAC5 Ser-279 phosphorylation is also crucial to prolonged forskolin-mediated nuclear accumulation, we evaluated the effects of 24-h forskolin exposure on the nucleocytoplasmic shuttling of WT HDAC5, S279A, and S279D. Prolonged forskolin treatment caused significant further increase of WT HDAC5 in the nucleus, whereas the S279A and S279D mutants both completely eliminated the increase (Fig. 7C), suggesting that prolonged forskolin-induced continuous nuclear import of HDAC5 also occurs in a Ser-279 phosphorylation-dependent manner.
FIGURE 7.
24-h forskolin exposure enhances HDAC5 nuclear signal in a Ser-279 phosphorylation-dependent manner. A, representative confocal images of cultured adult rabbit ventricular myocytes expressing WT HDAC5-GFP as control or treated with forskolin for 24 h. B, comparison of absolute base-line Fnuc/Fcyto of WT HDAC5-GFP after 1-h or 24-h exposure to forskolin (10 μm). C, comparison of cultured myocytes under a 24-h forskolin (10 μm) treatment using GFP-tagged WT HDAC5, S279A, and S279D. D, results of GFP-tagged WT HDAC5 translocation in cultured rabbit myocytes incubated with forskolin (10 μm) for 24 h followed by ET-1 (100 nm) or PE (20 μm) treatment for 1 h. ***, p < 0.0001. Error bars, S.E.
Next, we assessed whether chronic forskolin treatment can inhibit Gq-induced nuclear export in myocytes treated with forskolin for 24 h prior to the addition of ET-1 or PE. Forskolin pretreatment for 24 h did not block the HDAC5 nuclear export triggered by either ET-1 or PE (Fig. 7D), in contrast to our observation for acute β-AR/PKA stimulation. Our data provide evidence that prolonged forskolin exposure causes further increase in nuclear HDAC5-GFP signal that is dependent on its Ser-279 phosphorylation. However, chronic forskolin pretreatment does not block the Gq-mediated HDAC5 nuclear export, in contrast to the inhibitory effect of acute β-AR and PKA activation.
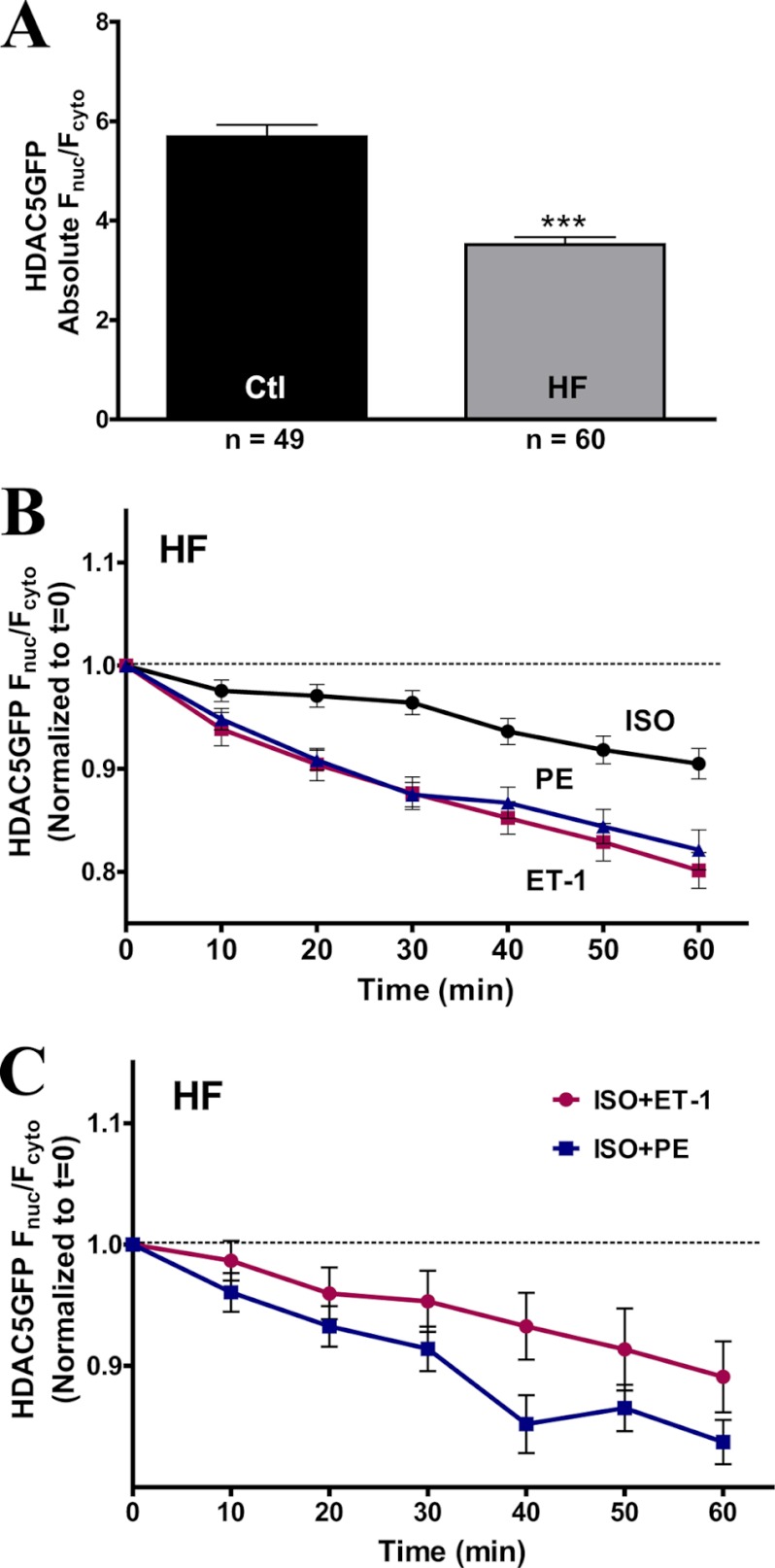
Absence of ISO-induced HDAC5 Nuclear Import in HF Rabbit Myocytes
Our current study demonstrates that acute β-AR or PKA activation can counter Gq-driven transcription activation by preventing nuclear export of HDAC5. We postulate that acute ISO/PKA-induced HDAC5 nuclear accumulation is altered in HF due to the well documented hyperadrenergic state in HF (41). To validate our hypothesis, we examined the HDAC5 translocation in HF rabbit myocytes using our GFP-tagged WT HDAC5 adenoviral construct. HF myocytes showed a more cytosolic HDAC5 expression, resulting in a lower base-line Fnuc/Fcyto ratio (Fig. 8A), an observation that matches previously reported data (27). Treatment with ISO for 1 h did not trigger HDAC5 nuclear accumulation in HF myocytes (Fig. 8B). Moreover, in HF, ISO caused a slight HDAC5 nuclear export, roughly half as much as ET-1 and PE in this state (Fig. 8B). Additionally, in HF, preincubation of ISO no longer prevented the ET-1- or PE-induced HDAC5 export (Fig. 8C). This is consistent with our prior observation that prolonged forskolin treatment did not prevent subsequent Gq-induced HDAC5 export (Fig. 7D). In summary, the HDAC5 nuclear import induced by acute β-AR stimulation is abolished in HF myocytes, coupled with a loss of protective effects against Gq-mediated hypertrophic response (as was seen for 24-h forskolin exposure).
FIGURE 8.
Absence of ISO-induced HDAC5 nuclear accumulation in HF rabbit myocytes. A, comparison of absolute base-line Fnuc/Fcyto in adult ventricular myocytes between aged-match controls and HF rabbits. B, GFP-tagged WT HDAC5 translocation in response to ISO (100 nm), ET-1 (100 nm), and PE (20 μm). C, GFP-tagged WT HDAC5 translocation at 1 h post-treatment with a 20-min preincubation of ISO (100 nm) followed by the addition of ET-1 (100 nm) or PE (20 μm) for 1 h. Error bars, S.E.
DISCUSSION
The current study highlights the physiological cross-interactions between β-AR and Gq-driven signaling at the level of HDAC5 shuttling in adult cardiac myocytes. We report that acute β-AR stimulation or PKA activation promotes nuclear accumulation of HDAC5 in contrast to Gq-induced nuclear export of HDAC5. In addition, acute pretreatment of ISO or forskolin effectively prevents the Gq-induced HDAC5 nuclear export, potentially preventing or delaying transcriptional activation in adult cardiac myocytes.
The Ser-259 and Ser-498 residues of HDAC5 are the major phosphorylation targets of CaMKII and PKD to initiate the 14-3-3-dependent nuclear export in neonatal and adult myocytes during Gq-driven hypertrophic signaling (15, 16, 19, 23, 24, 27). On the other hand, there are conflicting reports regarding the role of PKA activation during hypertrophic signaling. Moreover, the fundamental impact of HDAC5 phosphorylation by PKA in adult myocytes is not well defined.
Here we show that the non-phosphorylatable HDAC5 mutant, S279A, ablates ISO- or forskolin-induced HDAC5 nuclear import but does not perturb Gq-driven export. Conversely, mimicking PKA phosphorylation at the Ser-279 site resulted in constitutive HDAC5 nuclear import and effectively blocked HDAC5 nuclear export in response to Gq agonist stimulation. Moreover, the constitutive nuclear accumulation of the S279D mutant could not be further enhanced by PKA activation. The data suggest that β-AR- and PKA-mediated nuclear translocation of HDAC5 is predominantly Ser-279 phosphorylation-dependent. Our current findings in adult myocytes complement recent publications in which in vitro kinase assays identify PKA as the kinase that directly phosphorylates HDAC5 at Ser-279 in COS7 cells and neonatal rat ventricular myocytes (34). PKA activation negatively regulates α1-adrenergic mediated HDAC5 phosphorylation in neonatal rat ventricular myocytes (35).
Our immunoblot data indicate that forskolin pretreatment significantly reduces the level of Ser-498 phosphorylation of endogenous HDAC5 inducible by Gq agonist in adult rat myocytes. This observation is supplemented by our co-immumoprecipitation results detecting a decreased level of endogenous 14-3-3 binding to WT HDAC5-GFP in the presence of β-AR activation with the Gq agonist ET-1. Together, these observations show that β-AR or PKA activation prevents Gq-induced phosphorylation at Ser-498 and thus attenuates the consequent 14-3-3 association to prevent its nuclear export. In contrast, Carnegie et al. (30, 31) reported that an AKAP-Lbc complex allows PKA activation to potentiate PKD activation in vitro, thereby facilitating downstream HDAC5 nuclear export in HEK cells. It has also been suggested that ISO can induce nuclear HDAC5 export via a PKA-independent but oxidation-dependent pathway in adult rat myocytes (42). The discrepancies could be due to the nature of different cell types (they used HEK and cultured neonatal/adult rat ventricular myocytes) and the respective 14-3-3 isoforms present to associate with HDACs (21, 43, 44) Additionally, substrate specificity of cAMP/PKA signaling is achieved by compartmentalization of localized cAMP gradient via phosphodiesterase isoforms or β-arrestins and by association with various AKAP scaffolding subtypes in different cell types (45–57). Our study provides real-time observation of HDAC5-GFP translocation in adult myocytes to more accurately reflect the fundamentally dominant endogenous signaling pathway in adult ventricular myocytes.
The confocal data are further validated by our finding that MEF2 dependent transcriptional activity can be up-regulated upon Gq activation to promote hypertrophic signaling in adult cardiac myocytes. However, acute β-AR/PKA activation significantly blocks Gq-driven MEF2 activity, indicating that short term β-AR/PKA signaling counters the transcriptional activation triggered by neurohumoral stimuli.
Collectively, our data suggest that acute β-AR or PKA activation protects against hypertrophic stimulation during Gq activation by preventing nuclear export of HDAC5 in adult myocytes. This has significant implications in the regulation of cardiac physiology, in which short term sympathetic stimulation, such as exercise or fight or flight response, encourages beneficial compensations of increased cardiac output without triggering acute transcriptional changes. The protective effect of β-AR/PKA activation provides a short term window for cardiac myocytes to adjust quickly to external stimuli before attempting to reprogram its genetic control that is otherwise necessary during more severe long term stress conditions. This is further substantiated by our data on NE-induced HDAC5 translocation, where acute β-AR signaling is physiologically more dominant over α-AR to determine the net direction of HDAC5 shutting upon co-activation of both systems.
Chronic catecholamine stimulation is associated with decreased cardiac function and pathological cardiac remodeling, leading to hypertrophy and heart failure. Whereas we observe that acute β-AR/PKA activation protects against neurohumorally induced transcriptional activation, we show that the HDAC5-GFP nuclear signal is substantially elevated after a 24-h exposure to forskolin (1.6-fold over 1 h of treatment). Although this enhancement of nuclear HDAC5 is also dependent upon Ser-279 phosphorylation, prolonged forskolin treatment does not prevent the nuclear HDAC5 export induced by Gq agonist. Our findings suggest that Ser-279 phosphorylation by PKA is still important during chronic forskolin-induced elevation of nuclear HDAC5, yet the molecular mechanism of subsequent Gq-driven nuclear export after chronic forskolin exposure remains to be determined.
Although Ha et al. (34) identified PKA as a novel HDAC5 kinase to directly phosphorylate Ser-279 using an in vitro kinase assay, we importantly show the dynamics of this regulation in adult cardiac myocytes. An additional mechanistic issue remains as to how intracellular PKA localizes to HDAC5 during acute or chronic β-AR stimulation to alter HDAC5 translocation and transcriptional activity. One possibility is that an AKAP could be involved, a hypothesis that has been suggested (30, 31, 46). It will be of future interest to test whether a specific AKAP association is altered during acute versus chronic β-AR activation, or perhaps the neighboring proteins associated with PKA are differentially partnered with the AKAP.
We speculate that chronic PKA activation may recruit additional effects (e.g. PKA-dependent phosphorylation at additional HDAC5 sites), which restore the ability of PKD and CaMKII to phosphorylate Ser-259 and Ser-498 and drive HDAC5 nuclear export. This impact of dynamic HDAC5 phosphorylation on systemic regulation is further supported by a study in rodent striatum, in which reduced Ser-279 phosphorylation of HDAC5 during acute cocaine injection is restored after a 24-h exposure to cocaine (58). Moreover, a recent proteomic study indicates that HDAC5 contains ∼17 conserved serine/threonine sites available for phosphorylation (59). The impact of additional phosphorylation sites could alter potential interaction with other protein partners, such as kinases, phosphatases, or scaffolding complex. Hence, we cannot exclude the possibility that chronic Ser-279 phosphorylation may facilitate phosphorylation at Ser-259/Ser-498 or other sites to permit HDAC5 nuclear export after chronic PKA activation in cardiac myocytes.
Although the mechanistic details of this acute versus chronic PKA regulation of HDAC5 translocation will require further study, it represents a novel and elegant switch that fulfills a key physiological control point. In other words, in short term fight or flight responses, transcriptional activation by Gq-HDAC5 signaling is held in check, whereas in chronic PKA-related stress, such repression is relieved. Our findings in HF myocytes further support this time-dependent regulation of transcriptional activation, where protection against Gq-induced HDAC5 nuclear export occurs only during acute β-AR or PKA activation and not during sustained activation of PKA.
To the extent that HF constitutes a hyperadrenergic state, one might expect the HDAC5 Fnuc/Fcyto ratio to be elevated. However, in HF, that ratio is reduced versus control. This may have two causes: 1) the level of chronic PKA activation is probably much less in HF than we used experimentally (and down-regulation of β1-AR and elevated Gi and phosphatase function could also limit the PKA effect), and 2) HF is known to be accompanied by elevated levels of circulating Gq agonists (norepinephrine, ET-1, and angiotensin II). Moreover, ET-1 and PE trigger HDAC5 nuclear export in HF (Fig. 8), possibly due to the increased PKD and CaMKII activity in HF (27). The net result is that in HF there is reduced repression by nuclear HDAC5 of hypertrophic gene transcription and retained sensitivity to neurohumoral regulation via Gq-coupled receptor agonists. The dynamic interplay and opposing effects of β-AR versus Gq-coupled receptors on HDAC5 translocation may be important to consider in the context of β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers for HF patients. For example, it is possible that acute β-blockade alone could exacerbate Gq agonist effects in HF, and coordinated block of Gq and β-AR signaling (or Gq block first) could most effectively dampen this maladaptive signaling pathway. Patients on β-blockers might lose the “protective” effect of β-AR on HDAC5 nuclear import, leaving the Gq-mediated nuclear export unopposed. However, that may be partially ameliorated by the beneficial effects of chronic β-blockade to enhance residual β-AR responsiveness.
In conclusion, our study demonstrates the physiological interaction between the β-AR and Gq-coupled signaling pathways and their regulation on HDAC5 nucleocytoplasmic shuttling in adult cardiac myocytes. We report that acute β-AR and PKA activation favors HDAC5-dependent transcriptional repression by blocking neurohumoral activation effects (Fig. 9) and provides a time window for myocytes to adjust to stresses before resorting to epigenetic regulation. This could have teleological and energetic significance in keeping the cells transcriptionally silent during acute fight or flight. Accordingly, chronic stress, such as HF, permits neurohumoral signaling to mediate transcriptional activation by relieving HDAC5 transcriptional inhibition. Our findings illustrate the importance of acute versus chronic β-AR regulation in adult cardiac myocytes, in which acute sympathetic signaling is protective against Gq-driven hypertrophy, whereas chronic stress promotes pathological cardiac remodeling via HDAC5-dependent transcriptional regulation.
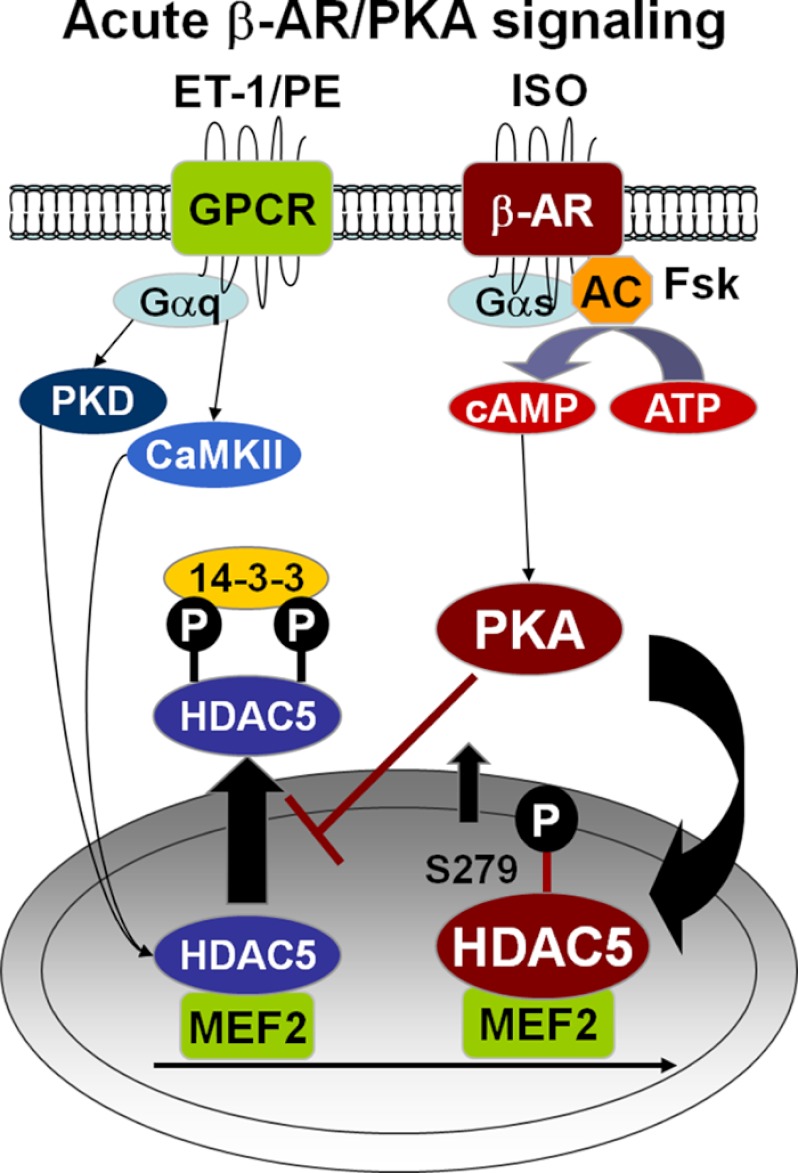
FIGURE 9.
Working hypothesis in adult cardiac myocytes. Acute β-AR/PKA signaling activates PKA to promote HDAC5 nuclear accumulation and prevent Gq-driven HDAC5 nuclear export by Ser-279 phosphorylation.
Acknowledgments
We thank Jeffrey Southard, William Ferrier, and Linda Talken for time and effort in performing the HF surgeries in rabbits. We especially thank Kenneth Ginsburg, C. Blake Nichols, and Matthew Stein for assistance in monitoring the HF rabbits and isolating rabbit myocytes. We are grateful for the AKAR adenovirus provided by Dr. Kevin Xiang at the University of California Davis. We also thank Jeff Erickson for critical comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant P01-HL80101 (to D. M. B.) and R01HL103933 (to J. B.). This work was also supported by American Heart Association Scientist Development Grant 0835312N (to J. B.) and American Heart Association Predoctoral Fellowship 09PRE2260256 (to C. W. C.).

This article contains supplemental Figs. 1 and 2.
- HDAC
- histone deacetylase
- CaMKII
- Ca2+-calmodulin protein kinase II
- PKD
- protein kinase D
- α- and β-AR
- α- and β-adrenergic receptor, respectively
- AKAP
- A-kinase anchoring protein
- ISO
- isoproterenol
- IP
- immunoprecipitation
- NE
- norepinephrine
- HF
- heart failure
- PKI
- PKA inhibitor 14–22 amide, cell-permeable, myristolated
- ET-1
- endothelin-1
- PE
- phenylephrine
- PdBu
- phorbol 12,13-dibutyrate.
REFERENCES
- 1. Johnson C. A., Turner B. M. (1999) Histone deacetylases. Complex transducers of nuclear signals. Semin. Cell Dev. Biol. 10, 179–188 [DOI] [PubMed] [Google Scholar]
- 2. Backs J., Olson E. N. (2006) Control of cardiac growth by histone acetylation/deacetylation. Circ. Res. 98, 15–24 [DOI] [PubMed] [Google Scholar]
- 3. Haberland M., Montgomery R. L., Olson E. N. (2009) The many roles of histone deacetylases in development and physiology. Implications for disease and therapy. Nat. Rev. Genet. 10, 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin M., Kettmann R., Dequiedt F. (2007) Class IIa histone deacetylases. Regulating the regulators. Oncogene 26, 5450–5467 [DOI] [PubMed] [Google Scholar]
- 5. McKinsey T. A., Olson E. N. (2005) Toward transcriptional therapies for the failing heart. Chemical screens to modulate genes. J. Clin. Invest. 115, 538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bush E. W., McKinsey T. A. (2009) Targeting histone deacetylases for heart failure. Expert Opin. Ther. Targets 13, 767–784 [DOI] [PubMed] [Google Scholar]
- 7. Parra M., Verdin E. (2010) Regulatory signal transduction pathways for class IIa histone deacetylases. Curr. Opin. Pharmacol. 10, 454–460 [DOI] [PubMed] [Google Scholar]
- 8. Yang X. J., Grégoire S. (2005) Class II histone deacetylases. From sequence to function, regulation, and clinical implication. Mol. Cell Biol. 25, 2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKinsey T. A. (2012) Therapeutic potential for HDAC inhibitors in the heart. Annu. Rev. Pharmacol. Toxicol. 52, 303–319 [DOI] [PubMed] [Google Scholar]
- 10. Bertos N. R., Wang A. H., Yang X. J. (2001) Class II histone deacetylases. Structure, function, and regulation. Biochem. Cell Biol. 79, 243–252 [PubMed] [Google Scholar]
- 11. Calalb M. B., McKinsey T. A., Newkirk S., Huynh K., Sucharov C. C., Bristow M. R. (2009) Increased phosphorylation-dependent nuclear export of class II histone deacetylases in failing human heart. Clin. Transl. Sci. 2, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang S., McKinsey T. A., Zhang C. L., Richardson J. A., Hill J. A., Olson E. N. (2004) Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol. Cell Biol. 24, 8467–8476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kee H. J., Kook H. (2011) Roles and targets of class I and IIa histone deacetylases in cardiac hypertrophy. J. Biomed. Biotechnol. 2011, 928326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin M., Kettmann R., Dequiedt F. (2009) Class IIa histone deacetylases. Conducting development and differentiation. Int. J. Dev. Biol. 53, 291–301 [DOI] [PubMed] [Google Scholar]
- 15. McKinsey T. A., Zhang C. L., Olson E. N. (2000) Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. U.S.A. 97, 14400–14405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKinsey T. A., Zhang C. L., Lu J., Olson E. N. (2000) Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 408, 106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu J., McKinsey T. A., Zhang C. L., Olson E. N. (2000) Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol. Cell 6, 233–244 [DOI] [PubMed] [Google Scholar]
- 18. McKinsey T. A., Zhang C. L., Olson E. N. (2002) MEF2. A calcium-dependent regulator of cell division, differentiation, and death. Trends Biochem. Sci. 27, 40–47 [DOI] [PubMed] [Google Scholar]
- 19. Grozinger C. M., Schreiber S. L. (2000) Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc. Natl. Acad. Sci. U.S.A. 97, 7835–7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKinsey T. A., Zhang C. L., Olson E. N. (2001) Identification of a signal-responsive nuclear export sequence in class II histone deacetylases. Mol. Cell Biol. 21, 6312–6321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellis J. J., Valencia T. G., Zeng H., Roberts L. D., Deaton R. A., Grant S. R. (2003) CaM kinase IIδC phosphorylation of 14-3-3β in vascular smooth muscle cells. Activation of class II HDAC repression. Mol. Cell Biochem. 242, 153–161 [PubMed] [Google Scholar]
- 22. Harrison B. C., Roberts C. R., Hood D. B., Sweeney M., Gould J. M., Bush E. W., McKinsey T. A. (2004) The CRM1 nuclear export receptor controls pathological cardiac gene expression. Mol. Cell Biol. 24, 10636–10649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison B. C., Kim M. S., van Rooij E., Plato C. F., Papst P. J., Vega R. B., McAnally J. A., Richardson J. A., Bassel-Duby R., Olson E. N., McKinsey T. A. (2006) Regulation of cardiac stress signaling by protein kinase d1. Mol. Cell Biol. 26, 3875–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vega R. B., Harrison B. C., Meadows E., Roberts C. R., Papst P. J., Olson E. N., McKinsey T. A. (2004) Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol. Cell Biol. 24, 8374–8385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu X., Zhang T., Bossuyt J., Li X., McKinsey T. A., Dedman J. R., Olson E. N., Chen J., Brown J. H., Bers D. M. (2006) Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Invest. 116, 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bossuyt J., Chang C. W., Helmstadter K., Kunkel M. T., Newton A. C., Campbell K. S., Martin J. L., Bossuyt S., Robia S. L., Bers D. M. (2011) Spatiotemporally distinct protein kinase D activation in adult cardiomyocytes in response to phenylephrine and endothelin. J. Biol. Chem. 286, 33390–33400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bossuyt J., Helmstadter K., Wu X., Clements-Jewery H., Haworth R. S., Avkiran M., Martin J. L., Pogwizd S. M., Bers D. M. (2008) Ca2+/calmodulin-dependent protein kinase IIδ and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ. Res. 102, 695–702 [DOI] [PubMed] [Google Scholar]
- 28. Chidsey C. A., Braunwald E., Morrow A. G., Mason D. T. (1963) Myocardial Norepinephrine Concentration in Man. Effects of Reserpine and of Congestive Heart Failure. N. Engl. J. Med. 269, 653–658 [DOI] [PubMed] [Google Scholar]
- 29. Movsesian M. A., Bristow M. R. (2005) Alterations in cAMP-mediated signaling and their role in the pathophysiology of dilated cardiomyopathy. Curr. Top. Dev. Biol. 68, 25–48 [DOI] [PubMed] [Google Scholar]
- 30. Carnegie G. K., Smith F. D., McConnachie G., Langeberg L. K., Scott J. D. (2004) AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol. Cell 15, 889–899 [DOI] [PubMed] [Google Scholar]
- 31. Carnegie G. K., Soughayer J., Smith F. D., Pedroja B. S., Zhang F., Diviani D., Bristow M. R., Kunkel M. T., Newton A. C., Langeberg L. K., Scott J. D. (2008) AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol. Cell 32, 169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Appert-Collin A., Cotecchia S., Nenniger-Tosato M., Pedrazzini T., Diviani D. (2007) The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates α1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 104, 10140–10145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Backs J., Worst B. C., Lehmann L. H., Patrick D. M., Jebessa Z., Kreusser M. M., Sun Q., Chen L., Heft C., Katus H. A., Olson E. N. (2011) Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J. Cell Biol. 195, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ha C. H., Kim J. Y., Zhao J., Wang W., Jhun B. S., Wong C., Jin Z. G. (2010) PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 107, 15467–15472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sucharov C. C., Dockstader K., Nunley K., McKinsey T. A., Bristow M. (2011) β-Adrenergic receptor stimulation and activation of protein kinase A protect against α1-adrenergic-mediated phosphorylation of protein kinase D and histone deacetylase 5. J. Card. Fail. 17, 592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bassani J. W., Bassani R. A., Bers D. M. (1995) Calibration of indo-1 and resting intracellular [Ca]i in intact rabbit cardiac myocytes. Biophys. J. 68, 1453–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bassani J. W., Bassani R. A., Bers D. M. (1994) Relaxation in rabbit and rat cardiac cells. Species-dependent differences in cellular mechanisms. J. Physiol. 476, 279–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pogwizd S. M., Qi M., Yuan W., Samarel A. M., Bers D. M. (1999) Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ. Res. 85, 1009–1019 [DOI] [PubMed] [Google Scholar]
- 39. Allen M. D., Zhang J. (2006) Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun. 348, 716–721 [DOI] [PubMed] [Google Scholar]
- 40. Liu S., Zhang J., Xiang Y. K. (2011) FRET-based direct detection of dynamic protein kinase A activity on the sarcoplasmic reticulum in cardiomyocytes. Biochem. Biophys. Res. Commun. 404, 581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Port J. D., Bristow M. R. (2001) Altered β-adrenergic receptor gene regulation and signaling in chronic heart failure. J. Mol. Cell Cardiol. 33, 887–905 [DOI] [PubMed] [Google Scholar]
- 42. Haworth R. S., Stathopoulou K., Candasamy A. J., Avkiran M. (2012) Neurohormonal regulation of cardiac histone deacetylase 5 nuclear localization by phosphorylation-dependent and phosphorylation-independent mechanisms. Circ. Res. 110, 1585–1595 [DOI] [PubMed] [Google Scholar]
- 43. Margariti A., Zampetaki A., Xiao Q., Zhou B., Karamariti E., Martin D., Yin X., Mayr M., Li H., Zhang Z., De Falco E., Hu Y., Cockerill G., Xu Q., Zeng L. (2010) Histone deacetylase 7 controls endothelial cell growth through modulation of β-catenin. Circ. Res. 106, 1202–1211 [DOI] [PubMed] [Google Scholar]
- 44. Faul C., Dhume A., Schecter A. D., Mundel P. (2007) Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol. Cell Biol. 27, 8215–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Berthouze M., Laurent A. C., Breckler M., Lezoualc'h F. (2011) New perspectives in cAMP-signaling modulation. Curr. Heart Fail. Rep. 8, 159–167 [DOI] [PubMed] [Google Scholar]
- 46. Diviani D., Dodge-Kafka K. L., Li J., Kapiloff M. S. (2011) A-kinase anchoring proteins. Scaffolding proteins in the heart. Am. J. Physiol. Heart Circ. Physiol. 301, H1742–H1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dodge-Kafka K. L., Bauman A., Mayer N., Henson E., Heredia L., Ahn J., McAvoy T., Nairn A. C., Kapiloff M. S. (2010) cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J. Biol. Chem. 285, 11078–11086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dodge-Kafka K. L., Langeberg L., Scott J. D. (2006) Compartmentation of cyclic nucleotide signaling in the heart. The role of A-kinase anchoring proteins. Circ. Res. 98, 993–1001 [DOI] [PubMed] [Google Scholar]
- 49. Dodge-Kafka K. L., Soughayer J., Pare G. C., Carlisle Michel J. J., Langeberg L. K., Kapiloff M. S., Scott J. D. (2005) The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature 437, 574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fischmeister R., Castro L. R., Abi-Gerges A., Rochais F., Jurevicius J., Leroy J., Vandecasteele G. (2006) Compartmentation of cyclic nucleotide signaling in the heart. The role of cyclic nucleotide phosphodiesterases. Circ. Res. 99, 816–828 [DOI] [PubMed] [Google Scholar]
- 51. Leroy J., Abi-Gerges A., Nikolaev V. O., Richter W., Lechêne P., Mazet J. L., Conti M., Fischmeister R., Vandecasteele G. (2008) Spatiotemporal dynamics of β-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes. Role of phosphodiesterases. Circ. Res. 102, 1091–1100 [DOI] [PubMed] [Google Scholar]
- 52. Mika D., Leroy J., Vandecasteele G., Fischmeister R. (2012) PDEs create local domains of cAMP signaling. J. Mol. Cell Cardiol. 52, 323–329 [DOI] [PubMed] [Google Scholar]
- 53. Mongillo M., McSorley T., Evellin S., Sood A., Lissandron V., Terrin A., Huston E., Hannawacker A., Lohse M. J., Pozzan T., Houslay M. D., Zaccolo M. (2004) Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ. Res. 95, 67–75 [DOI] [PubMed] [Google Scholar]
- 54. Mongillo M., Zaccolo M. (2006) A complex phosphodiesterase system controls β-adrenoceptor signalling in cardiomyocytes. Biochem. Soc. Trans. 34, 510–511 [DOI] [PubMed] [Google Scholar]
- 55. Nikolaev V. O., Bünemann M., Schmitteckert E., Lohse M. J., Engelhardt S. (2006) Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching β1-adrenergic but locally confined β2-adrenergic receptor-mediated signaling. Circ. Res. 99, 1084–1091 [DOI] [PubMed] [Google Scholar]
- 56. Perry S. J., Baillie G. S., Kohout T. A., McPhee I., Magiera M. M., Ang K. L., Miller W. E., McLean A. J., Conti M., Houslay M. D., Lefkowitz R. J. (2002) Targeting of cyclic AMP degradation to β2-adrenergic receptors by β-arrestins. Science 298, 834–836 [DOI] [PubMed] [Google Scholar]
- 57. Redden J. M., Dodge-Kafka K. L. (2011) AKAP phosphatase complexes in the heart. J. Cardiovasc. Pharmacol. 58, 354–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Taniguchi M., Carreira M. B., Smith L. N., Zirlin B. C., Neve R. L., Cowan C. W. (2012) Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron 73, 108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Greco T. M., Yu F., Guise A. J., Cristea I. M. (2011) Nuclear import of histone deacetylase 5 by requisite nuclear localization signal phosphorylation. Mol. Cell Proteomics 10, M110.004317 [DOI] [PMC free article] [PubMed] [Google Scholar]