Abstract
Homeodomain-interacting protein kinase 2 (HIPK2) is a nuclear serine/threonine kinase of the subfamily of dual-specificity Yak1-related kinase proteins. HIPK2 was first described as a homeodomain-interacting protein kinase acting as a corepressor for homeodomain transcription factors. More recently, it was reported that HIPK2 plays a role in p53-mediated cellular apoptosis and could also participate in the regulation of the cell cycle. US11 protein of herpes simplex virus type 1 is a multifunctional protein involved in the regulation of several processes related to the survival of cells submitted to environmental stresses by mechanisms that are not fully elucidated. In an attempt to better understand the multiple functions of US11, we identified cellular binding partners of this protein by using the yeast two-hybrid system. We report that US11 interacts with HIPK2 through the PEST domain of HIPK2 and that this interaction occurs also in human cells. This interaction modifies the subcellular distribution of HIPK2 and protects the cell against the HIPK2-induced cell growth arrest.
Homeodomain-interacting protein kinases (HIPKs) possess kinase domains that display strong sequence similarity with those of other protein kinases belonging to the family of dual-specificity Yak1-related kinases (DYRK). One main feature of DYRKs is their dual ability to autophosphorylate tyrosine residues and to phosphorylate their substrates on serine and threonine residues (17, 33). However, HIPKs are able to autophosphorylate and to phosphorylate their exogenous substrates on serine and threonine residues, but their phosphorylation on tyrosine residues might be mediated by so far uncharacterized kinases (17, 38). As such, HIPKs constitute a subfamily of DYRKs composed of at least five members: HIPK1, HIPK2, HIPK3 (21), PKM (47), and STANK (49). At present, HIPK2 is one of the best-characterized members of the HIPK family.
In addition to its kinase domain, HIPK2 contains a domain required for the interaction with several NK homeoproteins, a domain rich in proline, glutamic acid, serine, and threonine (PEST) and a domain rich in tyrosine and histidine. The function of these latter two domains is not yet clear, although PEST domains may be involved in the stability control of proteins (41). HIPK2 is localized mainly in nuclei and concentrates within a variable number of small nuclear domains. The targeting of HIPK2 into these domains is conditioned by its Ubc9-mediated posttranslational modification with the ubiquitin-like protein SUMO-1 (20). Under specific physiological conditions, HIPK2 is recruited to nuclear domain 10 (ND10), also called promyelocytic leukemia (PML) bodies. This recruitment requires the interaction of HIPK2 with the PML-3 protein (18). Moreover, HIPK2 is able to induce modification of ND10 structure and may play a role in ND10 dynamics during the cell cycle (12).
HIPK2 interacts with several NK homeodomain transcription factors that have important functions during embryonic development and organogenesis. In particular, HIPK2 is a component of a transcriptional corepressor complex containing NK-3, Groucho, and a histone deacetylase complex (6, 21). The formation and the activity of this complex is regulated by HIPK2. HIPK2 also intervenes in the transduction of extracellular signals from the cell surface to the nucleus, such as those induced by the tumor necrosis factor (25, 34, 51) or those mediated by CD43 and STAT3 (33, 49). Moreover, HIPK2 interacts with HMGI(Y) and regulates progression through the cell cycle (38). More recently, HIPK2 has been shown to be a novel activator of the p53 tumor suppressor protein. Indeed, human HIPK2 phosphorylates p53 on serine 46 after irradiation with UV and thus regulates p53-mediated induction of apoptosis (10, 18, 50). In addition, HIPK2 interacts with TP53INP1s to modulate the p53-mediated regulation of the cell cycle and apoptosis (46). HIPK2 interacts also with p73, a member of the p53 family (19).
The genome of herpes simplex virus type 1 (HSV-1) contains at least 79 genes, of which 42 are dispensable for the production of infectious viral particles in cell cultures. However, these dispensable genes likely play important roles in virus-host interactions, allowing the virus to adapt to its environment. For example, Us11 was initially described as one of these so-called nonessential genes (3, 27). The US11 protein is among the most-abundant viral proteins present in cells late in infection and is packaged in the tegument of the native virions to be delivered into cells after infection (2, 16, 42). Soon after infection, the US11 protein is found in the cytoplasm, either as heterogeneous oligomers or associated with ribosomes or both (8, 30, 39, 43). Later during infection, the US11 protein accumulates into nucleoli and is also found in RNP fibrils as well as in clusters of interchromatin granules. Retention of US11 within nucleoli and nuclear export are mediated by a unique motif embedded into the C-terminal part of the protein (5). In addition, this part of the protein contains a motif that confers to US11 an intercellular trafficking activity (22). Since US11 was first identified as a DNA-binding protein, strong evidences have been brought, showing that this protein also possesses RNA-binding activities that appear essential for several of its biological functions, such as control of mRNA transport and translation (7, 11) and regulation of PKR (double-stranded RNA-activated protein kinase) activity (4, 37). Moreover, even though US11 protein is dispensable for viral growth in cell cultures, it plays an important role in the replication of HSV-1 in the adrenal gland, an organ important for viral penetration in the spinal cord and brain (36), and in cells subjected to thermal stress (3). Furthermore, US11 enhances recovery of protein synthesis and survival in heat shock-treated cells by an unknown mechanism (9). Therefore, it is clear that US11 is a multifunctional protein involved in posttranscriptional regulation of gene expression and in biological processes related to the survival of cells following environmental stress.
To gain insight into the molecular mechanisms underlying the multiple functions of this protein, we decided to identify cellular factors that interact with the US11 protein. We have performed a yeast two-hybrid screen of a mouse library with US11 as a bait. We found that US11 interacts with HIPK2. The interaction was confirmed by coimmunoprecipitation experiments performed with HeLa cells and by kinase assays, demonstrating that US11 can be phosphorylated by HIPK2. As expected, a green fluorescent protein (GFP)-HIPK2 fusion was localized in numerous nuclear dots. This intracellular distribution was modified after coexpression of US11. More importantly, colony formation assays demonstrated that HIPK2-induced cell growth arrest was inhibited by US11.
MATERIALS AND METHODS
Cell culture and transfection.
HeLa cells were grown in Eagle's essential minimum medium supplemented with 5% fetal calf serum and 2 mM glutamine. HEK 293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 2 mM glutamine. Transfection of HeLa cells was carried out by the calcium phosphate precipitation procedure. The cells were plated on 35-mm-diameter petri dishes (105 cells/dish) and incubated at 37°C for 24 h. One hour prior to transfection, the medium was replaced with 1 ml of fresh medium. Transfection mix containing 1.5 μg of plasmid DNA was added to the cells. Experiments were performed 24 h after transfection.
Antibodies.
Antibodies used for Western blot analysis, immunoprecipitation, and immunofluorescence include anti-Flag M2 (Sigma-Aldrich), rabbit polyclonal anti-US11 (8), and Texas Red-labeled anti-rabbit immunoglobulin G (IgG) (Jackson ImmunoResearch).
Yeast two-hybrid system.
Plasmids, Saccharomyces cerevisiae strains, selective media, and the transformation protocol have already been described (48). For construction of the LexA wild-type US11 bait, the cDNA encoding the entire US11 sequence was inserted into the BTM116 vector, resulting in the production of a LexA wild-type US11 fusion protein. The S. cerevisiae L40 strain was transformed and selected in a medium lacking uracil and tryptophan. Selected colonies were tested for the expression of the LexA wild-type US11 fusion protein. For this analysis, yeast lysates were made by using glass beads in Laemmli buffer (23) and expression of the LexA wild-type US11 protein was analyzed by Western blot analysis with an anti-US11 antibody. A colony containing the LexA wild-type US11 bait was then transformed with the VP16 mouse embryonic day 9.5 and 10.5 cDNA library. Transformants were selected on medium lacking uracil, tryptophan, leucine, and histidine. Colonies containing a VP16 fusion protein interacting with the bait were selected on the basis of transactivation of the His3 reporter gene after 3 days of growth. Purified DNA isolated from positives clones was amplified by PCR with VP16-specific sense and antisense primers (48) and analyzed by enzymatic digestion and sequencing. Sequence comparisons were done with the BLAST program (http://www.ncbi.nlm.nih.gov).
For quantification of the interaction and test of the US11 mutant (US11mt) baits, the S. cerevisiae strain YRN974 with an integrated LexA operator GFP cassette was used (31). Ten thousand cells derived from four independent transformants were analyzed for fluorescence intensity with a Becton Dickinson FACScan flow cytometer (35).
Molecular cloning of HIPK2a and HIPK2b complete coding sequence and plasmid constructions.
The sequence corresponding to the complete coding sequence of HIPK2 protein was cloned by two sets of reverse transcription (RT)-PCR performed with OF-1 Swiss adult mouse heart total mRNA. The first 5′ part of the sequence, contained between positions 149 and 1985 according to the numbering of Kim et al. (21), was obtained with the forward primer 5′-TACGAAGGTATGGCCTCA-3′ and the reverse primer 5′-ATTGGTAGTTTAGTATGGAGAC-3′. The second part of the sequence, contained between positions 1648 and 3743, was cloned with the forward primer 5′-AAAGAAGATGCTGACCATCG-3′ and reverse primer 5′-AAACATATGGGCCTCCTCCAGTGTTTATA-3′, in which the sequence coding the endonuclease restriction site NdeI (underlined) was inserted. Both fragments were separately cloned in the pGEM-T easy vector (Promega), giving constructs pGEM-T1 (insert positions 149 to 1985) and pGEM-T3 (insert positions 1648 to 3743). Sequencing analysis revealed that 50% of the cloned insert positions 1648 to 3743 contained a deletion of 81 nucleotides compared to the sequence published previously (21). Plasmids carrying the shorter inserts were named pGEM-T3, whereas plasmids carrying the longer inserts were named pGEM-T5. The complete coding sequences were then reconstituted by insertion of the ClaI/NsiI fragments of pGEM-T5 or pGEM-T3 in pGEM-T1, giving constructs pGEM-HIPK2a and pGEM-HIPK2b, respectively.
pEGFP-HIPK2a and pEGFP-HIPK2b expression vectors.
The pEGFP-HIPK2a and pEGFP-HIPK2b plasmids were obtained by inserting the EcoRI Klenow-filled fragment of constructs pGEM-HIPK2a and pGEM-HIPK2b into the SmaI site of the pEGFP-C3 expression vector (Clontech). These two constructs were then digested with HindIII and recircularized to clone the HIPK2a and HIPK2b coding sequences in frame with that of the enhanced GFP (EGFP). These constructions were confirmed by nucleotide sequencing.
US11 and US11 mutant expression vectors.
The US11 expression vector named pG-9, containing the US11 coding sequence placed under the control of the cytomegalovirus (CMV) immediate-early promoter, and the corresponding control vector pG-8, in which the US11 coding sequence was removed, were previously cloned in our laboratory (1). Construction of the expression plasmids named pG61-US11-m6, pG61-US11-m12, and pG61-US11-m14, coding for modified forms of the US11 protein, are described in detail elsewhere (5).
pSG5-Flag-HIPK2/PEST expression vector.
The sequence coding for Flag-M2 peptide was inserted into the EcoRI/BglII sites of the pSG5 vector (Stratagene). The full-length sequence contained in a positive two-hybrid VP16 clone was amplified by PCR with oligonucleotides 5′-GAGTTTGAGCAGATGTTTA-3′ and 5′-GGAAGATCTGTGAATTCG-3′, which contained a BglII site (underlined). This amplified fragment was then restriction digested with BamHI and BglII enzymes and inserted into the pSG5-Flag construct, giving the pSG5-Flag-HIPK2/PEST plasmid.
p3Flag-HIPK2b and p3Flag-HIPK2b AS expression vector.
The p3Flag-HIPK2b and p3Flag-HIPK2b antisense (AS) expression vectors were obtained by inserting the EcoRI fragment of construct pGEM-HIPK2b into the EcoRI site of plasmid 3XFLAG-CMV-10 (Sigma). This gave the HIPK2b sequence cloned in both orientations. The plasmid p3Flag-HIPK2b contained the HIPK2b cDNA in the sense orientation, and the plasmid p3Flag-HIPK2b AS contained the HIPK2b cDNA in the antisense orientation.
Western blot analysis and immunoprecipitation.
Cells were harvested 48 h after transfection. Cells in phosphate-buffered saline (PBS) were scraped and pelleted by centrifugation. The cell pellet was resuspended in 1.5 ml of PBS and divided into three equal fractions. After centrifugation, cells from 2 fractions were lysed in 400 μl of radioimmunoprecipitation assay (RIPA) buffer for immunoprecipitation (50 mM Tris-HCl [pH 7.2], 1% NP-40, 1% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], and 150 mM NaCl, supplemented with protease inhibitor [Complete; Roche Molecular Biochemicals]). Cells from the third fraction were resuspended in Laemmli buffer without glycerol and without bromophenol blue to allow quantification of proteins. Proteins (10 μg) from total cell lysates were separated by one-dimensional polyacrylamide gel electrophoresis (PAGE) in a gel containing 15% polyacrylamide and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore).
Proteins of interest were identified by incubating the membranes with primary antibodies (anti-Flag M2 or anti-US11) in TBS-T buffer (20 mM Tris-HCl [pH 7.4], 130 mM NaCl, 0.1% Tween 20). After incubation with a peroxidase-labeled secondary antibody, immunoreactive bands were revealed by enhanced chemiluminescence (Amersham). For immunoprecipitation, cell extracts were incubated at 4°C for 30 min in RIPA buffer and centrifuged at 75,000 rpm (TLA 100.3 rotor) in a TL-100 Beckman centrifuge for 30 min at 4°C. Supernatants were then incubated with the anti-Flag M2 or the anti-US11 antibodies overnight at 4°C. Immunocomplexes were immobilized on protein A-Sepharose beads (CL4B; Amersham Pharmacia Biotech) by a 30-min incubation at 4°C. The beads were collected and washed three times with RIPA buffer. Bound proteins were eluted and analyzed by Western blot analysis with the anti-Flag M2 or anti-US11 antibodies.
In vitro kinase assay.
For the catalytic kinase assay, HEK 293 cells were transfected with the p3Flag-HIPK2b expression vector and lysed 24 h after transfection in the following RIPA buffer (50 mM Tris-HCl [pH 7.5], 300 mM NaCl, 150 mM KCl, 1% NP-40, 5 mM EDTA, 1 mM dithiothreitol) supplemented with protease inhibitor (Complete; Roche Molecular Biochemicals). 3Flag-HIPK2b was immunoprecipitated as described above, and protein A-Sepharose beads were washed three times in kinase buffer (20 mM HEPES [pH 7.4], 10 mM MgCl2, 200 μM sodium orthovanadate). For the kinase reaction, immunoprecipitated 3Flag-HIPK2b was incubated with 1 μg of glutathione S-transferase (GST) or GST-US11-m55 or with 1 or 2 μg of GST-US11 (5) in 30 μl of kinase buffer supplemented with 5 μCi of [γ-32P]ATP and 50 μM unlabeled ATP for 30 min at 30°C. The reaction was stopped by adding 5 μl of 6× Laemmli buffer, and reaction products were analyzed directly by SDS-PAGE. 32P-labeled proteins were then detected by autoradiography.
Immunohistochemistry and indirect immunofluorescence.
HeLa cells were transfected with a combination of the following expression vectors as indicated on the figures: pEGFP-HIPK2a, pEGFP-HIPK2b, pG-9, pG61-US11-m6, pG61-US11-m12, and pG61-US11-m14. At the end of the transfection time (48 h), cells were fixed with 3% paraformaldehyde in PBS for 30 min at room temperature and washed with a 10 mM glycine-PBS solution. The cells were then permeabilized for 5 min with a solution of 1% Triton X-100 in PBS, washed with 10 mM glycine-PBS, and incubated in a 25 mM glycine-PBS solution for 30 min. The cells were then incubated with an anti-US11 antibody overnight, washed, and incubated with a secondary anti-rabbit IgG antibody conjugated with Texas Red. Epifluorescence was visualized with an Axiophot microscope from Zeiss coupled with a charge-coupled device camera (LH 750; Lhesa Electronic) at excitation wavelengths of 489 nm for GFP and 596 nm for Texas Red.
Semiquantitative RT-PCR.
A one-step RT-PCR kit (Qiagen) was used for amplification of specific RNA from a mouse total RNA collection (Ambion). HIPK2a and HIPK2b mRNAs and 18S rRNA were reverse transcribed and amplified in the same reaction with primers 18S rRNA sense (5′-ATGCGGCGGCGTTATTC-3′) and antisense (5′-GCGCGGGCGGTGTGTA-3′) and HIPK2a/HIPK2b mRNA sense (5′-GTCACCATGACACACCTGCT-3′) and antisense (5′-AGGGGGACACACGATGAGAG-3′). PCR products were separated by agarose gel electrophoresis and quantified with a gel analyzer (Bio-1D Vilbert Lourmat) after ethidium bromide staining.
Colony formation assay.
Cells plated in 60-mm-diameter petri dishes were transfected by the calcium phosphate method with different combinations of the following plasmids as indicated on the figures: p3Flag-HIPK2b, p3Flag-HIPK2b AS, pG-9, and pG-8. The total amount of transfected DNA was 4.5 μg. At 24 h after transfection, cells were cultivated in medium containing 1 mg of G418/ml for 2 weeks before staining of the surviving colonies with crystal violet. Colonies were counted by using ImageMaster 2D (Pharmacia).
Theoretical structure of the mouse HIPK2 gene.
Mouse genomic draft DNA sequences (accession numbers AC069142 and AC079129) homologous to HIPK2a and HIPK2b cDNAs were found by using the BLAST program. Unordered pieces were ordered with the SIM4 program (15) to draw the murine HIPK2 gene structure.
Nucleotide sequence accession number.
The accession numbers (available from EMBL, GenBank, and DDBJ) for the HIPK2a and HIPK2b sequences reported in this paper are AF333791 and AF333792, respectively.
RESULTS
Interaction of US11 with the PEST domain of HIPK2.
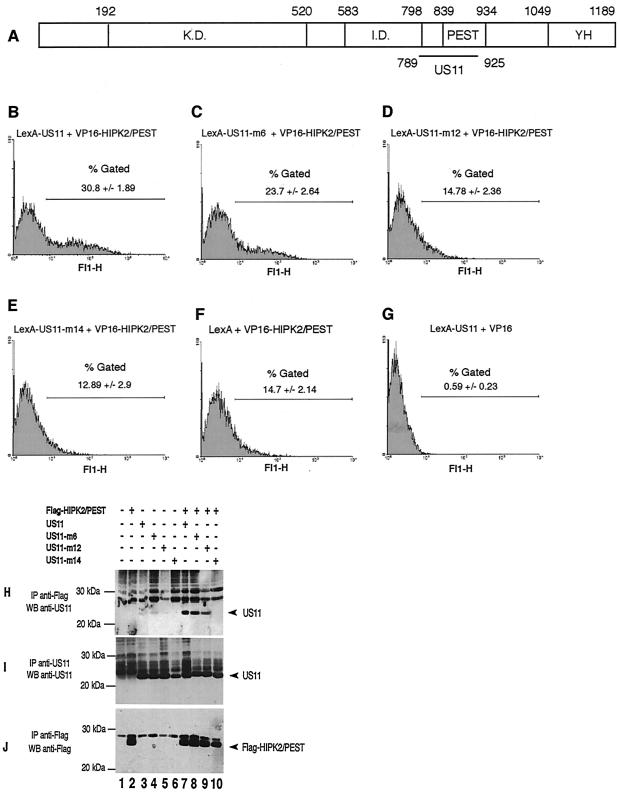
A yeast two-hybrid system was used to identify cellular proteins that could interact with the US11 protein. In this system, the bait protein, LexA-US11, was a fusion protein made of the DNA binding domain of the LexA operator and the entire US11 protein. The prey proteins were fusion proteins made of the activator domain of VP16 and of fragments of proteins of about 100 amino acids derived from a mouse embryo cDNA library (48). Using this system, we have tested 20 million prey fusion proteins for the ability to interact with the US11 protein. Among those, 400 were shown to interact with US11 protein, and 41 of the 400 were identified as fusion proteins containing the region encompassing amino acids 789 to 925 of HIPK2 (data not shown).
As shown in Fig. 1A, this region overlaps with the PEST domain of HIPK2 (amino acids 839 to 934) and the nuclear speckle retention signal (amino acids 860 to 967). However, because US11 and the PEST domain of HIPK2 both exhibited a high proline content, and because these domains rich in proline may interact with each other nonspecifically (53), it was determined whether a single or a few amino acid substitutions within the US11 protein sequence could affect its ability to interact with VP16-HIPK2/PEST. Three expression vectors coding for modified forms of the LexA-US11 wild-type protein, designated LexA-US11-m6, LexA-US11-m12, and LexA-US11-m14, were constructed (for a detailed description of these modified forms, see reference 5) and individually transfected together with that coding for VP16-HIPK2/PEST in the yeast strain YRN974. In this strain, the reporter gene encodes the GFP under the control of a LexA-binding-site-containing promoter, and the efficiency of the interaction between fusion proteins is estimated by numbering the fluorescent cells together with measuring the intensity of their fluorescence with a flow cytometer. LexA-US11, as expected, and also LexA-US11-m6 interacted strongly with VP16-HIPK2/PEST, since 31 and 23.7%, respectively, of the cells displayed a very high level of fluorescence (Fig. 1B and C). Conversely, the interaction of LexA-US11-m12 and LexA-US11-m14 with VP16-HIPK2/PEST was greatly impaired, since only 14.78 and 12.89% of the cells displayed a high level of fluorescence (Fig. 1D and E), percentages very close to that obtained when expressing only the LexA part of the fusion protein (Fig. 1F). The percentage of highly fluorescent cells was also very low when expressing LexA-US11 together with the DNA binding domain of VP16 only (Fig. 1G). Therefore, we concluded that the interaction observed between LexA-US11 and VP16-HIPK2/PEST occurred indeed via the US11 and HIPK2 parts of the fusion proteins.
FIG. 1.
(A) Schematic representation of the HIPK2 coding sequence. K.D., kinase domain; I.D., interaction domain for homeoproteins; PEST, proline-, glutamic acid-, serine-, and threonine-rich domain; YH, tyrosine- and histidine-rich domain. Numbers indicate amino acid positions. The horizontal black line marks the delineation of the interaction domain of HIPK2 with US11. (B to G) Interaction of LexA-US11 and LexA-US11 mutant forms with VP16-HIPK2/PEST. The protein-protein interaction was measured by the yeast two-hybrid analyses with the GFP gene as a reporter. Yeast strain YRN974 with an integrated LexA operator-GFP gene was used for these analyses. (B) Yeast cells were transformed with expression vectors encoding LexA-US11 and VP16-HIPK2/PEST proteins. (C to E) Yeast cells were transformed with expression vectors encoding VP16-HIPK2/PEST combined with various vectors coding for different LexA-US11 mutants, i.e., LexA-US11-m6, LexA-US11-m12, and LexA-US11-m14. Mutations carried by these proteins are detailed in reference 5. (F) Yeast cells expressing LexA alone and VP-16-HIPK2/PEST. (G) Yeast cells expressing LexA-US11 and VP-16 alone. (H to J) Interaction of the PEST domain of HIPK2 with US11 and US11 mutants in human cells. HeLa cells were cotransfected with empty vectors (lane 1) or the indicated expression vectors containing a cDNA encoding Flag-HIPK2/PEST (lanes 2 and 7 to 10), US11 (lanes 3 and 7), US11-m6 (lanes 4 and 8), US11-m12 (lanes 5 and 9), or US11-m14 (lanes 6 and 10). Cell lysates were subjected to immunoprecipitation (IP) with an anti-Flag antibody (H and J) or an anti-US11 antibody (I). Purified proteins were analyzed by Western blotting with an anti-US11 (H and I) or an anti-Flag (J) antibody. Arrowheads indicate the positions of US11 (H and I) or Flag-HIPK2/PEST (J). Molecular masses are shown on the left in kilodaltons. +, present; −, absent.
To validate these results, we next determined whether a specific interaction between the native form of the US11 protein and the PEST domain of HIPK2 could be obtained after the synthesis in eucaryotic cells (Fig. 1H to J). An expression vector coding for a Flag-tagged HIPK2/PEST domain was constructed and cotransfected in HeLa cells, either alone or together with expression vectors coding for US11, US11-m6, US11-m12, and US11-m14. Forty-eight hours after transfection, proteins interacting with Flag-HIPK2/PEST were purified by coimmunoprecipitation with an anti-Flag antibody and analyzed by Western blotting with an anti-US11 protein antibody (Fig. 1H). US11 and US11-m6 proteins were efficiently coimmunoprecipitated with Flag-HIPK2/PEST (Fig. 1H, lanes 7 and 8), whereas coimmunoprecipitation of US11-m12 was much less efficient (Fig. 1H, lane 9). US11-m14 was not coimmunoprecipitated with Flag-HIPK2/PEST (Fig. 1H, lane 10), even though both proteins, Flag-HIPK2/PEST and US11-m14, were effectively synthesized (Fig. 1I and J, lanes 10). Because US11-m6 and US11-m14 exhibit, respectively, only 1 or 2 proline residue substitutions of 35 present in US11 and because their abilities to interact with the PEST domain of HIPK2 are very different, it can be concluded that the overall proline content of the two proteins does not account for their interaction. Moreover, the results obtained for US11-m12, in which only an arginine residue has been replaced by a glutamine, clearly demonstrated that the interaction of US11 with HIPK2 could be impaired without changing the proline content of both proteins. Therefore, these results demonstrate that the US11 protein interacts specifically with the PEST domain of HIPK2.
Structural organization of HIPK2 gene and mRNA expression.
To analyze further the interaction between US11 and HIPK2, we designed an experimental strategy to isolate the entire coding sequence of HIPK2 by RT-PCR. This experimental strategy allowed us to isolate and to characterize two slightly different HIPK2 cDNAs from the hearts of adult Swiss mice. The deduced sequences from these two cDNAs were designated HIPK2a and HIPK2b (GenBank accession numbers AF333791 and AF333792, respectively). The sequence of HIPK2a corresponds to that of nuclear body-associated kinase 1a (NBAK1a; accession number AF170301), also named PKM, and the sequence of HIPK2b corresponds to the sequence described by Kim et al. (21), also named NBAK1b or STANK (accession numbers AF170302 and AF273680, respectively). The sequence of HIPK2a displays a 27-amino-acid deletion in the region of the molecule initially described by Kim et al. (21) which contains the domain interacting with homeoproteins. This difference might result from an alternative splicing of HIPK2 pre-mRNA.
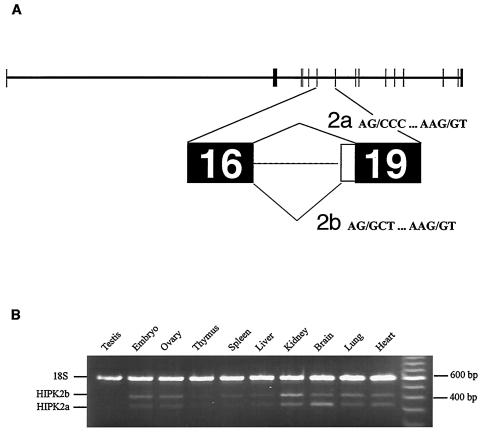
To test this possibility, we decided to elucidate the theoretical structure of a portion of the HIPK2 gene to determine whether potential alternative splicing sites are found within this gene. For this, large fragments of murine genomic DNA encompassing HIPK2 sequences were found in GenBank and contiguously ordered according to the HIPK2 coding sequence (see Materials and Methods). Sequences of every intron-exon junction (data not shown) allowed us to validate the intron-exon arrangement proposed for the mouse HIPK2 gene, since all of these sequences are in very good agreement with those known to be potential splicing sites (44). This partial HIPK2 gene model correlated with the model predicted by the model maker software (http://www.ncbi.nlm.nih.gov/mapview). Finally, the presence of two different acceptor splicing sites at the boundary of putative intron-exon 19, according to the numbering of model maker software, supports the hypothesis that HIPK2a and HIPK2b are derived from differently spliced mRNAs (Fig. 2A).
FIG. 2.
(A) Structural organization of the murine HIPK2 gene. The bioinformatic analysis is described in Materials and Methods. Exons are represented by filled boxes. Potential alternative splicing occurring between exons 16 and 19 according to the numbering of model maker software (http://www.ncbi.nlm.nih.gov/mapview) are labeled 2a and 2b. (B) Analysis of the HIPK2 gene expression in different murine tissues by RT-PCR. Total mRNA (Clontech) was reverse transcribed, and the HIPK2a and HIPK2b cDNA and 18S rRNA were amplified with primers described in Materials and Methods. Positions of the DNA fragments corresponding to 18S, HIPK2b, and HIPK2a are indicated to the left of the figure. Sizes of the DNA fragments are indicated to the right of the figure in base pairs.
We then determined whether both mRNAs coding for either HIPK2a or HIPK2b were present in mouse embryos and in different tissues of adult mice. For this, fragments of DNA corresponding to either HIPK2a or HIPK2b and to the 18S rRNAs were obtained by simultaneous amplification by RT-PCR with total RNAs extracted from embryos and from various mouse tissues. DNA samples were separated by electrophoresis and stained with ethidium bromide (Fig. 2B). The ratios between the amount of DNA obtained from 18S rRNA and that obtained from both HIPK2 mRNAs were calculated (data not shown). This analysis showed that the two forms of HIPK2 mRNAs were expressed in all of the tissues analyzed. Furthermore, this analysis showed that both mRNAs are generally present in approximately equal amounts, except in the kidney and the brain, where HIPK2b mRNA was the major and the minor form, respectively.
Subcellular distribution of HIPK2a and HIPK2b when coexpressed or not with US11.
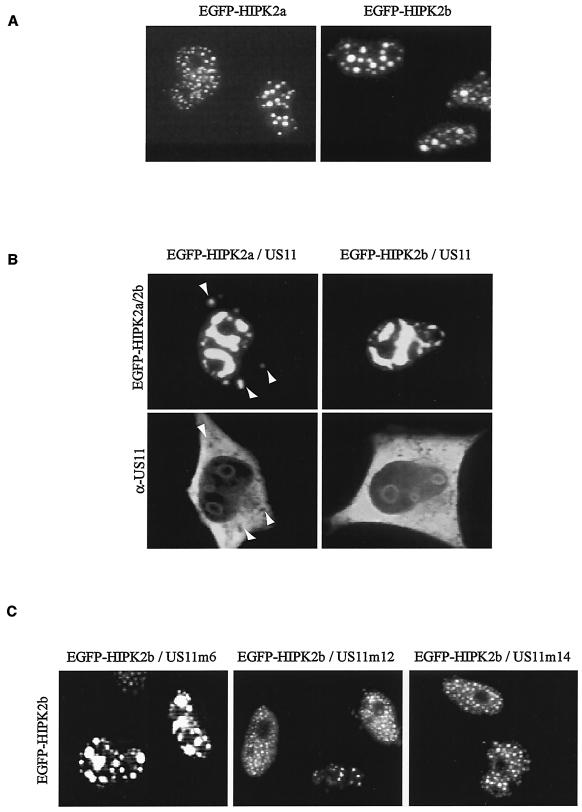
With the cDNAs coding for HIPK2a and HIPK2b being cloned, we then analyzed the intracellular distribution of both proteins. Two expression vectors coding for fusion proteins made of the EGFP and either HIPK2a or HIPK2b were constructed and transfected into HeLa cells. As shown in Fig. 3A, at 48 h after transfection both proteins display a similar, if not identical, intracellular distribution in numerous nuclear dots excluded from nucleoli and almost undetectable in the cytoplasm. This intracellular distribution is in agreement with that already described for HIPK2 (21, 47). In addition, confocal microscopy analyses allowed us to verify that some of the nuclear dots containing HIPK2 also contained PML (as already demonstrated by others; data not shown) (10, 12, 47).
FIG. 3.
(A) Intracellular localization of HIPK2a and HIPK2b. EGFP-HIPK2a and EGFP-HIPK2b expression vectors were transiently expressed in HeLa cells. The EGFP fluorescence signal was visualized 48 h after transfection. (B) Intracellular distribution of HIPK2a and HIPK2b when coexpressed with US11. US11 disrupts the nuclear localization of HIPK2a and HIPK2b. HeLa cells were transfected with expression vectors encoding US11 protein and a vector encoding either EGFP-HIPK2a or EGFP-HIPK2b. At 48 h after transfection, cells were fixed and stained with an anti-US11 antibody and Texas Red-conjugated anti-rabbit IgG. Arrows indicate HIPK2a found in the cytoplasm. (C) Intracellular distribution of HIPK2b when coexpressed with different US11 mutants. HeLa cells were transfected with expression vector encoding EGFP-HIPK2b combined with expression vector coding for either US11-m6, US11-m12, or US11-m14. The EGP-HIPK2b fluorescence signal was visualized 48 h after transfection.
To gain insights into the functional significance of the interaction between HIPK and US11, we then investigated whether the intracellular distribution of one of these proteins might be affected after cosynthesis of the other. For this, HeLa cells were transfected with expression vectors coding for either EGFP-HIPK2a or EGFP-HIPK2b together with a vector coding for wild-type US11 protein. At different times after transfection, expression of the transgenes was monitored by direct visualization of EGFP fusion proteins and by immunofluorescence for the US11 protein (data not shown). These experiments demonstrated that, at each time analyzed after transfection, the typical pattern of intracellular distribution of each protein was dramatically different when proteins were synthesized together. For example, as shown in Fig. 3B, upper panel, at 48 h after transfection, HIPK2a and HIPK2b did not display a fine punctate nuclear pattern but rather were found within nuclear structures and tended to occupy the entire space and always excluding the nucleoli.
Another striking feature of the US11-induced HIPK2 pattern was the presence within the cytoplasm of dots containing HIPK2 (compare Fig. 3B, upper panels, with 3A). The usual nuclear distribution of US11 protein described many times (39, 43), concentrated into nucleoli and almost undetectable in the nucleoplasm, was also modified by coexpression of the HIPK2 and US11 genes, since in these conditions the US11 protein was detectable in regions surrounding the nucleoli and spread throughout the nucleoplasm (Fig. 3B, lower panels). The same modifications of intracellular distribution of both proteins were also observed when Flag-HIPK2 fusion proteins were used instead of EGFP-HIPK2 fusion proteins (data not shown). To evaluate whether HIPK2 interaction with the US11 protein could account for this unusual behavior, the intracellular distribution of EGFP-HIPK2b was determined after coexpression with vectors coding for modified forms of US11, which we have shown to interact differently with the PEST domain of HIPK2 (Fig. 1). Interestingly, US11-m6, which interacts with the PEST domain of HIPK2 with the same efficiency as the wild-type US11 protein, induced a modification of intracellular distribution of HIPK2 similar to that induced by the US11 wild-type protein (Fig. 3C). Conversely, US11-m12, which interacts with the PEST domain of HIPK2 less efficiently than the US11 wild-type protein, and US11-m14, which does not interact with this domain, did not significantly alter this pattern. Together, these results demonstrate that the US11 protein induces an intracellular redistribution of HIPK2, and this effect is mediated through US11 protein-HIPK2 interaction.
Phosphorylation of US11 by HIPK2b.
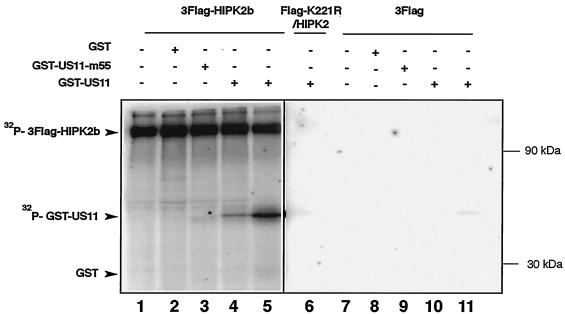
US11 is a protein that is phosphorylated in vivo. This prompted us to investigate whether HIPK2 can phosphorylate US11. To perform the experiment, 3Flag-HIPK2b produced in HEK 293 cells was subjected to an immune complex kinase assay with GST-US11 and the GST-US11-m55 mutant as substrates. As shown in Fig. 4, 3Flag-HIPK2b undergoes autophosphorylation (Fig. 4, lanes 1 to 5) and efficiently phosphorylates GST-US11 (Fig. 4, lanes 4 and 5) but not GST alone (Fig. 4, lane 2). Moreover, the GST-US11-m55 mutant, in which the main phosphorylation site has been mutated, was only slightly phosphorylated (Fig. 4, lane 3). Additionally, control experiments performed with the 3Flag expression vector, as well as with a kinase-defective HIPK2b (Flag-K221R/HIPK2b), show that the immunoprecipitated 3Flag-HIPK2b was not contaminated by another cellular kinase (Fig. 4, lanes 6 to 11). These results thus demonstrated that HIPK2b could phosphorylate US11 in vitro.
FIG. 4.
Kinase activity of HIPK2b. HEK 293 cells were transfected with either 3Flag-HIPK2b, Flag-K221R/HIPK2, or 3Flag expression vector. The corresponding proteins were immunoprecipitated with anti-Flag antibody, and their activities were determined by immune kinase complex assay with GST, GST-US11-m55, or GST-US11 as a substrate. The figure shows an autoradiogram of proteins separated by SDS-PAGE. Lanes 1 to 5, experiment performed with 3Flag-HIPK2b expression vector; lane 6, experiment performed with Flag-K221R/HIPK2 expression vector; lanes 7 to 11, experiment performed with 3Flag expression vector. Lanes 4 and 5 contain, respectively, 1 and 2 μg of GST-US11. Positions of the radiolabeled proteins are indicated to the left of the figure. Molecular masses are indicated to the right of the figure. +, present; −, absent.
Inhibition of HIPK2-induced cell cycle arrest by US11.
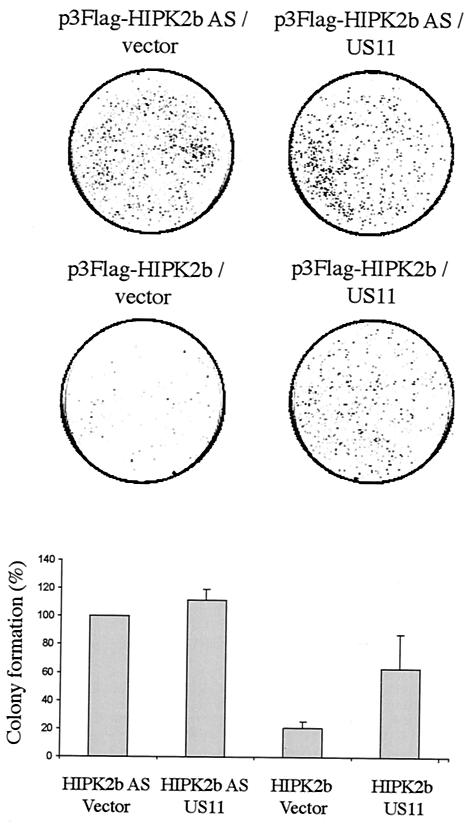
HIPK2 induces cell growth arrest, as seen by a decrease of cell proliferation with colony formation assays (10, 18, 19). Moreover, we have demonstrated that cells expressing US11 exhibited an increased survival capacity to thermal stress compared to cells that did not express this gene and that US11 could inhibit some cell signaling pathways leading to apoptosis (9; C. Diaz-Latoud, personal communication). To investigate further the functional relevance of US11-HIPK2 interactions, we performed colony formation assays. The wild-type p53-expressing HEK 293 cells were transfected with 3Flag-HIPK2b expression vectors, allowing also the expression of the Neor gene together with and without expression vectors coding for US11. As a negative control, HEK 293 cells were transfected with a vector encoding an antisense mRNA of HIPK2b (HIPK2b AS) with or without US11 expression vectors. Twenty-four hours after the transfection, cells were selected for resistance to G418 during 2 weeks of incubation, and the number of colonies was estimated for each experimental condition (Fig. 5). As expected, HIPK2b prevented cell growth, since the number of G418-resistant colonies was reduced to 17% of those obtained after transfection of the negative control vectors, HIPK2b AS alone and HIPK2b AS with the US11 expressing vector (Fig. 5). Conversely, when US11 was coexpressed with HIPK2b, the number of colonies represented 60% of those obtained after the transfection of the control vectors. This result clearly indicates that US11 impaired HIPK2 growth arrest.
FIG. 5.
HIPK2 cell growth arrest antagonized by US11 expression. HEK 293 cells were transfected with plasmids p3Flag-HIPK2b and p3Flag-HIPK2b AS combined with either expression vector for US11 or the corresponding empty vector. At 24 h after transfection, cells were selected with G418 and surviving colonies were stained with crystal violet. The combination of the vectors used for transfection is indicated above each corresponding box. Colony formation with the control vector p3Flag-HIPK2b AS was set as 100%. Results are means ± standard deviations of the results from three independent experiments (lower panel).
DISCUSSION
Us11 of HSV-1 was initially described as a so-called nonessential gene, i.e., not essential for the production of infectious viral particles in cell cultures (3, 27). However, even though the US11 protein is dispensable in culture, it seems to play an important role in the replication of HSV-1 in different cells under various environmental conditions (9, 52). To define the function of US11, we looked for its cellular protein binding partners with the yeast two-hybrid system. From this we isolated a cDNA whose sequence encompassed the PEST domain of the murine HIPK2 protein (21). Coimmunoprecipitation of US11 with transiently expressed Flag-tagged HIPK2/PEST provided further evidence for the tight association of US11 with HIPK2 in mammalian cells. The specificity of the interaction between US11 and HIPK2 was determined by using modified forms of US11 containing either a single or a few amino acid substitutions that interacted very slightly or did not interact at all with HIPK2/PEST in both experimental systems (i.e., coimmunoprecipitation as well as the two-hybrid system). US11 has been shown to be phosphorylated in vivo by a cellular kinase that remains unknown (45). We investigated whether US11 could be phosphorylated by HIPK2, and our results demonstrate that HIPK2b phosphorylates US11 in vitro. Therefore, we suggest that HIPK2 may be one of the cellular kinases capable of phosphorylating US11 in vivo.
To isolate the full coding sequence of the mouse HIPK2, we cloned two cDNAs that corresponded to two different proteins, named HIPK2a and HIPK2b, that differed slightly in length. The major difference between HIPK2a and HIPK2b is a 27-amino-acid deletion within HIPK2a overlapping the sequence coding for the domain of HIPK2 that interacts with homeoproteins (21). A BLAST analysis indicated that the longer cDNA corresponding to HIPK2b gave rise to a protein identical to HIPK2, previously identified in mice (21). The shorter cDNA corresponding to HIPK2a gave rise to a protein identified as PKM in hamsters (47). It may be that these cDNA sequences, coding for the two isoforms of HIPK2, were derived from the same alternatively spliced pre-mRNA. The presence of two potential acceptor splicing sites at the putative intron-exon 19 boundary of the prediction model favors this possibility. Moreover, transcripts coding for HIPK2a and HIPK2b were detected in all murine tissues tested. To our knowledge, this is the first report of the differential expression and function of the two isoforms of HIPK2.
HIPK2 localizes to distinct subcellular structures, i.e., ND10 and other nuclear speckles of unknown function (10, 18, 21, 47; this study). We have demonstrated that the US11 protein of HSV-1 interacts with the PEST domain of HIPK2, which is a domain common to both HIPK2a and HIPK2b. Because this domain encompasses the speckle retention signal, we investigated whether the interaction between US11 and HIPK2a or HIPK2b could disturb the subcellular localization of these proteins. Interestingly, US11 completely abolished the dot-like pattern of HIPK2a and HIPK2b and induced the formation of bigger structures that spread through the entire nucleoplasm, however, always excluding the space occupied by nucleolar structures. HIPKs are SUMOlated proteins that interact with the SUMO-conjugating enzyme Ubc9. The SUMOlation of HIPKs regulates the partitioning of HIPKs within the nuclear speckles. Interaction between HIPK2 and Ubc9 occurs at a region of the PEST sequence (20) that matches perfectly with the interaction domain of HIPK2 with US11. Two possibilities are apparent. First, by interacting with the PEST domain of HIPK2, US11 interferes with the SUMOlation of HIPK2 by Ubc9 and thus disturbs its nuclear localization. Alteration of the ratio between un-SUMOlated and SUMOlated HIPKs thus modifies the proportion of nuclear matrix-associated HIPKs (SUMOlated form) versus the soluble non-SUMOlated form of the protein. This possibility is conceivable, since we have found that US11 interacts with Ubc9 in the yeast two-hybrid system (unpublished data). Second, PEST sequences are predicted to regulate the stability of proteins (41). By interacting with the PEST domain of HIPK2, US11 might interfere with its degradation mechanism. Whether the degradation of HIPKs occurs via the proteasome or another pathway still remains to be determined.
Viruses depend on host cells for their replication and have developed different strategies to affect various signaling pathways and counteract apoptosis. Particularly, HSV-1 gene expression, DNA replication, and encapsidation all take place within nuclei in globular domains designated as replication compartments (26, 28, 29, 40). Formation of these replication compartments requires a profound reorganization of preexisting nuclear domains of the infected cell that proceeds in several sequential steps. ND10 is among the nuclear domains modified during HSV-1 infection. During early infection, HSV-1 disrupts ND10 by inducing degradation of the PML protein, a major component of ND10 (see reference 13 for a review). In addition to PML protein, ND10 contains several other proteins, such as HIPK2, p53, pRB, CBP, and TRADD (32, 34), that play a crucial role in transcription and apoptotic pathway regulation. Since HSV-1 disrupts ND10 and ND10 is implicated in the induction of apoptosis, we addressed the functional implication of US11-HIPK2 interaction on cell proliferation. In our colony formation assays with HEK 293 cells, US11 antagonized the HIPK2 inhibition of proliferation. HIPK2 is activated by UV irradiation and triggers p53-induced apoptosis (10, 18, 19). Since UV irradiation is one of the most powerful agents to activate latent HSV-1, we propose that in HSV-1-infected cells subjected to UV irradiation, US11 interferes with the HIPK2-induced apoptosis in addition to its interference with the HIPK2-induced cell growth arrest, thus allowing HSV-1 to replicate. If this is the case, US11 can be considered a new candidate for HSV-1 inhibition of cellular apoptosis, like US3 or ICP0 (14, 24). However, further work remains to be performed to correlate US11 inhibition of cell growth arrest and the HIPK2/p53 pathway. Moreover, it will be of particular interest to determine whether both HIPK2a and HIPK2b proteins are involved in the same pathways and interact with the same partners.
Interestingly, US11 is a potent inhibitor of PKR activity and blocks PKR-mediated apoptosis (4, 37). Our study suggests a novel and complementary function for US11, in addition to its already described antiapoptotic role. US11 interacting with HIPK2 counteracts the inhibition of cell proliferation by HIPK2. Thus, US11, by suppressing apoptosis and antagonizing cell growth arrest, appears to be more than a nonessential gene and, in fact, seems to be crucial for HSV-1 replication under various environmental conditions.
Acknowledgments
We are grateful to Anne Vojtek and Stan Hollenberg for the murine embryonic VP16 library and materials for the yeast two-hybrid screen. We thank R. K. Niedenthal and T. Tamura for the generous gift of the YRN974 yeast strain and A. Fusco and G. Pierantoni for the generous gift of the K221R/HIPK2 expression vector. We thank R. W. Currie for critical reading of the manuscript.
This work was supported by the Institut National de la Santé et de la Recherche Médical (INSERM).
REFERENCES
- 1.Besse, S., J.-J. Diaz, E. Pichard, K. Kindbeiter, J.-J. Madjar, and F. Puvion-Dutilleul. 1996. In situ hybridization and immuno-electron microscope analyses of the Us11 gene of herpes simplex virus type 1 during transient expression. Chromosoma 104:434-444. [DOI] [PubMed] [Google Scholar]
- 2.Bhat, R. A., and B. Thimmappaya. 1983. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc. Natl. Acad. Sci. USA 80:4789-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, S. M., and J. Harland. 1987. Three mutants of herpes simplex virus type 2: one lacking the genes Us10, Us11, Us12 and two in which Rs has been extended by 6 kb to 0, 91 map units with loss of Us sequences between 0, 94 and the Us/TRs junction. J. Gen. Virol. 68:1-18. [DOI] [PubMed] [Google Scholar]
- 4.Cassady, K. A., and M. Gross. 2002. The herpes simplex virus type 1 U(S)11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J. Virol. 76:2029-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catez, F., M. Erard, N. Schaerer-Uthurralt, K. Kindbeiter, J.-J. Madjar, and J.-J. Diaz. 2002. A unique motif for nucleolar retention and nuclear export regulated by phosphorylation. Mol. Cell. Biol. 22:1126-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, C. Y., Y. H. Kim, H. J. Kwon, and Y. Kim. 1999. The homeodomain protein NK-3 recruits Groucho and a histone deacetylase complex to repress transcription. J. Biol. Chem. 274:33194-33197. [DOI] [PubMed] [Google Scholar]
- 7.Diaz, J.-J., M. Duc Dodon, N. Schaerer-Uthurralt, D. Simonin, K. Kindbeiter, L. Gazzolo, and J.-J. Madjar. 1996. Post-transcriptional transactivation of human retroviral envelope glycoprotein expression by herpes simplex virus Us11 protein. Nature 379:273-277. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, J.-J., D. Simonin, T. Massé, P. Deviller, K. Kindbeiter, L. Denoroy, and J.-J. Madjar. 1993. The herpes simplex virus type 1 Us11 gene product is a phosphorylated protein found to be non-specifically associated with both ribosomal subunits. J. Gen. Virol. 74:397-406. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Latoud, C., J.-J. Diaz, N. Fabre-Jonca, K. Kindbeiter, J.-J. Madjar, and A.-P. Arrigo. 1997. Herpes simplex virus Us11 protein enhances recovery of protein synthesis and survival in heat shock treated HeLa cells. Cell Stress Chaperones 2:119-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Orazi, G., B. Cecchinelli, T. Bruno, I. Manni, Y. Higashimoto, S. Saito, M. Gostissa, S. Coen, A. Marchetti, G. Del Sal, G. Piaggio, M. Fanciulli, E. Appella, and S. Soddu. 2002. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 4:11-19. [DOI] [PubMed] [Google Scholar]
- 11.Duc Dodon, M., I. Mikaelian, A. Sergeant, and L. Gazzolo. 2000. The herpes simplex virus 1 Us11 protein cooperates with suboptimal amounts of human immunodeficiency virus type 1 (HIV-1) Rev protein to rescue HIV-1 production. Virology 270:43-53. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt, O. G., C. Boutell, A. Orr, E. Ullrich, O. Haller, and R. D. Everett. 2003. The homeodomain-interacting kinase PKM (HIPK-2) modifies ND10 through both its kinase domain and a SUMO-1 interaction motif and alters the posttranslational modification of PML. Exp. Cell Res. 283:36-50. [DOI] [PubMed] [Google Scholar]
- 13.Everett, R. D. 1999. A surprising role for the proteasome in the regulation of herpesvirus infection. Trends Biochem. Sci. 24:293-295. [DOI] [PubMed] [Google Scholar]
- 14.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Florea, L., G. Hartzell, Z. Zhang, G. M. Rubin, and W. Miller. 1998. A computer program for aligning a cDNA sequence with a genomic DNA sequence. Genome Res. 8:967-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gressner, A. M., and I. G. Wool. 1974. The phosphorylation of liver ribosomal proteins in vivo: evidence that only a single small subunit protein (S6) is phosphorylated. J. Biol. Chem. 249:6917-6925. [PubMed] [Google Scholar]
- 17.Hofmann, T. G., A. Mincheva, P. Lichter, W. Droge, and M. Lienhard Schmitz. 2000. Human homeodomain-interacting protein kinase-2 (HIPK2) is a member of the DYRK family of protein kinases and maps to chromosome 7q32-q34. Biochimie 82:1123-1127. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann, T. G., A. Moller, H. Sirma, H. Zentgraf, Y. Taya, W. Droge, H. Will, and M. L. Schmitz. 2002. Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat. Cell Biol. 4:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Kim, E. J., J. S. Park, and S. J. Um. 2002. Identification and characterization of HIPK2 interacting with p73 and modulating functions of the p53 family in vivo. J. Biol. Chem. 277:32020-320208. [DOI] [PubMed] [Google Scholar]
- 20.Kim, Y. H., C. Y. Choi, and Y. Kim. 1999. Covalent modification of the homeodomain-interacting protein kinase 2 (HIPK2) by the ubiquitin-like protein SUMO-1. Proc. Natl. Acad. Sci. USA 96:12350-12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, Y. H., C. Y. Choi, S. J. Lee, M. A. Conti, and Y. Kim. 1998. Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J. Biol. Chem. 273:25875-25879. [DOI] [PubMed] [Google Scholar]
- 22.Koshizuka, T., H. Takakuwa, F. Goshima, T. Murata, and Y. Nishiyama. 2001. The US11 gene product of herpes simplex virus has intercellular trafficking activity. Biochem. Biophys. Res. Commun. 288:597-602. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, X., Y. Wang, K. M. Debatin, and H. Hug. 2000. The serine/threonine kinase HIPK2 interacts with TRADD, but not with CD95 or TNF-R1 in 293T cells. Biochem. Biophys. Res. Commun. 277:513-517. [DOI] [PubMed] [Google Scholar]
- 26.Liptak, L. M., S. L. Uprichard, and D. M. Knipe. 1996. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures. J. Virol. 70:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longnecker, R., and B. Roizman. 1986. Generation of an inverting herpes simplex virus 1 mutants lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and α 47 gene. J. Virol. 58:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukonis, C. J., J. Burkham, and S. K. Weller. 1997. Herpes simplex virus type 1 prereplicative sites are a heterogeneous population: only a subset are likely to be precursors to replication compartments. J. Virol. 71:4771-4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukonis, C. J., and S. K. Weller. 1996. Characterization of nuclear structures in cells infected with herpes simplex virus type 1 in the absence of viral DNA replication. J. Virol. 70:1751-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLean, C. A., F. J. Rixon, and H. S. Marsden. 1987. The products of gene Us11 of herpes simplex virus type 1 are DNA-binding and localize to the nucleoli of infected cells. J. Gen. Virol. 68:1921-1937. [DOI] [PubMed] [Google Scholar]
- 31.Mancini, A., R. Niedenthal, H. Joos, A. Koch, S. Trouliaris, H. Niemann, and T. Tamura. 1997. Identification of a second Grb2 binding site in the v-Fms tyrosine kinase. Oncogene 15:1565-1572. [DOI] [PubMed] [Google Scholar]
- 32.Matera, A. G. 1999. Nuclear bodies: multifaceted subdomains of the interchromatin space. Trends Cell Biol. 9:302-309. [DOI] [PubMed] [Google Scholar]
- 33.Matsuo, R., W. Ochiai, K. Nakashima, and T. Taga. 2001. A new expression cloning strategy for isolation of substrate-specific kinases by using phosphorylation site-specific antibody. J. Immunol. Methods 247:141-151. [DOI] [PubMed] [Google Scholar]
- 34.Morgan, M., J. Thorburn, P. P. Pandolfi, and A. Thorburn. 2002. Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms. J. Cell Biol. 157:975-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niedenthal, R. K., L. Riles, M. Johnston, and J. H. Hegemann. 1996. Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast 12:773-786. [DOI] [PubMed] [Google Scholar]
- 36.Nishiyama, Y., R. Kurachi, T. Daikoku, and K. Umene. 1993. The US 9, 10, 11, and 12 genes of herpes simplex virus type 1 are of no importance for its neurovirulence and latency in mice. Virology 194:419-423. [DOI] [PubMed] [Google Scholar]
- 37.Peters, G. A., D. Khoo, I. Mohr, and G. C. Sen. 2002. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 76:11054-11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierantoni, G. M., M. Fedele, F. Pentimalli, G. Benvenuto, R. Pero, G. Viglietto, M. Santoro, L. Chiariotti, and A. Fusco. 2001. High mobility group I (Y) proteins bind HIPK2, a serine-threonine kinase protein which inhibits cell growth. Oncogene 20:6132-6141. [DOI] [PubMed] [Google Scholar]
- 39.Puvion-Dutilleul, F. 1987. Localization of viral-specific 21kDa protein in nucleoli of herpes simplex infected cells. Eur. J. Cell Biol. 43:487-498. [PubMed] [Google Scholar]
- 40.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857-868. [DOI] [PubMed] [Google Scholar]
- 41.Rechsteiner, M., and S. W. Rogers. 1996. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21:267-271. [PubMed] [Google Scholar]
- 42.Roller, R. J., L. L. Monk, D. Stuart, and B. Roizman. 1996. Structure and function in the herpes simplex virus 1 RNA-binding protein Us11: mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. J. Virol. 70:2842-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein Us11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp, P. A. 1987. Splicing of messenger RNA precursors. Science 235:766-771. [DOI] [PubMed] [Google Scholar]
- 45.Simonin, D., J.-J. Diaz, K. Kindbeiter, P. Pernas, and J.-J. Madjar. 1995. Phosphorylation of herpes simplex virus type 1 Us11 protein is independent of viral genome expression. Electrophoresis 16:1317-1322. [DOI] [PubMed] [Google Scholar]
- 46.Tomasini, R., A. A. Samir, A. Carrier, D. Isnardon, B. Cecchinelli, S. Soddu, B. Malissen, J. C. Dagorn, J. L. Iovanna, and N. J. Dusetti. 2003. TP53INP1s and homeodomain-interacting protein kinase-2 (HIPK2) are partners in regulating p53 activity. J. Biol. Chem. 278:37722-37729. [DOI] [PubMed] [Google Scholar]
- 47.Trost, M., G. Kochs, and O. Haller. 2000. Characterization of a novel serine/threonine kinase associated with nuclear bodies. J. Biol. Chem. 275:7373-7377. [DOI] [PubMed] [Google Scholar]
- 48.Vojtek, A. B., and S. M. Hollenberg. 1995. Ras-Raf interaction: two-hybrid analysis. Methods Enzymol. 255:331-342. [DOI] [PubMed] [Google Scholar]
- 49.Wang, W., V. Link, and J. M. Green. 2000. Identification and cloning of a CD43-associated serine/threonine kinase. Cell. Immunol. 205:34-39. [DOI] [PubMed] [Google Scholar]
- 50.Wang, Y., K. M. Debatin, and H. Hug. 2001. HIPK2 overexpression leads to stabilization of p53 protein and increased p53 transcriptional activity by decreasing Mdm2 protein levels. BMC Mol. Biol. 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, Y., T. G. Hofmann, L. Runkel, T. Haaf, H. Schaller, K. Debatin, and H. Hug. 2001. Isolation and characterization of cDNAs for the protein kinase HIPK2. Biochim. Biophys. Acta 1518:168-172. [DOI] [PubMed] [Google Scholar]
- 52.Ward, P. L., and B. Roizman. 1994. Herpes simplex genes: the blueprint of a successful human pathogen. Trends Genet. 10:267-274. [DOI] [PubMed] [Google Scholar]
- 53.Williamson, M. 1994. The structure and function of proline-rich regions in proteins. Biochem. J. 297:249-260. [DOI] [PMC free article] [PubMed] [Google Scholar]