Abstract
Tripeptidyl aldehyde proteasome inhibitors have been shown to effectively increase viral capsid ubiquitination and transduction of recombinant adeno-associated virus type 2 (rAAV-2) and rAAV-5 serotypes. In the present study we have characterized a second class of proteasome-modulating agents (anthracycline derivatives) for their ability to induce rAAV transduction. The anthracycline derivatives doxorubicin and aclarubicin were chosen for analysis because they have been shown to interact with the proteasome through a mechanism distinct from that of tripeptidyl aldehydes. Our studies demonstrated that doxorubicin and aclarubicin also significantly augmented rAAV transduction in airway cell lines, polarized human airway epithelia, and mouse lungs. Both tripeptidyl aldehyde and anthracycline proteasome-modulating agents similarly augmented nuclear accumulation of rAAV in A549 and IB3 airway cell lines. However, these two cell types demonstrated cell specificity in the ability of N-acetyl-l-leucyl-l-leucyl-l-norleucine (LLnL) or doxorubicin to augment rAAV transduction. Interestingly, the combined administration of LLnL and doxorubicin resulted in substantially increased transduction (>2,000-fold) following apical infection of human polarized epithelia with either rAAV-2 or rAAV-5. In summary, the cell type specificity of LLnL and doxorubicin to induce rAAV transduction, together with the ability of these compounds to synergistically enhance rAAV transduction in polarized airway epithelial induction, suggests that these two classes of compounds likely modulate different proteasome functions that affect rAAV transduction. Findings from this study provide new insights into how modulation of proteasome function can be effectively used to augment rAAV transduction in airway epithelia for gene therapy of cystic fibrosis.
Adeno-associated virus (AAV) is a nonpathogenic parvovirus with a 4.7-kb single-stranded DNA genome. This virus has been effectively developed into a highly promising vector system for gene therapy of genetic diseases (5). Several serotypes of the AAV family have been identified and cloned (7). While these various serotypes have very similar genomic organizations, their infectious tropisms vary greatly as a result of evolutionary diversity among capsid proteins. The identification of optimal AAV serotypes for a given tissue target has been an area of intense interest to gene therapy. Inefficiencies in recombinant AAV (rAAV) transduction can be attributed to multiple steps involving virus-host cell interactions, including receptor abundance, intracellular barriers that limit nuclear accumulation of the virus, and rate-limiting conversion of single-stranded viral genomes to expressible double-stranded forms (12). Vectoring approaches have been used to solve barriers related to receptor abundance (9, 18) and genomic conversion (27). However, a less-studied approach to improve rAAV transduction involves enhancement of intracellular processing of the virus.
Cystic fibrosis (CF), a recessive genetic disease that affects 1 in every 3,000 Caucasian births, results from a gene defect in the CF transmembrane regulator (CFTR). CF has been a model disease for the clinical testing of rAAV-mediated gene therapy (1, 34-36). The lung is the primary target for gene therapy of CF, since pathology in this organ is most affected by the CFTR defect. Phase I and II trials for CF lung disease with rAAV type 2 (rAAV-2) have demonstrated a promising safety profile with persistence of viral DNA genomes in airway epithelia. However, transduction in these trials was sufficiently low enough to preclude the detection of transgene-derived CFTR mRNA (1). These findings are reminiscent of in vitro studies of polarized human airway epithelia in which postentry barriers appeared to be primarily responsible for low efficiencies of rAAV-2 transduction from the apical membrane (3, 13, 14). Such studies have demonstrated that transgene expression following apical infection with rAAV-2 is much less efficient than basolateral infection, despite the fact that internalization of viral DNA from these two membranes does not differ significantly (14). These studies have implied that impaired intracellular processing of rAAV-2 is responsible for the observed reduced transduction from the apical membrane of human airway epithelia.
Recently, it has been reported that rAAV-5 vectors more effectively transduce murine lungs. Intranasal delivery of pseudotyped AAV-2 genomes with AAV-5 capsids (rAAV-2/5) results in a 250-fold-higher level of transgene expression than rAAV-2 capsid-mediated infection of mouse lungs (2, 40). Although it is generally accepted that in vivo delivery of rAAV-5 infects mouse lungs more efficiently than rAAV-2, data from in vitro comparisons between rAAV-2 and rAAV-5 in human cell lines and primary polarized human airway epithelia remains inconclusive (10, 40). Previously, it was reported that tripeptidyl aldehyde proteasome inhibitors, such as LLnL (N-acetyl-l-leucyl-l-leucyl-l-norleucine, also called MG101) or Z-LLL (carbobenzoxy-l-leucyl-l-leucyl-leucinal, also called MG132), could enhance rAAV-2 transduction in vitro in human polarized epithelia (10, 14) and in vivo in mouse lungs (14). Although the mechanism by which proteasome inhibitors enhance rAAV transduction remains unclear, several general observations suggest that proteasome inhibitors act to enhance rAAV capsid processing. Evaluation of viral DNA suggests that proteasome inhibitors do not act by simply preventing enzymatic degradation of internalized rAAV virions (14, 39). Furthermore, treatment of cells with proteasome inhibitors increases ubiquitination of both rAAV-2 and rAAV-2/5 capsid proteins (39). Lastly, in vitro reconstitution assays have demonstrated that AAV-2 and AAV-5 capsid proteins are substrates for conjugation with ubiquitin (39). Cumulatively, these data suggest that the ubiquitin/proteasome pathway is involved in AAV infection. In support of these findings, the ubiquitin/proteasome pathway has been shown to be critical in the life cycle of the parvovirus minute virus of mice (MVMp) (30).
With the goal of transitioning the application of proteasome inhibitors to clinical trials with rAAV, we sought to evaluate two anthracycline compounds currently used for cancer chemotherapy: doxorubicin (adriamycin) and aclarubicin (aclacinomycin A). In the present study, we evaluated the ability of doxorubicin and aclarubicin to augment rAAV-2 and rAAV-5 transduction in airway cell models. Aclarubicin is a non-peptide inhibitor with discrete selectivity for the chymotrypsin-like proteolytic activity of the 20S proteasome. Doxorubicin is reported to inhibit proteasome activity in the same manner (16). Results from the present study demonstrated that both tripeptidyl aldehyde and anthracycline proteasome inhibitors similarly enhanced rAAV transduction in immortalized human airway cell lines. However, doxorubicin was the most effective at enhancing rAAV-2 and rAAV-5 transduction in polarized human airway epithelia and mouse lungs. Interestingly, rAAV-2 and rAAV-5 demonstrated species-specific differences in transduction efficiency in the presence of proteasome inhibitors. rAAV-2/5 was more effective than rAAV-2 in mouse lungs, and rAAV-2 was more effective than rAAV-2/5 in human polarized airway epithelia. Furthermore, our findings demonstrated a dramatic synergistic inductive effect on rAAV transduction (2,000-fold) to human polarized airway epithelia when doxorubicin and LLnL were coadministered at the time of AAV infection. These studies provide further insights into the use of proteasome-modulating agents as vehicles for the enhancement of rAAV transduction in the airway.
MATERIALS AND METHODS
Cell culture.
IB3 and A549 immortalized human airway cell lines were used for in vitro studies of rAAV transduction. IB3 cells were derived from a CF patient, and A549 cells were derived from a human lung carcinoma. IB3 and A549 cells were cultured as monolayers in Dulbecco's modified Eagle medium (DMEM), supplemented with 10% fetal bovine serum and penicillin-streptomycin, and maintained in a 37°C incubator at 5% CO2. Polarized human airway epithelia were generated as previously described from lung transplant airway tissue (15). Epithelia were grown on 12-mm Milicell membrane inserts (Millipore) and differentiated at an air-liquid interface.
Recombinant AAV vectors and viral infections.
Viral stocks of rAAV were generated, as previously described, with an adenovirus-free system and ion-exchange high-performance liquid chromatography purification (21). AV2.Luc and AV2/5.Luc recombinant viruses utilized the same AAV-2 inverted terminal repeat proviral genome, containing a luciferase reporter driven by the cytomegalovirus (CMV) immediate-early promoter. Full-length rAAV vectors (AV2.eGFP and AV2/5.eGFP) and half-sized self-complementary rAAV (scAV2.eGFP) (10, 38) were also used to evaluate AAV transduction in polarized airway epithelia. These vectors also expressed the enhanced green fluorescent protein (eGFP) gene under the direction of the CMV promoter.
IB3 and A549 cells were grown in 12-well plates to 70% confluence prior to infection, with rAAV at a multiplicity of infection (MOI) equal to 500 particles/cell. Viral infection was performed in 0.5 ml of serum-free DMEM in the absence or presence of proteasome-modulating agents. At 2 h postinfection, 0.5 ml of DMEM-20% fetal bovine serum was added to bring the final serum level to 10%. The chemicals and virus were left in the medium for an additional 22 h. At 24 h postinfection, luciferase assays were performed to quantify gene expression, as previously described (39). For dose-response curves performed with various concentrations of doxorubicin and/or LLnL, a matrix of two drug concentrations was achieved in 96-well plates. Infections were performed as described for 12-well plates, but they utilized 100 μl of culture medium. The tripeptidyl aldehyde inhibitor LLnL, which was purchased from Boston Biochem (Boston, Mass.), was dissolved in dimethyl sulfoxide as a 40 mM stock solution. Z-LLL, doxorubicin, and aclarubicin were purchased from Calbiochem (La Jolla, Calif.). Z-LLL was dissolved in ethanol as a 400 mM stock, and doxorubicin and aclarubicin were dissolved in phosphate-buffered saline (PBS) as 500 μM stock solutions.
rAAV infections of fully differentiated polarized human airway epithelia were performed, as described previously (14), by applying 109 particles of rAAV in 50 μl of medium directly onto the apical surface of airway epithelia. When the infection was initiated, proteasome inhibitors were added directly to the viral inoculum at the desired concentration. Viral infections were performed for a 24-h period, after which the media and virus were removed from the apical compartment and cultures were returned to an air-liquid interface. At the time the virus was removed from the apical compartment, the lower chamber was replaced with fresh media (lacking proteasome inhibitors).
Four-week-old C57BL/6 mice were infected with AV2.Luc or AV2/5.Luc by nasal aspiration. rAAV vectors were diluted with PBS and supplemented with or without proteasome inhibitors. Forty microliters of virus inoculum containing 2 × 1010 particles of rAAV was intranasally instilled into each mouse. Final proteasome inhibitor concentrations in the viral inoculum were 400 μM for Z-LLL and 200 μM for doxorubicin. It was necessary to use Z-LLL for in vivo studies due to the limited solubility of LLnL (14). Additionally, our in vitro studies with polarized airway epithelia indicate that the effective concentration of Z-LLL is 10-fold lower than that for LLnL (see Fig. 2). Three sequential infections were performed at 24-h intervals. Mice were sacrificed 2 weeks after the final infection, and the lungs and trachea were collected for luciferase expression assays.
FIG. 2.
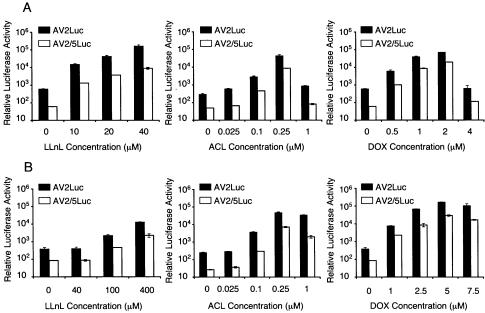
Proteasome-modulating agents augment rAAV transduction from the apical membrane of polarized human airway epithelia. Primary bronchial human airway epithelia were cultured and differentiated at an air-liquid interface on transwell filters 1 cm in diameter. Particles (109) of AV2.Luc or AV2/5.Luc in a volume of 50 μl were applied to the apical surface of airway epithelia in the presence or absence of LLnL (40 μM), Z-LLL (4 μM), aclarubicin (ACL; 0.25 μM), or doxorubicin (DOX; 5 μM). Luciferase activity was measured at 3 days (A) and 17 days (B) postinfection with a luminometer setting of 80% sensitivity. Data represent the means (± standard errors of the means) of the relative luciferase activity (per well) results from three independent experiments.
Human bronchial xenografts were generated from primary human bronchial airway cells and transplanted into denuded rat tracheas, as previously described (15). The fully differentiated xenografts (4 weeks posttransplantation) were infected with 1011 particles (in a volume of 100 μl) of AV2.Luc in the absence or presence of proteasome inhibitors (Z-LLL and doxorubicin). At 16 h after infection, the xenografts were irrigated with 1 ml of Ham's F-12 medium, followed by air. Proteasome inhibitors were administered by two methods. (i) Z-LLL and doxorubicin were applied to the lumen of xenograft airways at the time of infection. In this context, AV2.Luc was mixed together with Z-LLL and doxorubicin at various concentrations (100 μM Z-LLL and 50 or 200 μM doxorubicin). (ii) A lipid-encapsulated formulation of doxorubicin (called Doxil) was also tested. Doxil was administered on the day after infection by tail vein injection at a dose of 200 μl of a 2-mg/ml stock solution per mouse. The dosage was repeated on the second day after infection to give a cumulative dose of 800 μg/mouse. Negative-control mice were injected twice with 200 μl of saline. Two weeks after infection, the grafts were harvested and luciferase assays were performed.
Subcellular fractionation of rAAV DNA.
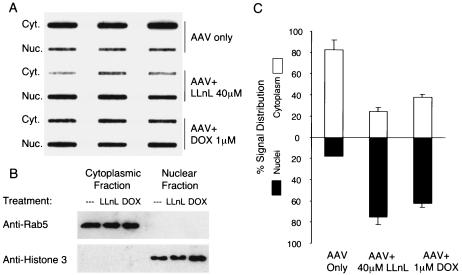
IB3 cells (5 × 105) were seeded in six-well plates a day prior to infection. Infections were performed as described above; however, an increased MOI of 10,000 particles/cell was used. Viral infections with AV2.eGFP were performed in the presence or absence of LLnL (40 μM) or doxorubicin (1 μM) for 16 h. Cells were then washed twice with medium and refed with fresh medium (with the continued presence of proteasome inhibitors when appropriate for the experimental sample). Cultures were then incubated for an additional 8 h. At 24 h postinfection, cells were trypsinized, washed twice with 10 ml of PBS, and then transferred to a microcentrifuge tube in 1 ml of PBS. Cytoplasmic and nuclear fractions were then isolated from the cell pellet by using the method described by Xiao et al. (37). The purities of each fraction were tested by immunoblotting for the presence of Rab5 (an early endosomal marker) with an anti-Rab5 polyclonal antibody (Santa Cruz) and for the presence of the nuclear antigen histone 3 with an anti-histone 3 polyclonal antibody (Molecular Probes). Viral DNA in each sample was extracted by the Hirt method following proteinase K digestion. Viral DNA was visualized by slot blotting with a 32P-labeled eGFP probe. DNA hybridization was quantified by phosphorimager scanning and Bio-Rad software analysis.
Image acquisition and analysis of GFP expression.
To evaluate whether second-strand synthesis of AAV genomes was rate limiting in the presence of doxorubicin, full-length AV2.eGFP and self-complementary scAV2.eGFP were used to infect polarized human airway epithelia from the apical surface. Doxorubicin was applied to the basolateral surface at the time of infection or 13 days postinfection. GFP expression was longitudinally examined in each epithelium up to 30 days postinfection by quantitative morphometry, as described previously (10). The image acquisition parameters were identical for all time-course comparisons, and a blank culture filter well was used as the background for normalization. Ten images were randomly captured in the plane of focus for each experimental sample at different time points. The acquired images were analyzed by National Institutes of Health Image J, version 1.27. The value of total eGFP expression was determined by multiplying the mean area of fluorescence by its mean intensity.
RESULTS
Anthracycline proteasome-modulating agents enhance rAAV transduction in immortalized human airway cell lines and polarized airway epithelia.
The ubiquitin/proteasome system has increasingly been recognized as an integral component of parvovirus transduction (14, 30). Previously, it was reported that the tripeptidyl aldehyde proteasome inhibitors LLnL and Z-LLL can augment both rAAV-2 and rAAV-5 transduction in a variety of human cell lines with various efficiencies, ranging from 10-fold to several hundredfold (39). This study, which also demonstrated enhanced ubiquitination of denatured, purified rAAV virions, proposed that ubiquitination may be a signal for uncoating and processing of rAAV. In support of these findings, proteasome inhibitors also inhibit replication of the MVMp, which suggests that proteolytic processing of MVMp by the ubiquitin/proteasome pathway may be critical for capsid disassembly or nuclear translocation (30).
The use of tripeptidyl aldehyde proteasome inhibitors to enhance rAAV2 transduction from the apical membrane of polarized airway epithelial cells may have useful applications in the gene therapy of CF (14). The proteasome is a complex composed of multiple subunits with unique proteolytic enzyme components. Therefore, the proteasome can be functionally modified by a number of chemical inhibitors that affect its chymotrypsin-, caspase-, and trypsin-like activities. Furthermore, competitive inhibition of substrate binding can be achieved with peptidyl substrate analogs, such as LLnL or Z-LLL. Recently, two clinically used anticancer agents, doxorubicin and aclarubicin, have been recognized as proteasome inhibitors. They belong to the anthracycline class, which distinguishes them from LLnL and Z-LLL. We hypothesized that the assessment of various classes of proteasome-modulating agents might be useful in optimizing approaches to enhance rAAV transduction. Furthermore, the identification of clinically approved proteasome inhibitors capable of augmenting rAAV transduction would allow for more rapid application in gene therapy clinical trials.
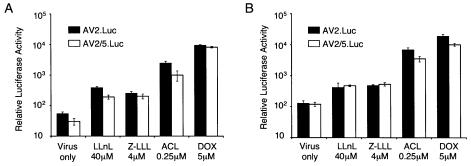
To this end, we compared the effects of tripeptidyl aldehyde and anthracycline-derivative proteasome inhibitors on both rAAV-2 and rAAV-5 transduction in two human airway cell lines (Fig. 1 and Table 1). As expected, the tripeptidyl aldehyde inhibitor LLnL augmented rAAV-2 and rAAV-5 transduction of a luciferase reporter gene in both IB3 and A549 cells. Interestingly, the two types of proteasome-modulating agents demonstrated cell type specificity in the degree to which they augmented rAAV transduction. At the concentration of 40 μM LLnL, no enhancement of rAAV transduction was seen in A549 cells (Fig. 1). This same dose, however, dramatically enhanced transduction of IB3 cells (270-fold for rAAV-2 and 150-fold for rAAV-5). In contrast, <30-fold induction of either rAAV-2 or rAAV-5 transduction was observed in A549 cells when 10-fold-higher concentrations of LLnL (400 μM) were applied at the time of infection. These findings, which highlight the varying dose responses of LLnL in two airway cell lines, suggest that cell type-specific dependencies exist in the ability of LLnL to induce rAAV transduction.
FIG. 1.
Proteasome-modulating agents augment rAAV transduction in airway cell lines. The effects of proteasome-modulating agents LLnL, aclarubicin (ACL), and doxorubicin (DOX) on AV2.Luc and AV2/5.Luc transduction of immortalized human airway cell lines IB3 (A) and A549 (B) were evaluated. Proteasome-modulating agents were coadministered with each rAAV vector (MOI of 500 particles/cell) at the time of infection, and transduction was evaluated 24 h later. Various concentrations of each chemical were evaluated, as indicated on each graph. Luciferase activity was measured with a luminometer at 47.7% sensitivity. Data represent the means (± standard errors of the means) of the relative luciferase activity (per well) results from four independent experiments.
TABLE 1.
Induction of luciferase expression with proteasome-modulating agentsa
| Cell line | Mean ± SEM induction (fold) of luciferase activity in the presence of:
|
|||||
|---|---|---|---|---|---|---|
| Aclarubicin
|
Doxorubicin
|
LLnL
|
||||
| rAAV-2 | rAAV-5 | rAAV-2 | rAAV-5 | rAAV-2 | rAAV-5 | |
| IB3 | 150.0 ± 13.9 | 324.1 ± 3.0 | 118.2 ± 0.6 | 324.13 ± 3.6 | 273 ± 16.2 | 149 ± 12.6 |
| A549 | 187 ± 10.7 | 265.4 ± 8.8 | 426.4 ± 3.3 | 339.4 ± 10.4 | 29.5 ± 6.1 | 23.12 ± 1.2 |
| Polarized airway epithelia | 54.1 ± 4.5 | 29.5 ± 3.2 | 147.9 ± 29.7 | 84.5 ± 12.7 | 3.3 ± 0.8 | 3.8 ± 0.5 |
Data are derived from four (IB3 and A549) and three (polarized cell) independent experiments. Luciferase assays were performed at 24 h postinfection with IB3 and A549 cells and at 17 days postinfection with human polarized airway cells. Induction was calculated with the following formula for each experimental point: relative luciferase activity with proteasome-modulating agent/relative luciferase activity without proteasome-modulating agent. The optimal doses of each compound are as follows: aclarubicin, 0.25 μM for all cell lines; doxorubicin, 2 μM for IB3 cells and 5 μM for A549 cells and polarized airway epithelia; LLnL, 40 μM for IB3 cells and polarized airway epithelia and 400 μM for A549 cells.
Anthracycline anticancer agents, aclarubicin and doxorubicin, have been documented as nonpeptide proteasome inhibitors that hinder the degradation of ubiquitinated proteins (16). The 20S proteasome contains at least three distinct proteolytic activities, including chymotrypsin-like, trypsin-like, and caspase-like (cleaving after acid residues) activities (23). In contrast to tripeptidyl aldehydes, which inhibit both chymotrypsin- and caspase-like activities of the proteasome (26), anthracycline derivatives, such as doxorubicin and aclarubicin, specifically inhibit only chymotrypsin-like activity of the 20S proteasome (11, 24). When we tested the effects of aclarubicin and doxorubicin on rAAV transduction, we found that both dramatically augmented rAAV-2 and rAAV-5 transduction in IB3 and A549 cells (Fig. 1). In contrast to LLnL, aclarubicin and doxorubicin showed much higher induction in A549 cells. They also enhanced transgene expression from rAAV5 with slightly higher efficiencies than LLnL. Of note, doxorubicin and aclarubicin induction of rAAV transduction was not specific to airway cell lines. Application of these drugs at the time of infection also significantly enhanced transduction in HeLa cells, 293 cells, and primary fibroblasts from humans and ferrets (data not shown).
The aldehyde proteasome inhibitors LLnL and Z-LLL have been shown to induce both rAAV-2 and rAAV-5 transduction from the apical membrane of human polarized airway epithelia approximately 10-fold (10, 14). Interestingly, this enhancement appears to be specific to apical entry pathways, with no significant long-term enhancement of rAAV transduction from the basolateral membrane (10). Together with our present findings, these results suggest that cell phenotypes, such as polarity and cell type, may influence the effectiveness of certain proteasome inhibitors to induce rAAV transduction. Hence, we next sought to compare the effectiveness of tripeptidyl aldehyde and anthracycline proteasome inhibitors to induce rAAV2 or rAAV-5 transduction from the apical membrane of polarized airway epithelia by using luciferase reporter vectors (Fig. 2). Consistent with previous observations, LLnL and Z-LLL administration at the time of viral infection marginally augmented rAAV transduction (three- to sevenfold) by 17 days postinfection. In contrast, anthracycline proteasome inhibitors enhanced both rAAV-2 and rAAV-5 transduction with much higher efficiencies. Aclarubicin or doxorubicin administrations were 8- to 45-fold more effective at inducing rAAV-2 or rAAV-5 transduction from the apical surface than LLnL (Fig. 2 and Table 1). A single application of doxorubicin at the time of infection augmented rAAV-2 transduction 147-fold and rAAV-5 transduction 84-fold by 17 days postinfection. Similarly, aclarubicin induced rAAV-2 transduction 54-fold and rAAV-5 transduction 30-fold.
Combined administration of doxorubicin and LLnL or Z-LLL synergistically augments rAAV transduction from the apical membrane of polarized human airway epithelia.
Studies evaluating how LLnL and doxorubicin alter proteasome activity have suggested that their mechanisms may be quite different. Although both compounds inhibit the chymotrypsin-like activity of the 20S proteasome, their sites of interaction with the proteasome are unique. The tripeptidyl aldehyde analog, LLnL or Z-LLL, blocks the catalytic center of proteases within the proteasome (28, 33), whereas anthracycline compounds are noncompetitive inhibitors that bind at an as-yet-unidentified site on the 20S proteasome (16). The high-affinity binding of doxorubicin to the 20S proteasome is thought to result in distortion of the protease catalytic site, obstructing its access to the protein substrate (25). To investigate whether doxorubicin and LLnL act through similar mechanisms to enhance rAAV transduction, we assessed whether these compounds demonstrated additive or synergistic effects when used in combination. We reasoned that if the two compounds acted through similar mechanisms, involving only chymotrypsin-like inhibitory activity of the 20S proteasome, then when used in combination, their net effect on rAAV transduction would be no greater than the sum of each individually.
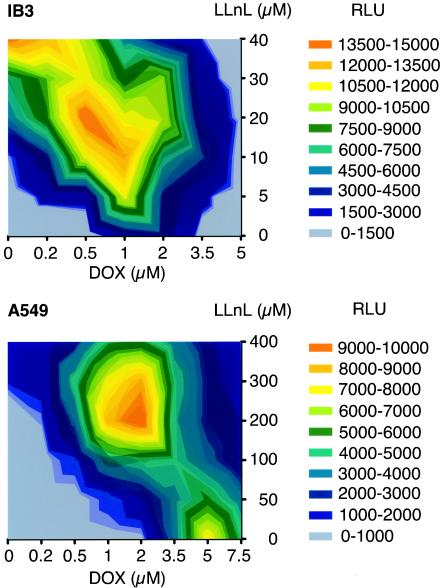
We first investigated the combined effect of LLnL and doxorubicin on rAAV transduction in A549 and IB3 cells. These studies were performed with matrix dose-response assays in 96-well plates and with luciferase transgene expression as an indicator of transduction. As shown in Fig. 3, A549 and IB3 cells demonstrated unique rAAV2 transduction profiles at various doses of LLnL and/or doxorubicin. A549 cells gave two peaks of rAAV2 transduction in the presence of 5 μM doxorubicin alone or 1.5 μM doxorubicin combined with 200 μM LLnL. In contrast, peak levels of rAAV2 transduction in IB3 cells were observed at doses of 40 μM LLnL alone or 20 μM LLnL-0.5 μM doxorubicin. Although the induction with combined proteasome inhibitors was not considered synergistic, these studies demonstrated that lower doses of each proteasome inhibitor could be combined to maximally augment rAAV transduction.
FIG. 3.
Matrix dose-response assays of rAAV-2 transduction with combined application of LLnL and doxorubicin. IB3 (A) and A549 (B) cells, cultured in 96-well plates, were infected with 500 particles/cell of AV2.Luc. Different concentrations of each proteasome inhibitor alone or in combination (as indicated on each axis) were applied at the time of infection. At 24 h postinfection, luciferase activity was measured with a luminometer at 47.7% sensitivity with 1/10 of the total cell lysate. The level of luciferase expression in the diagrams represents the total from each well. The color signifies the range of the mean relative luciferase activity (measured in relative luciferase units [RLU]) per well for four independent experiments.
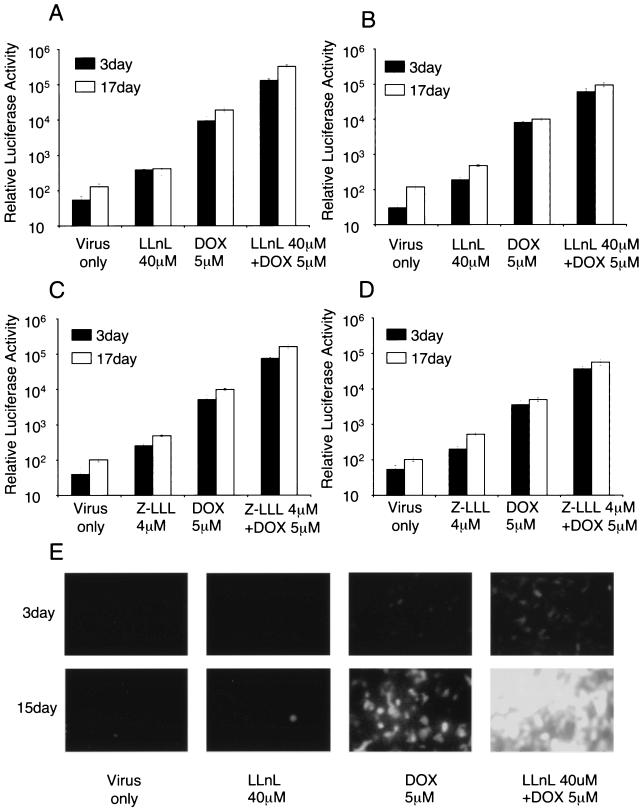
Studies comparing LLnL and/or doxorubicin administration at the time of rAAV infection from the apical surface of polarized airway epithelia demonstrated significant synergistic augmentation of both rAAV-2 and rAAV-5 transduction (Fig. 4A and B). In the case of rAAV-2 infection, combined doxorubicin and LLnL treatments augmented transduction by a dramatic 2,500-fold by 17 days postinfection (Fig. 4A). This was significantly higher than the 148-fold induction produced by doxorubicin alone and the 3.3-fold induction by LLnL alone. Similar synergistic effects between LLnL and doxorubicin were also observed with rAAV-5 vectors; however, the overall maximal expression in the presence of LLnL-doxorubicin remained below that seen with rAAV-2 (Fig. 4B). We also tested the combined application of Z-LLL and doxorubicin on rAAV-2 and rAAV-5 transduction; the same pattern of synergistic induction was observed (Fig. 4C and D). To investigate the percentage of cells transduced under each of these conditions, we repeated the analysis, comparing the various chemical compounds with GFP reporter rAAV vectors. Consistent with the results obtained from the luciferase vectors, the combination of LLnL and doxorubicin treatments maximally enhanced rAAV-2 transduction in >95% of epithelial cells, far greater than with either compound alone (Fig. 4E). Similar inductive effects were seen with rAAV-5, but the overall percentage of cells was slightly lower than with rAAV-2 (data not shown).
FIG. 4.
Combined administration of proteasome-modulating agents can synergistically induce rAAV transduction from the apical surface of polarized human airway epithelia. Particles (109) of AV2.Luc (A and C) or AV2/5.Luc (B and D) were applied to the apical surface of polarized human airway epithelial cultures in the absence or presence of various combinations of LLnL (40 μM), Z-LLL (4 μM), and/or doxorubicin (DOX; 5 μM). Luciferase expression was assayed at 3 and at 17 days postinfection. Data represent the means (± standard errors of the means) of the relative luciferase activity (per well) results from three independent experiments. (E) Similar results were observed following apical infection with AV2.GFP under the above conditions. Representative fluorescent photomicrographs of GFP expression at 3 and 15 days postinfection are shown for the labeled conditions.
Doxorubicin and LLnL both enhance nuclear accumulation of rAAV in airway cells.
Studies evaluating rAAV trafficking with the fluorescent and radiolabeled virus have demonstrated that once endocytosed, the virus moves relatively slowly to the nucleus and accumulates in a perinuclear compartment (4, 14, 19, 20, 37). In polarized human airway epithelia, in situ localization of the 35S-labeled rAAV-2 particles after infection from the apical surface demonstrated enhanced nuclear accumulation in the presence of LLnL (14). In the present study, we sought to determine whether doxorubicin similarly enhanced nuclear accumulation of rAAV-2, as seen with LLnL. To more quantitatively assess transport of rAAV into the nucleus, we utilized subcellular fractionation techniques, as previously described in studies with HeLa cells (37). Due to a limited quantity of samples with polarized airway epithelia, IB3 cells were used as the target cell line. Furthermore, this cell line demonstrated the greatest responsiveness to both LLnL and doxorubicin, by which functional augmentation in transport could be assessed. Following nuclear and cytoplasmic fractionation, DNA blot hybridization and phosphorimager quantification were used to assess doxorubicin- or LLnL-induced changes in the distribution of viral DNA. The purities of the cytoplasmic and nuclear fractions were tested by immunoblotting against Rab5 (an early endosomal marker) and histone 3 (a nuclear antigen marker). Results from this analysis confirmed that Rab5 was limited to the cytoplasmic fraction, whereas histone 3 was limited to the nuclear fraction. Quantification of viral DNA in these two fractions demonstrated that both 40 μM LLnL and 1 μM doxorubicin significantly enhanced nuclear accumulation of viral DNA by 24 h postinfection (Fig. 5). This result was similar to the observed enhancement of rAAV movement to the nucleus in adenovirus-infected HeLa cells (37) and LLnL-treated polarized airway epithelia (14). The ability of either doxorubicin or LLnL to facilitate rAAV nuclear translocation supports the notion that the ubiquitin/proteasome system plays a key role in controlling rAAV intracellular processing.
FIG. 5.
LLnL and doxorubicin both facilitate translocation of rAAV to the nucleus. IB3 cells were infected with AV2eGFP (MOI of 1,000 particles/cell) in the presence or absence of 40 μM LLnL or 1 μM doxorubicin (DOX). At 24 h postinfection, cytoplasmic (Cyt.) and nuclear (Nuc.) fractions were isolated (n = 3 infections for each experimental point). (A) Viral DNA in each fraction was detected by slot blot hybridization against a 32P-labeled eGFP probe and visualized with a Bio-Rad phosphorimager. (B) Purities of the cytoplasmic and nuclear fractions were confirmed by immunoblotting against the cytoplasmic marker Rab5 and nuclear antigen histone 3. (C) The percentage distributions of the viral genome signals in the nuclear and cytoplasmic fractions were calculated based on the mean (± standard error of the mean) signals for three experimental points. The 32P signal was quantified with Bio-Rad software.
Doxorubicin induces rAAV transduction without directly enhancing the efficiency of second-strand synthesis.
Previously, it was reported that the tripeptidyl aldehyde proteasome inhibitor LLnL augments transduction of human airway epithelia by both self-complementary and full-length rAAV vectors (10). These studies attempted to address whether second-strand synthesis of viral genomes was rate-limiting in airway epithelia and/or was affected by tripeptide proteasome inhibitors. Conclusions from this study suggested that intracellular trafficking, not second-strand genome conversion, was the major rate-limiting step hindering rAAV-2 and rAAV-5 transduction of polarized airway epithelia from the apical surface. The observation that self-complementary and full-length AAV vectors demonstrated similar transduction profiles in polarized airway epithelia differed from previous observations of HeLa cells and other cell lines (10, 27).
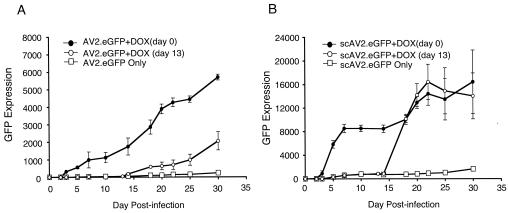
In the present study, we sought to utilize a similar approach to assess whether second-strand synthesis becomes rate-limiting at the level of transduction achieved with applied doxorubicin. Bearing in mind that doxorubicin was also known to be a DNA topoisomerase inhibitor, we hypothesized that treatment with this agent could also enhance rAAV transduction by altering second-strand synthesis of viral genomes. In contrast to the full-length AV2.eGFP vector, the self-complementary rAAV vector scAV2.eGFP does not require second-strand synthesis of its genome to express GFP (10). Since intracellular trafficking should be identical for both full-length and self-complementary AAV vectors, the extent to which doxorubicin differentially induces transduction by full-length or self-complementary AAV vectors could be used to directly infer any potential effects doxorubicin might have on gene conversion.
Apical transduction with either full-length AV2.eGFP or self-complementary scAV2.eGFP was monitored over a 30-day period by image acquisition of GFP fluorescence. Two experimental protocols were used to evaluate the effect of doxorubicin on apical transduction of airway epithelia: (i) doxorubicin was applied to epithelia for 16 h at the time of infection or (ii) airway epithelia were infected in the absence of doxorubicin and then doxorubicin was transiently applied to epithelia at 13 days postinfection for a 24 h period. Several interesting findings resulted from these experiments (Fig. 6). First, in the presence of doxorubicin, the onset of GFP expression was significantly faster for scAV2.eGFP than for the full-length AV2.eGFP vector. Second, the overall level of GFP expression was approximately 2.5-fold greater by 30 days for scAV2.eGFP than for AV2.eGFP. These findings support the notion that doxorubicin likely enhances the movement of viral genomes to the nucleus of airway epithelia to a point where gene conversion becomes rate-limiting for AV2.eGFP full-length vectors. Since the level to which doxorubicin induced scAV2.eGFP or AV2.eGFP transduction at any of the time points evaluated did not significantly differ, we concluded that doxorubicin has a minimal influence on the rate of AAV second-strand synthesis.
FIG. 6.
Doxorubicin induces rAAV transduction without directly enhancing the efficiency of second-strand synthesis. Polarized human airway epithelia grown at the air-liquid interface were infected with 5 × 109 particles of full-length AV2.eGFP (A) or self-complementary scAV2.eGFP (B) from the apical surface at day 0. GFP expression was quantified at the time points indicated on the graphs by fluorescent microscopy and the following calculation: the mean area of GFP fluorescence multiplied by the mean intensity of fluorescence. Ten images were acquired randomly from each experimental point. The following experimental protocols were performed: (i) rAAV infection without doxorubicin (DOX), (ii) rAAV infection in the presence of 5 μM doxorubicin, and (iii) rAAV infection without doxorubicin and subsequent application of 5 μM doxorubicin for 24 h at 13 days postinfection. Results depict the means ± standard errors of the means for three independent epithelia for each experimental point.
To further address whether doxorubicin enhances rAAV transduction in airway epithelia at a pre- or post-gene conversion stage, we sought to determine whether doxorubicin applied at 13 days postinfection could efficiently rescue gene expression from scAV2.eGFP or AV2.eGFP vectors. We hypothesized that if the virus progressively moved to an intracellular compartment following apical infection and was trapped in an inactive state prior to gene conversion, then application of doxorubicin could more rapidly rescue gene expression from self-complementary vectors, since second-strand synthesis would not be rate-limiting. As shown in Fig. 6, application of doxorubicin at 13 days postinfection with scAV2.eGFP restored gene expression within 3 days to a level equivalent to that seen in epithelia receiving scAV2.eGFP and doxorubicin at the same time. This result is consistent with viral accumulation at a proteasome-dependent block that is lifted by the addition of doxorubicin. In contrast, addition of doxorubicin at 13 days postinfection with full-length AV2.eGFP led to a significantly slower rate of GFP gene expression rescue. These data are consistent with rate-limiting, second-strand synthesis for full-length rAAV vectors that persist after application with doxorubicin. The extent to which second-strand synthesis becomes rate-limiting is dependent on the number of viral genomes in the nucleus at any one time. The application of doxorubicin at 13 days postinfection has synchronized accumulation of viral genomes in the nucleus and hence amplifies rate-limiting aspects of second-strand synthesis for full-length, but not self-complementary, vectors.
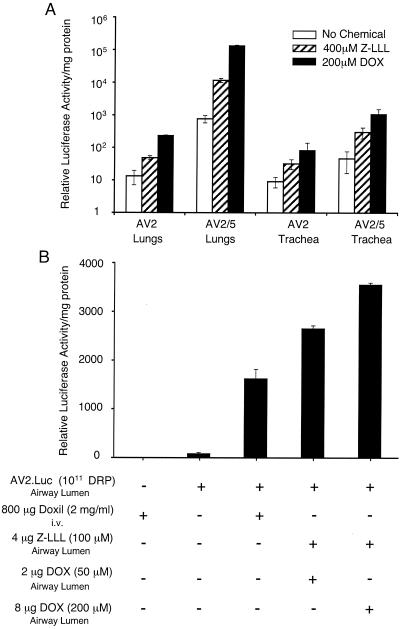
Doxorubicin and Z-LLL augmented both rAAV-2 and rAAV-5 transduction in mouse lungs and human bronchial xenografts.
In vitro studies have demonstrated that aldehyde or anthracycline proteasome inhibitors can enhance both rAAV-2 and rAAV-5 transduction. To further evaluate the use of doxorubicin as a vehicle to enhance rAAV transduction in the lung, we performed additional studies with mice. Previous data have shown that Z-LLL can efficiently enhance rAAV-2-mediated transduction in mouse lungs (14). However, similar studies have not been performed for rAAV-5. Z-LLL was chosen for use in vivo since it is more soluble than LLnL. Results from in vivo comparisons of doxorubicin and Z-LLL are shown in Fig. 7A. As previously demonstrated, AV2/5.Luc was approximately 60-fold more effective at transducing mouse lungs than AV2.Luc vector in the absence of applied proteasome inhibitors. However, analysis of the trachea demonstrated less difference in transduction between these two serotypes. These findings suggest that perhaps certain cell types found more distally in the mouse lung are considerably more susceptible to rAAV-2/5 transduction. Interestingly, observations of increased transduction with rAAV-2/5 in mouse lungs contrasted with findings in polarized human airway epithelia, in which levels of gene transfer were nearly equivalent between these two vector serotypes. Coapplication of Z-LLL or doxorubicin with either AV2.Luc or AV2/5.Luc led to enhancement of transduction in both the lung and trachea. Doxorubicin more efficiently induced rAAV transduction for both serotypes than did Z-LLL. These findings also reflect the in vitro studies comparing doxorubicin and LLnL in polarized human airway epithelia. Interestingly, rAAV-2/5 transduction of mouse lungs was more effectively induced (174-fold) by doxorubicin than that of rAAV-2 (17-fold). A similar trend was also observed with Z-LLL in mouse lungs. This finding appears to be the opposite of that seen in polarized human airway epithelia with these two serotypes: rAAV-2 was slightly more effective than rAAV-2/5 on human airway epithelia in the presence of proteasome inhibitors by 17 days postinfection. Cumulatively, these findings demonstrate for the first time that rAAV-2/5 transduction in mouse lungs can be augmented by the addition of proteasome inhibitors at the time of infection. Furthermore, these studies demonstrate that doxorubicin is more effective at augmenting gene transfer to mouse lungs with rAAV than previously tested aldehyde proteasome inhibitors.
FIG. 7.
Proteasome inhibitors augment rAAV transduction to mouse lungs and human bronchial xenografts in vivo. (A) Aerosol delivery of rAAV with Z-LLL or doxorubicin to the mouse airway. C57BL/6 mice were infected with a total of 6 × 1010 particles of AV2.Luc or AV2/5.Luc through nasal aspiration in the absence or presence of either 400 μM Z-LLL or 200 μM doxorubicin (DOX). Viruses and the proteasome inhibitor were mixed together (40 μl of inoculum/mouse) and delivered through the nose three times on sequential days. Two weeks after the final infection, the mouse lungs and trachea were harvested and homogenized in a Promega luciferase reporter lysis buffer. Samples were normalized for protein concentration, and luciferase activity was measured in a luminometer at 80% sensitivity. The values represent the mean (± standard error of the mean) relative luciferase activities in both the lungs and trachea (n = 3). (B) Human bronchial xenografts were infected with 1011 particles (in a volume of 100 μl) of AV2.Luc. The administration of proteasome inhibitors was either through a local application mixed with virus and applied to the lumen of grafts at the time of infection (doxorubicin and Z-LLL) or through systemic application to the mouse by tail vein injection 1 and 2 days after rAAV infection of the airway lumen (Doxil). Total microgram doses and working concentrations of drugs are summarized below the chart (+, present; −, absent). The xenograft airways were harvested at 2 weeks postinfection, and luciferase assays were performed on the entire airway. Results depict the means ± standard errors of the means for three independent xenografts for each experimental point.
Although Z-LLL or doxorubicin was capable of augmenting rAAV transduction in the mouse lung, the combined application of these two drugs did not provide greater efficacy than the application of doxorubicin alone (data not shown). We are currently unsure whether the differences in proteasome inhibitor synergism seen between the human polarized airway epithelial and in vivo mouse models are due to species-specific differences or pharmacokinetic differences in drug clearance.
We utilized a human bronchial xenograft model as an alternative in vivo model to evaluate the application of proteasome inhibitors to enhance rAAV transduction. Given the fact that doxorubicin has associated cardiac toxicities, we also sought to assess whether the less cardiotoxic, pegylated, liposomal formulation of doxorubicin (6, 17) (called Doxil) was also effective at inducing rAAV transduction to human xenograft airways following systemic delivery of the drug. This lipid-formulated doxorubicin was systemically delivered by tail vein injection 1 and 2 days after rAAV infection of xenograft airways. The effectiveness of systemic Doxil administration was compared to Z-LLL-doxorubicin administered directly to the lumen of the airway. As shown in Fig. 7B, all routes and doses of proteasome inhibitors in this study effectively induced rAAV-mediated luciferase transgene expression at 14 days postinfection by 25- to 50-fold of that seen in the absence of the inhibitors. The direct application of Z-LLL and doxorubicin to the airway was marginally (twofold) more effective than Doxil delivered systemically. However, it should be emphasized that the effective total dose of proteasome inhibitor was significantly reduced by direct administration to the airway (4 μg of Z-LLL-8 μg of DOX) compared to systemic circulation (Doxil, containing 800 μg of doxorubicin).
DISCUSSION
It was previously reported that the tripeptidyl aldehyde proteasome inhibitors LLnL and Z-LLL can significantly enhance rAAV-2 transduction both in polarized mouse and human airway models. In the present study, we have evaluated a new class of proteasome-modulating agents, the anthracycline derivatives doxorubicin and aclarubicin. Currently, doxorubicin and aclarubicin are both clinically used as anticancer drugs (8, 32), and as such, they are useful compounds to test for potential applications in gene therapy. Our studies with airway cell lines and polarized human airway epithelia have demonstrated that doxorubicin and aclarubicin effectively augment both rAAV-2 and rAAV-5 transduction. Interestingly, studies with A549 and IB3 cells demonstrated cell line specificity with regard to whether tripeptidyl or anthracycline compounds maximally induced rAAV transduction. Furthermore, the effective dose at which each of these compounds maximally augmented rAAV transduction varied between the two cell lines. This cell specificity suggested that tripeptidyl or anthracycline compounds may alter proteasome function and rAAV transduction through slightly different mechanisms.
Cell-specific responses to various classes of proteasome inhibitors suggested the possibility that combined administration of these two types of proteasome-modulating agents might be capable of synergistically inducing rAAV transduction. In support of this hypothesis, analysis of rAAV transduction with a matrix of doxorubicin and LLnL dose combinations demonstrated unique transduction profiles in IB3 and A549 cells. Combined administration of the two drugs at lower concentrations demonstrated greater efficacy than those of either drug alone. Although only a moderate increase in the effectiveness of rAAV transduction was observed in cell lines at lower doses when LLnL and doxorubicin were administered together, polarized human airway epithelia demonstrated a dramatic synergistic induction in rAAV-2 and rAAV-5 transduction when both LLnL and doxorubicin were administered at the time of infection. Such augmentation in rAAV transduction, which exceeded 2,000-fold, was stable for the length of the experiment (17 days). These findings suggest that tailoring the dosage of combined proteasome-modulating agents could be useful in increasing the efficacy of rAAV transduction for different target tissues.
Previously, it was reported that the tripeptidyl inhibitor Z-LLL increased rAAV-2 transduction to mouse lungs in vivo. In the present study, we have extended these observations to include rAAV-5. We have also demonstrated that doxorubicin induced rAAV transduction to mouse lungs with both serotypes more effectively than Z-LLL. Interestingly, our findings comparing rAAV-5 and rAAV-2 in human and mouse polarized airway models suggest that species-specific differences exist in the optimal serotype for gene delivery. Although rAAV-5 was notably more effective in mouse lungs in vivo, this difference was far less evident in human polarized epithelia. Furthermore, in the presence of proteasome inhibitors, rAAV-2 outperformed rAAV-5 in human polarized airway epithelia. The implications of this finding are important from a clinical standpoint regarding the optimal serotype for CF gene therapy. Whether such differences are due to model system dependencies and/or species differences in airway cell biology remains to be determined.
Doxorubicin and aclarubicin are compounds that affect multiple cellular processes. In addition to being proteasome inhibitors, doxorubicin and aclarubicin are also DNA topoisomerase inhibitors. In fact, the clinical application of these anthrathycline cytostatics as antitumor agents is mainly based on their activity as DNA topoisomerase inhibitors. Although topoisomerase inhibitors, such as champtothecin and etoposide, augment rAAV-2 transduction in human primary fibroblasts, they do not augment apical rAAV transduction in polarized human airway epithelia (14). Additionally, the mechanism by which topoisomerase inhibition is thought to enhance rAAV transduction is through increased second-strand genome conversion. However, studies comparing the effects of doxorubicin on transduction of polarized airway epithelia with full-length and self-complementary rAAV vectors suggest that doxorubicin most likely has a minimal direct effect, if any, on second-strand synthesis. These results are consistent with previous findings, which demonstrated that LLnL does not appear to influence rAAV gene conversion in polarized human airway epithelia (10). However, in contrast with this previous study (in which the levels of transduction were 50-fold lower), doxorubicin appears to mobilize a sufficient amount of the virus to the nucleus such that second-strand genome conversion becomes rate-limiting for full-length rAAV vectors. This is most evident in studies comparing full-length to self-complementary vectors when doxorubicin was applied at 13 days following apical infection. Additionally, doxorubicin did not significantly influence transcription of transgenes directly. Doxorubicin treatment of IB3 or A549 cells transfected with different rAAV proviral plasmids showed no obvious changes in transgene expression (data not shown). This was true for three independent, promoter-driven transgenes, including the CMV immediate-early promoter, Rous sarcoma virus long terminal repeat, and the AAV inverted terminal repeat. Furthermore, LLnL and doxorubicin also both enhanced nuclear accumulation of rAAV in IB3 cells. Hence, based on the current information available, it appears that both anthracycline derivatives and tripeptidyl proteasome inhibitors act to enhance rAAV transduction by increasing the efficiency of intracellular processing.
Unlike LLnL, which inhibits protease activity in the 20S proteasome by competitively blocking the enzyme catalytic center as an aldehyde peptide analog, doxorubicin and aclarubicin are reversible, noncompetitive inhibitors that act only on the chymotypsin-like protease activity of the 20S proteasome. Although the precise binding sites of doxorubicin are not yet known, the aglycone and sugar moieties of the molecule have been demonstrated to be essential for inhibition (16). Doxorubicin appears to bind to an allosteric site, distorting the catalytic site and obstructing its access to the scissile bond in the target protein. Although it is presently unclear how proteasome-modulating agents, such as LLnL and doxorubicin, alter rAAV interactions with the proteasome, it appears from the present study that modulating the proteasome function with multiple small molecules can synergistically enhance rAAV transduction. A better understanding of how the ubiquitin/proteasome system is involved in rAAV transduction and of how proteasome inhibition alters intracellular trafficking and endosomal processes in polarized airway epithelia will help improve the application of proteasome modulation for gene therapy of airway diseases such as CF.
Doxorubicin is a cytotoxic, anthracycline, chemotherapeutic agent used clinically. Hence, its toxicity in humans has been extensively studied (22, 29). The cardiotoxicity of doxorubicin is the most limiting factor in its application for human use, and the total lifelong dose influences the cumulative cardiac toxicity of this compound (31). Although the lipid formulation of doxorubicin (Doxil) reduces cardiac toxicity by limiting uptake by the heart, this approach may not be well suited for CF lung gene therapy, since the dose of doxorubicin required is 100-fold higher than direct airway delivery. Preclinical toxicology will be required to support a lung delivery trial which uses doxorubicin to enhance rAAV transduction.
Acknowledgments
This work was supported by NIH RO1 HL58340 (J.F.E.) and Targeted Genetics Corporation.
We gratefully acknowledge P. Karp, P. Weber, and J. Zabner of the Gene Therapy Center Cells and Tissue Core (DK54759). We also thank Leah Williams for editorial assistance with the manuscript.
REFERENCES
- 1.Aitken, M. L., R. B. Moss, D. A. Waltz, M. E. Dovey, M. R. Tonelli, S. C. McNamara, R. L. Gibson, B. W. Ramsey, B. J. Carter, and T. C. Reynolds. 2001. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 12:1907-1916. [DOI] [PubMed] [Google Scholar]
- 2.Auricchio, A., E. O'Connor, D. Weiner, G. Gao, M. Hildinger, L. Wang, R. Calcedo, and J. Wilson. 2002. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Investig. 110:499-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bals, R., W. Xiao, N. Sang, D. J. Weiner, R. L. Meegalla, and J. M. Wilson. 1999. Transduction of well-differentiated airway epithelium by recombinant adeno-associated virus is limited by vector entry. J. Virol. 73:6085-6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett, J. S., R. Wilcher, and R. J. Samulski. 2000. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J. Virol. 74:2777-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berns, K. I., and C. Giraud. 1996. Adeno-associated virus (AAV) vectors in gene therapy. Springer, Berlin, Germany.
- 6.Burstein, H. J., M. J. Ramirez, W. P. Petros, K. D. Clarke, M. A. Warmuth, P. K. Marcom, U. A. Matulonis, L. M. Parker, L. N. Harris, and E. P. Winer. 1999. Phase I study of Doxil and vinorelbine in metastatic breast cancer. Ann. Oncol. 10:1113-1116. [DOI] [PubMed] [Google Scholar]
- 7.Carter, B. J., H. B. Burnstein, and R. W. Peluso. AAV vectors for gene therapy, in press. Marcel Dekker, Inc., New York, N.Y.
- 8.Casper, E. S., R. J. Gralla, and C. W. Young. 1981. Clinical phase I study of aclacinomycin A by evaluation of an intermittent intravenous administration schedule. Cancer Res. 41:2417-2420. [PubMed] [Google Scholar]
- 9.Davidson, B. L., C. S. Stein, J. A. Heth, I. Martins, R. M. Kotin, T. A. Derksen, J. Zabner, A. Ghodsi, and J. A. Chiorini. 2000. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA 97:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, W., Z. Yan, R. Zak, M. Saavedra, D. M. Rodman, and J. F. Engelhardt. 2003. Second-strand genome conversion of adeno-associated virus type 2 (AAV-2) and AAV-5 is not rate limiting following apical infection of polarized human airway epithelia. J. Virol. 77:7361-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong, J., M. Naito, T. Tatsuta, H. Seimiya, O. Johdo, and T. Tsuruo. 1995. Difference between the resistance mechanisms of aclacinomycin- and adriamycin-resistant P388 cell lines. Oncol. Res. 7:245-252. [PubMed] [Google Scholar]
- 12.Duan, D., Y. Yue, and J. F. Engelhardt. 2002. Adeno-associated virus. Marcel Dekker, Inc., New York, N.Y.
- 13.Duan, D., Y. Yue, Z. Yan, P. B. McCray, and J. F. Engelhardt. 1998. Polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia. Hum. Gene Ther. 9:2761-2776. [DOI] [PubMed] [Google Scholar]
- 14.Duan, D., Y. Yue, Z. Yan, J. Yang, and J. F. Engelhardt. 2000. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J. Clin. Investig. 105:1573-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan, D., Y. Zhang, and J. F. Engelhardt. 1998. Gene delivery to the airway, p. 13.9.1-13.9.34. In N. C. Dracopoli, J. L. Haines, B. R. Korf, D. T. Moir, C. C. Morton, C. E. Seidman, J. G. Seidman, and D. R. Smith (ed.), Current protocols in human genetics, John Wiley & Sons, Inc., New York, N.Y.
- 16.Figueiredo-Pereira, M. E., W. E. Chen, J. Li, and O. Johdo. 1996. The antitumor drug aclacinomycin A, which inhibits the degradation of ubiquitinated proteins, shows selectivity for the chymotrypsin-like activity of the bovine pituitary 20 S proteasome. J. Biol. Chem. 271:16455-16459. [DOI] [PubMed] [Google Scholar]
- 17.Gabizon, A., H. Shmeeda, and Y. Barenholz. 2003. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin. Pharmacokinet. 42:419-436. [DOI] [PubMed] [Google Scholar]
- 18.Gao, G. P., M. R. Alvira, L. Wang, R. Calcedo, J. Johnston, and J. M. Wilson. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99:11854-11859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen, J., K. Qing, H. J. Kwon, C. Mah, and A. Srivastava. 2000. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. J. Virol. 74:992-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen, J., K. Qing, and A. Srivastava. 2001. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. J. Virol. 75:4080-4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaludov, N., B. Handelman, and J. A. Chiorini. 2002. Scalable purification of adeno-associated virus type 2, 4, or 5 using ion-exchange chromatography. Hum. Gene Ther. 13:1235-1243. [DOI] [PubMed] [Google Scholar]
- 22.Keizer, H. G., H. M. Pinedo, G. J. Schuurhuis, and H. Joenje. 1990. Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol. Ther. 47:219-231. [DOI] [PubMed] [Google Scholar]
- 23.Kisselev, A. F., T. N. Akopian, K. M. Woo, and A. L. Goldberg. 1999. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J. Biol. Chem. 274:3363-3371. [DOI] [PubMed] [Google Scholar]
- 24.Kiyomiya, K., M. Kurebe, H. Nakagawa, and S. Matsuo. 2002. The role of the proteasome in apoptosis induced by anthracycline anticancer agents. Int. J. Oncol. 20:1205-1209. [PubMed] [Google Scholar]
- 25.Kiyomiya, K., S. Matsuo, and M. Kurebe. 1998. Proteasome is a carrier to translocate doxorubicin from cytoplasm into nucleus. Life Sci. 62:1853-1860. [DOI] [PubMed] [Google Scholar]
- 26.Lee, D. H., and A. L. Goldberg. 1998. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 8:397-403. [DOI] [PubMed] [Google Scholar]
- 27.McCarty, D. M., P. E. Monahan, and R. J. Samulski. 2001. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 8:1248-1254. [DOI] [PubMed] [Google Scholar]
- 28.Mellgren, R. L. 1997. Specificities of cell permeant peptidyl inhibitors for the proteinase activities of mu-calpain and the 20 S proteasome. J. Biol. Chem. 272:29899-29903. [DOI] [PubMed] [Google Scholar]
- 29.Muller, I., D. Niethammer, and G. Bruchelt. 1998. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity. Int. J. Mol. Med. 1:491-494. [DOI] [PubMed] [Google Scholar]
- 30.Ros, C., C. J. Burckhardt, and C. Kempf. 2002. Cytoplasmic trafficking of minute virus of mice: low-pH requirement, routing to late endosomes, and proteasome interaction. J. Virol. 76:12634-12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singal, P. K., and N. Iliskovic. 1998. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 339:900-905. [DOI] [PubMed] [Google Scholar]
- 32.Stebbing, J., and A. Gaya. 2002. Pegylated liposomal doxorubicin (Caelyx) in recurrent ovarian cancer. Cancer Treat. Rev. 28:121-125. [DOI] [PubMed] [Google Scholar]
- 33.Vinitsky, A., C. Michaud, J. C. Powers, and M. Orlowski. 1992. Inhibition of the chymotrypsin-like activity of the pituitary multicatalytic proteinase complex. Biochemistry 31:9421-9428. [DOI] [PubMed] [Google Scholar]
- 34.Wagner, J. A., A. H. Messner, M. L. Moran, R. Daifuku, K. Kouyama, J. K. Desch, S. Manley, A. M. Norbash, C. K. Conrad, S. Friborg, T. Reynolds, W. B. Guggino, R. B. Moss, B. J. Carter, J. J. Wine, T. R. Flotte, and P. Gardner. 1999. Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 109:266-274. [DOI] [PubMed] [Google Scholar]
- 35.Wagner, J. A., M. L. Moran, A. H. Messner, R. Daifuku, C. K. Conrad, T. Reynolds, W. B. Guggino, R. B. Moss, B. J. Carter, J. J. Wine, T. R. Flotte, and P. Gardner. 1998. A phase I/II study of tgAAV-CF for the treatment of chronic sinusitis in patients with cystic fibrosis. Hum. Gene Ther. 9:889-909. [DOI] [PubMed] [Google Scholar]
- 36.Wagner, J. A., I. B. Nepomuceno, A. H. Messner, M. L. Moran, E. P. Batson, S. Dimiceli, B. W. Brown, J. K. Desch, A. M. Norbash, C. K. Conrad, W. B. Guggino, T. R. Flotte, J. J. Wine, B. J. Carter, T. C. Reynolds, R. B. Moss, and P. Gardner. 2002. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 13:1349-1359. [DOI] [PubMed] [Google Scholar]
- 37.Xiao, W., K. H. Warrington, Jr., P. Hearing, J. Hughes, and N. Muzyczka. 2002. Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2. J. Virol. 76:11505-11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan, Z., T. C. Ritchie, D. Duan, and J. F. Engelhardt. 2002. Recombinant AAV-mediated gene delivery using dual vector heterodimerization. Methods Enzymol. 346:334-357. [DOI] [PubMed] [Google Scholar]
- 39.Yan, Z., R. Zak, G. W. Luxton, T. C. Ritchie, U. Bantel-Schaal, and J. F. Engelhardt. 2002. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J. Virol. 76:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zabner, J., M. Seiler, R. Walters, R. M. Kotin, W. Fulgeras, B. L. Davidson, and J. A. Chiorini. 2000. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J. Virol. 74:3852-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]