Abstract
The high pathogenicity of Lassa virus is assumed to involve resistance to the effects of interferon (IFN). We have analyzed the effects of alpha IFN (IFN-α), IFN-γ, and tumor necrosis factor alpha (TNF-α) on replication of Lassa virus compared to the related, but less pathogenic, lymphocytic choriomeningitis virus (LCMV). Three low-passage Lassa virus strains (AV, NL, and CSF), isolated from humans with mild to fulminant Lassa fever, were tested. Lassa virus replication was inhibited by IFN-α and IFN-γ, but not TNF-α, in Huh7 and Vero cells. The degree of IFN sensitivity of a Lassa virus isolate did not correlate with disease severity in human patients. Furthermore, cytokine effects observed for Lassa virus and LCMV (strains CH-5692, Armstrong, and WE) were similar. To address the mechanisms involved in the IFN effect, we used cell lines in which overexpression of IFN-stimulated proteins promyelocytic leukemia protein (PML) and Sp100 could be induced. Both proteins reside in PML bodies, a cellular target of the LCMV and Lassa virus Z proteins. Overexpression of PML or Sp100 did not affect replication of either virus. This, together with the previous finding that PML knockout facilitates LCMV replication in vitro and in vivo (M. Djavani, J. Rodas, I. S. Lukashevich, D. Horejsh, P. P. Pandolfi, K. L. Borden, and M. S. Salvato, J. Virol. 75:6204-6208, 2001; W. V. Bonilla, D. D. Pinschewer, P. Klenerman, V. Rousson, M. Gaboli, P. P. Pandolfi, R. M. Zinkernagel, M. S. Salvato, and H. Hengartner, J. Virol. 76:3810-3818, 2002), describes PML as a mediator within the antiviral pathway rather than as a direct effector protein. In conclusion, the high pathogenicity of Lassa virus compared to LCMV is probably not due to increased resistance to the effects of IFN-α or IFN-γ. Both cytokines inhibit replication which is relevant for the design of antiviral strategies against Lassa fever with the aim of enhancing the IFN response.
Lassa virus belongs to the Old World complex of the family Arenaviridae and is the etiologic agent of Lassa fever in humans. Its natural host is the African rodent Mastomys natalensis. Transmission of the virus from its natural host to humans causes a systemic disease, Lassa fever, which is associated with bleeding and organ failure; there is a high fatality rate for hospitalized patients with Lassa fever (34). Lassa fever is endemic in the West African countries of Sierra Leone, Guinea, Liberia, and Nigeria. The drug ribavirin has been shown to be effective if it is administered early after infection (35). However, several recent cases of Lassa fever in Europe have demonstrated that even state-of-the-art intensive care cannot prevent a fatal outcome (42). In contrast to the highly pathogenic Lassa virus, the related lymphocytic choriomeningitis virus (LCMV), which is carried by Mus musculus, causes only a mild febrile illness and benign meningitis in humans. Similarly, there are other Old World arenaviruses, such as Ippy, Mobala, and Mopeia virus which do not appear to be associated with significant human disease. The molecular or immunological mechanisms underlying the differences in pathogenicity are not understood.
Type I (alpha interferon [IFN-α] and IFN-β) and type II (IFN-γ) IFNs are the central mediators of the innate immune response by inducing an antiviral state within a cell. IFN signaling involves binding of IFN to the respective receptor (type I or type II), activation of STAT1 and/or STAT2, leading to the formation of active transcription factor complexes, and the subsequent activation of IFN-stimulated genes (41). Another cytokine which shows antiviral activity to some viruses is tumor necrosis factor alpha (TNF-α) (5, 10, 29, 33, 45). The TNF-α-mediated antiviral response involves activation of the NF-κB transcription factor (10).
LCMV is susceptible to IFN in murine cells (37), and there is evidence that IFN plays an important role in the clearance of LCMV in mice (23, 38). Moreover, there is a correlation between the relative resistance of particular LCMV strains to murine IFN and their propensity to establish a persistent infection in mice (37). There is little analogous data available for Lassa virus. In one published study in which low concentrations of IFN-α were used, the researchers concluded that Lassa virus is resistant to the effect of IFN (39).
Besides the abovementioned association between IFN susceptibility of LCMV strains and course of infection (37), there are several examples in the literature for an association between IFN susceptibility and pathogenicity among related viruses or strains (13, 44, 46, 49). Many viruses modulate their susceptibility to the IFN system by expressing IFN antagonists (41), and it is conceivable that the activity of antagonists differs from strain to strain. Other viruses interfere with the TNF-α pathway (3, 19, 40). A prominent example for a correlation between pathogenicity and susceptibility to IFN and TNF-α is influenza A virus (44). Lethal H5N1 strains were totally resistant to the antiviral effects of IFN-α, IFN-γ, and TNF-α in vitro, while the replication of common non-H5N1 strains of influenza A virus was strongly suppressed by all three cytokines. Amino acid changes in the influenza A virus NS1 protein, which is known to counteract the IFN response (32), appear to determine the IFN susceptibility phenotype (44).
The present study focused on three questions. (i) Is Lassa virus resistant or susceptible to the effects of IFN-α, IFN-γ, and TNF-α in cells of human and nonhuman primate origin? (ii) Does the highly pathogenic phenotype of Lassa virus correlate with increased resistance to these cytokines compared to the less pathogenic LCMV? (iii) Do the IFN-stimulated proteins promyelocytic leukemia protein (PML) and Sp100 play a role as effector proteins in the IFN response to Lassa virus and LCMV?
Virus strains and cell lines.
The study was performed with a representative set of three low-passage strains of Lassa virus belonging to three phylogenetic lineages (11, 25) (Table 1). The strains were isolated from humans with different clinical courses of Lassa fever. A fulminant Lassa fever was caused by strain AV (42). A less severe disease was caused by strain NL (42). Strain CSF was isolated from a patient with a mild and unusual course of disease (25). For comparison, three strains of LCMV were tested. One of the three strains was a low-passage isolate from a pygmy marmoset (Cebuella pygmaea) with callitrichid hepatitis (2), while the other two strains were the classical laboratory strains Armstrong and WE (kindly provided by Michael Bruns, Heinrich-Pette-Institute, Hamburg, Germany) (Table 1). Experiments with Lassa virus and LCMV were performed under biosafety level 4 and biosafety level 3 conditions, respectively. Virus stocks were quantified by an immunological focus assay (4), because plaques were poorly produced by all virus strains. Vero cells in 24-well plates were inoculated with 100 μl of serial 10-fold dilutions of virus stock. The inoculum was removed after 1 h and replaced by a 1% methylcellulose medium overlay. After 5 days of incubation, cells were fixed with 4% formaldehyde, permeabilized with 0.5% Triton X-100, blocked with 10% fetal calf serum, and washed. Cell foci infected with Lassa virus and LCMV were detected with Lassa virus nucleoprotein (NP)-specific monoclonal antibody L2F1 diluted 1:50 (26) and LCMV NP-specific monoclonal antibody 53 diluted 1:800 (kindly provided by Michael Bruns), respectively. After the cells were washed and incubated with peroxidase-labeled anti-mouse immunoglobulin G antibody (Dianova) diluted 1:3,000, foci were detected with 3,3′,5,5′-tetramethylbenzidine (TMB). Titers are expressed as plaque (focus)-forming units (PFU) per milliliter.
TABLE 1.
Viruses used in this study
| Strain | Geographic origin | Phylogenetic lineage | Source of isolate | Associated disease | No. of passages after isolation | Reference(s) |
|---|---|---|---|---|---|---|
| Lassa virus | ||||||
| AV | Ghana, Ivory Coast, or Burkina Faso | V | Human (serum) | Multiorgan failure, bleeding, and hypovolemic shock | 1 | 24, 42 |
| NL | Sierra Leone | IV | Human (serum) | Lassa fever without multiorgan failure and bleeding | 1 | 42 |
| CSF | Nigeria | III | Human (cerebrospinal fluid) | Encephalopathy without fever | 1 | 25 |
| LCMV | ||||||
| CH-5692 | Germany | Cebuella pygmaea (spleen) | Callitrichid hepatitis | 1 | 2 | |
| Armstrong E-350 | United States | Laboratory strain | Several | 1 | ||
| WE | United States | Laboratory strain (from cerebrospinal fluid sample of a human with meningitis) | Several | 43 |
Two cell lines were used for the experiments. The highly differentiated human hepatoma cell line Huh7 was chosen, because the liver is a major target organ of Lassa virus (36, 50). Experiments were also performed in Vero cells, which are of nonhuman primate origin and are defective in the induction of IFN genes (18), preventing auto- and paracrine effects due to endogenously expressed IFN.
Functionality of the cell culture system as tested with VSV.
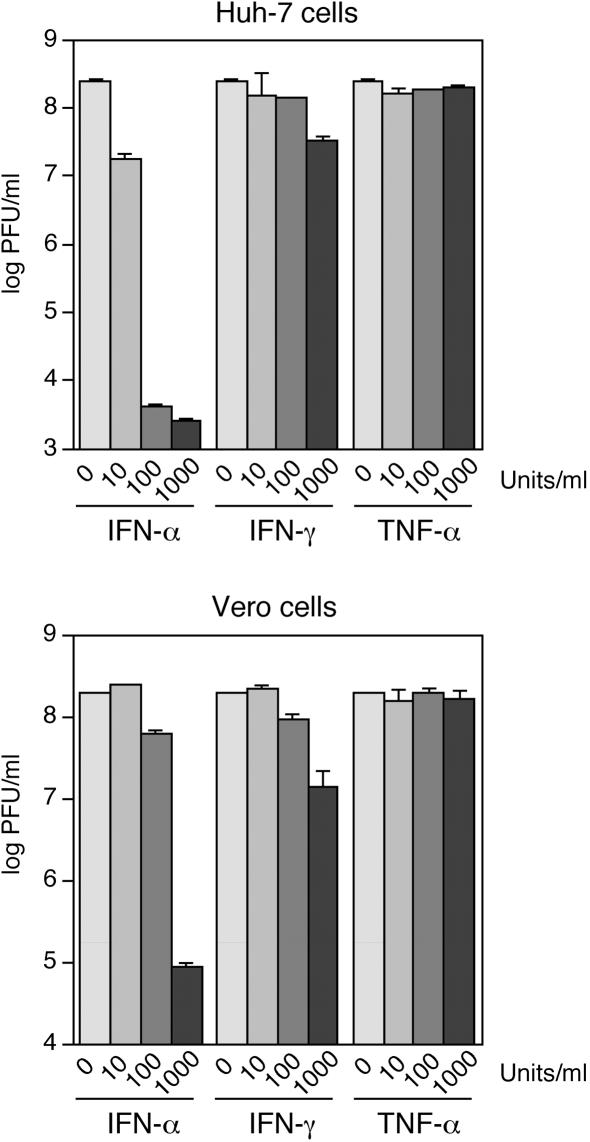
The functionality of the cell culture system was verified using vesicular stomatitis virus (VSV). Huh7 and Vero cells were incubated with various concentrations (0, 10, 100, and 1,000 U/ml) of natural human IFN-α (affinity-purified mixture of different IFN-α species), recombinant human IFN-γ, and recombinant human TNF-α (Biochrom AG). The cells were infected 24 h later with VSV strain Indiana at a multiplicity of infection (MOI) of 0.01, and fresh medium supplemented with the cytokines was added. Supernatants were taken 24 h postinfection, and the virus titer was determined by a plaque test on Vero cells. IFN-α reduced the VSV titer by up to 4 log units, while IFN-γ caused only a reduction of about 1 log unit, indicating that the IFN signaling and effector pathways are functional (Fig. 1). In agreement with previous observations (13, 39), these data also show that human IFN is active on cells originating from African green monkeys. Since TNF-α showed no effect, the functionality of the TNF-α pathway was measured by transfection of a TNF-α response plasmid containing NF-κB sites upstream of a luciferase reporter gene. Huh7 and Vero cells were transfected with the reporter plasmid pNFkB-Luc (Clontech) and either treated with 1,000 U of TNF-α per ml of medium (about 10 ng/ml) or left untreated; 5 h later, cells were harvested, and luciferase activity was measured. TNF-α treatment led to increases in luciferase activity of 3.4 ± 1.1-fold (n = 6) and 1.5 ± 0.1-fold (n = 6) in Huh7 and Vero cells, respectively, indicating that the cells respond to TNF-α.
FIG. 1.
Sensitivity of VSV to different concentrations of human IFN-α, IFN-γ, and TNF-α in Huh7 and Vero cells. Cells were infected at an MOI of 0.01, and the virus titer was determined in the supernatant 24 h later by plaque assay. The values for two experiments are shown on a logarithmic scale.
Growth kinetics of Lassa virus and LCMV strains.
The growth kinetics of the Lassa virus and LCMV strains were determined to optimize the conditions of the virus replication assay. To facilitate rapid processing of a large number of samples, a quantitative real-time PCR was established for measurement of virus RNA concentration in the cell culture supernatant. Huh7 and Vero cells were plated at a density of 4 × 104 cells/well of a 24-well plate. The cells were infected 24 h later with Lassa virus or LCMV at an MOI of 0.01 in 100 μl. The inoculum was removed after 1 h and replaced by fresh medium. For RNA preparation (8), 140-μl aliquots of supernatant were taken at regular intervals, mixed with 560-μl portions of chaotropic lysis buffer AVL (Qiagen), and incubated at room temperature for 15 min. The lysate was added to 100 mg of diatomaceous silica (Sigma-Aldrich) suspended in 560 μl of ethanol and incubated with agitation for 30 min at room temperature. The diatomaceous silica was pelleted by centrifugation, and the pellet was washed three times, first with 500 μl of AW1 buffer (Qiagen), then with 500 μl of AW2 buffer (Qiagen), and finally with 400 μl of acetone. The pellet was dried at 56°C, and the RNA was eluted with 100 μl of water. Virus RNA concentration was measured using the Brilliant single-step quantitative reverse transcriptase (RT) PCR kit (Stratagene) with SybrGreen as a reporter dye. The 20-μl reaction mixture contained 2 μl of RNA, 1× RT-PCR buffer, 2.5 mM MgCl2, 800 μM deoxynucleoside triphosphate, 0.8 μg of bovine serum albumin, 5 μM dye-binding molecule SGS (C. Drosten, patent pending), SybrGreen (Molecular Probes) diluted 1:10,000, 1.25 U of StrataScript RT, 1 U of SureStart Taq, and 0.25 μM concentrations of primers LCMV-S 13+ and LCMV-S 322− (2) to detect LCMV or 0.2 μM primer 36E2 and 0.3 μM primer 80F2 (16) to detect Lassa virus. Both pairs of primers target the S RNA of the virus. The reactions were run on a LightCycler instrument as follows: (i) 20 min at 45°C; (ii) 12 min at 95°C; (iii) 10 precycles, with 1 precycle consisting of 10 s at 95°C, 10 s at 60°C (which decreased 0.8°C/cycle), and 20 s at 72°C; (iv) 25 cycles, with 1 cycle consisting of 5 s at 95°C, 10 s at 52°C (LCMV) or 55°C (Lassa virus), 30 s at 65°C, and 20 s of fluorescence read at 83°C (LCMV) or 80°C (Lassa virus); and (v) melting curve analysis. The PCR target regions were cloned into pT-Adv (AdvanTAge PCR cloning kit; Clontech) and transcribed in vitro (MEGAscript; Ambion). The in vitro transcripts were quantified and used in the PCR to generate standard curves to quantify the virus RNA in supernatant. Both RT-PCRs showed a broad dynamic range and allowed simultaneous detection of different strains and discrimination between the strains in the melting point analysis (data not shown).
Similar growth curves were obtained for both cell lines, although replication of all low-passage isolates, particularly Lassa virus CSF and LCMV CH-5692, was somewhat slower (log phase up to 72 h) than replication of the two highly cell culture-adapted LCMV strains, Armstrong and WE (log phase up to 48 h) (Fig. 2 and data not shown). There was a good correlation between the RNA concentration and the titer of infectious particles as determined by RT-PCR and immunological focus assay (Fig. 2, compare the top and bottom graphs), respectively, indicating that real-time PCR is suitable for measuring virus growth in our cell culture system. About 100 molecules of S RNA per PFU were found, suggesting an excess of noninfectious virus particles. The ratio of S RNA molecules to infectious particles increased slightly at later time points (Fig. 2, top) which may be attributable to an increasing release of defective particles or loss of infectivity of virions in medium. Pilot experiments with 100 U of IFN-α per ml revealed no significant inhibitory effect with any virus, so the assays were performed with 1,000 U of IFN per ml.
FIG. 2.
Growth kinetics of Lassa virus AV. Vero cells were infected at different MOIs, and the concentration of S RNA molecules (top graph) and infectious particles (bottom graph) in supernatant was measured by real-time PCR and an immunological focus assay, respectively. The table at the top of the figure shows the relation between both measurements, expressed as S RNA molecules per PFU.
Effects of IFN-α, IFN-γ, and TNF-α alone and in combination on replication of Lassa virus and LCMV.
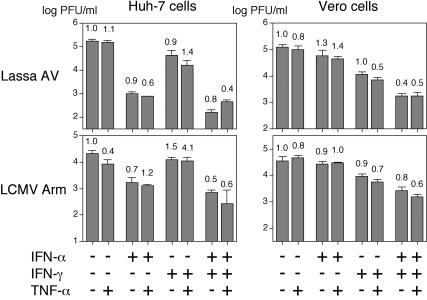
Huh7 and Vero cells were plated in 24-well plates and incubated with human IFN-α, IFN-γ, and TNF-α (1,000 U/ml) or combinations thereof for 24 h before virus infection or were left untreated. Cells were infected at an MOI of 0.01 and further incubated with the same cytokines as in the priming phase. Virus RNA concentration in supernatant was measured by real-time PCR after 48 or 72 h (Lassa virus CSF and LCMV CH-5692). The data are summarized in Fig. 3. IFN-α reduced Lassa virus growth by about 1 log unit in Huh7 cells, while the effect was lower in Vero cells. IFN-γ inhibited Lassa virus growth by about 0.5 log unit in both cell lines. The combination of both IFNs reduced the virus RNA concentration by about 1 log unit in both cell lines. Additive effects of IFN-α plus IFN-γ were observed only in Vero cells. TNF-α alone or in combination with IFN had no consistent effect. The strongest effects with IFN-α and IFN-γ were seen with Lassa virus AV, which had been associated with the most severe clinical course, while the lowest effects were seen with Lassa virus CSF, which had been associated with a mild course without signs of a systemic disease. Most importantly, the LCMV strains had effects comparable to those seen with the Lassa virus strains, particularly Lassa virus AV. The only remarkable difference was the synergistic inhibitory effect of IFN-α plus IFN-γ on LCMV strain WE, which was evident in both cell lines. There was no correlation between the S RNA molecule/PFU ratio in the initial virus stock (see the legend to Fig. 3) and the level of cytokine sensitivity, indicating that the fraction of noninfectious particles in the inoculum does not influence the outcome of the experiment.
FIG. 3.
Sensitivity of Lassa virus and LCMV to IFN-α, IFN-γ, and TNF-α. Huh7 and Vero cells were preincubated with human IFN-α, IFN-γ, or TNF-α (1,000 U/ml) (+) or combinations thereof for 24 h before virus infection or left untreated. Cells were infected with Lassa virus strains AV, NL, and CSF as well as LCMV strains CH-5692 (CH), Armstrong (Arm), and WE at an MOI of 0.01 and incubated again with the same cytokines as in the priming phase. All virus stocks used in the experiment had roughly comparable ratios of S RNA molecule/PFU. The S RNA molecule/PFU ratios for the different viruses on Huh-7 cells were as follows: 1.1 × 103 for AV, 2.5 × 103 for NL, 6.8 × 103 for CSF, 3.3 × 104 for CH-5692, 6.5 × 103 for Armstrong, and 1.2 × 104 for WE. The S RNA molecule/PFU ratios for the different viruses on Vero cells were as follows: 1.2 × 103 for AV, 3.1 × 104 for NL, 3.5 × 103 for CSF, 2.2 × 104 for CH-5692, 2.6 × 104 for Armstrong, and 1.7 × 103 for WE. Virus RNA concentration in supernatant was measured by real-time PCR after 48 or 72 h (Lassa virus CSF and LCMV CH-5692). Means ± standard deviations (error bars) for three experiments are shown on a logarithmic scale. To facilitate comparison, the data are shown as relative values, with the RNA concentrations in the untreated cells being given the value 1, corresponding to 0 on a log scale.
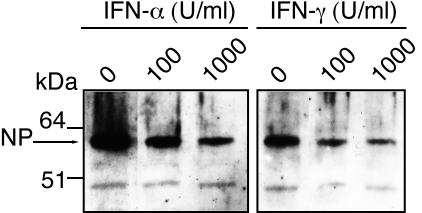
Additional experiments were performed with Lassa virus AV and LCMV Armstrong to substantiate these data. In the first experiment, the assay described above was repeated with both cell lines, and in addition to the virus RNA concentration, the concentration of infectious particles was determined by an immunological focus assay. The inhibitory effects of IFN-α and IFN-γ on Lassa virus and LCMV were confirmed in this assay (Fig. 4). Furthermore, there was no significant change in the specific infectivity of the released virus particles (expressed as PFU per S RNA molecule) due to the treatment with the cytokines (Fig. 4). This indicates that neither IFN-α, IFN-γ, nor TNF-α treatment leads to a relative overproduction of defective virus particles. Thus, lethal hypermutation caused by the IFN-stimulated, RNA-specific enzyme adenosine deaminase (ADAR1) (41) is unlikely to play a role. Immunoblot analysis of Lassa virus-infected Vero cells also demonstrated that the reduction due to IFN-α and IFN-γ treatment in the released particles is accompanied by a decrease in the intracellular NP level (Fig. 5).
FIG. 4.
Influence of IFN-α, IFN-γ, and TNF-α on the titer of infectious particles and the specific infectivity of released particles of Lassa virus AV and LCMV Armstrong. The experiment was performed as indicated in the legend to Fig. 3, except the concentration of infectious particles in the supernatant (PFU/ml) was measured by an immunological focus assay. Mean and standard deviations (n = 3) are shown on a logarithmic scale. To determine the specific infectivity of the released particles, the virus RNA concentration (number of S RNA molecules per milliliter) was measured by real-time PCR, and the PFU/S RNA molecule ratio was calculated. The values for specific infectivity are the means of three determinations and are depicted above the columns. To facilitate comparison, the PFU/S RNA molecule value without treatment was defined as 1.
FIG. 5.
Immunoblot analysis of intracellular nucleoprotein (NP) of Lassa virus AV. Huh7 cells were preincubated with various concentrations (0, 100, and 1,000 U/ml) of human IFN-α or IFN-γ for 24 h before infection with Lassa virus AV at an MOI of 0.01. Cells were further incubated with the same cytokines as in the priming phase and harvested after 48 h. Cytoplasmic lysate was prepared using Nonidet P-40 (NP) lysis buffer. The lysate was resolved in a polyacrylamide gradient gel (4 to 16% NuPAGE Bis-Tris gel; Invitrogen) and blotted onto a nitrocellulose membrane (Protran; Schleicher & Schuell). Nucleoprotein was detected by standard immunoblotting techniques using rabbit anti-NP (diluted 1:1,000), peroxidase-labeled anti-rabbit immunoglobulin G (Dianova), and SuperSignal Pico chemiluminescence substrate (Pierce).
In another experiment, Vero cells were pretreated with cytokines for 24 h, inoculated with about 200 PFU/well for 1 h, and overlaid with 1% methylcellulose in medium to prevent further virus spread. Infected foci were immunologically detected as described for the immunological focus assay. Pretreatment with both IFN-α and IFN-γ reduced the number of foci by about 30% (Fig. 6). Thus, IFN completely blocked virus replication in a fraction of cells. There was a slightly additive effect of both IFNs, while TNF-α had no effect. Again, no remarkable differences were seen between Lassa virus and LCMV in these sets of experiments (Fig. 4 and 6).
FIG. 6.
Influence of IFN-α, IFN-γ, and TNF-α on Lassa virus AV and LCMV Armstrong (Arm) as measured by the focus reduction test. Vero cells were pretreated with cytokines (+) for 24 h, inoculated with about 200 PFU/well, and overlaid with 1% methylcellulose. Infected foci were immunologically detected as described for the immunological focus assay. (A) Representative wells. (B) Quantitative presentation of the data. Means ± standard deviations (error bars) of three experiments are shown, with the control values (without treatment) defined as 1.
Relevance of the IFN-stimulated proteins PML and Sp100.
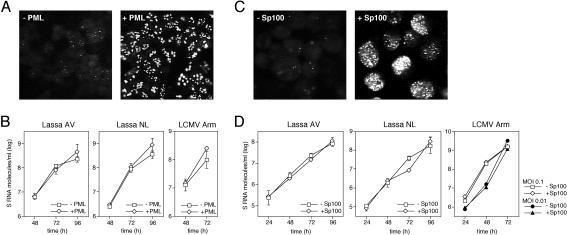
PML and Sp100 are IFN-stimulated proteins which colocalize in so-called ND10 nuclear domains (15, 21, 22). PML has been shown to interact with Z protein of Lassa virus and LCMV (9). In an attempt to understand the mechanisms of IFN-mediated inhibition of Lassa virus and LCMV replication, we examined whether overexpression of PML or Sp100 has an antiviral effect. To this end, cell lines allowing tetracycline (TET)-induced overexpression of the PML-L (PML-III according to the revised nomenclature [28]) and Sp100-AltC isoforms were used (HeLa-PML++ and HeLa-SpAltC++) (47, 48). The TET-induced overexpression of these proteins resembles IFN-stimulated overexpression (22). The cells were seeded and cultured in the presence or absence of TET. After 3 days, they were infected with Lassa virus AV or NL or LCMV Armstrong at an MOI of 0.01, and virus growth kinetics were determined by real-time PCR as described above. At the end of each experiment, the overexpression of PML and Sp100 was monitored by immunofluorescence using PML- and Sp100-specific antibodies, respectively (Fig. 7A and C). There was no evidence that overexpression of PML or Sp100 affects the replication of Lassa virus and LCMV (Fig. 7B and D).
FIG. 7.
Influence of PML and Sp100 overexpression on the replication of Lassa virus and LCMV. Cell lines HeLa-PML++ and HeLa-SpAltC++ (47, 48) allowing TET-induced overexpression of the PML-L and Sp100-AltC isoforms were cultured in the presence or absence of TET. After 3 days, cells were infected with Lassa virus AV or NL or LCMV Armstrong at an MOI of 0.01. Virus growth kinetics were determined by real-time PCR. (A) Endogenous PML expression (−PML) versus TET-induced PML overexpression (+PML) in HeLa-PML++ cells as tested by immunofluorescence using rat PML-specific antibodies at the end of each experiment. (B) Virus growth kinetics on PML-induced (+PML) cells versus noninduced (−PML) cells. Mean and standard deviations (n = 3) are shown. (C) Endogenous Sp100 expression (−Sp100) versus TET-induced Sp100 overexpression (+Sp100) in HeLa-SpAltC++ cells as tested by immunofluorescence using rat Sp100-specific antibodies at the end of each experiment. (D) Virus growth kinetics on Sp100-induced (+Sp100) versus noninduced (−Sp100) cells. Means ± standard deviations (error bars) of three experiments are shown.
The results with PML were further substantiated by focus reduction test on TET-induced and noninduced HeLa-PML++ cells infected with about 100 PFU of Lassa virus AV or LCMV Armstrong. Foci were detected as described above for the immunological focus assay. Overexpression did not reduce the number of foci (Lassa virus AV foci on noninduced cells [set at 100%] versus induced cells, 100 ± 24 versus 153 ± 21, respectively; LCMV Armstrong foci on noninduced cells [set at 100%] versus induced cells, 100 ± 28 versus 123 ± 19, respectively).
Summary and discussion.
This study demonstrates that Lassa virus is sensitive to the antiviral effects of IFN-α and IFN-γ, but not TNF-α, in liver and kidney cell lines of human and nonhuman primate origin, respectively. This finding is relevant to designing antiviral strategies against Lassa fever aimed at enhancing the IFN response. Analogous concepts have been found to be promising in treating infections with filoviruses (12) and flaviviruses (30) in animal models.
There was no correlation between the severity of disease caused by a Lassa virus isolate and the level of susceptibility to IFN. Contrary to expectations, replication of Lassa virus AV, which caused a fulminant and fatal Lassa fever (42), was actually slightly more inhibited by IFN-α and IFN-γ than the other two strains, which were associated with considerably less severe disease (25, 42). Although the clinical course in a patient with Lassa fever cannot be assumed to represent the pathogenicity of the virus isolate, it provides a clue as to the virus phenotype. Previous studies have demonstrated that the disease severity in the human patient (except pregnant women and newborns) correlates approximately with the virulence of a Lassa virus isolate in an animal model (27).
No effect could be demonstrated for TNF-α, though the NF-κB pathway was clearly activated upon treatment in Huh-7 cells. NF-κB activation as tested with the reporter plasmid was lower but highly reproducible in experiments in Vero cells, suggesting that the monkey cells were less responsive to the human TNF-α. However, it is not known how the level of activation of the reporter plasmid correlates with the induced antiviral state. For example, lower doses of TNF-α than those used in our study showed clear antiviral effects on Sin Nombre virus in Vero cell clone E6 (29).
Most importantly, in none of the experimental settings were there remarkable differences between Lassa virus and LCMV in their response to IFN-α, IFN-γ, and TNF-α. This contrasts to the situation with influenza A virus in which a clear correlation between virulence and resistance to these cytokines was demonstrated (44). It is concluded from the data presented here that increased resistance to IFN is unlikely to be the major determinant of pathogenicity of Lassa virus in humans. The data do not exclude the possibility that both Lassa virus and LCMV interfere similarly with the IFN signaling pathway. Indeed, the relative resistance of all arenavirus strains to the antiviral effects of IFN-α compared to VSV, which was much more strongly inhibited, may point to the presence of IFN-antagonistic activity in Lassa virus and LCMV. The clue to the pathogenicity of Lassa virus may reside in a reduced induction of the IFN cascade upon infection, as has been suggested with pathogenic versus nonpathogenic hantaviruses (20).
In a first attempt to characterize the mechanisms underlying the IFN-mediated inhibition of Lassa virus and LCMV, whether overexpression of PML and Sp100 exerts antiviral effects was tested. No evidence for a direct role of these proteins in the IFN-mediated inhibition of virus replication was found, although the experimental setting resembles the IFN-stimulated overexpression of these proteins. This finding seemingly contrasts with a recent report that LCMV replicates to somewhat higher level in mouse embryonic fibroblast cells lacking PML (PML−/− cells) (17). However, these and our findings both fit the hypothesis that PML is a mediator in the antiviral pathways rather than an effector protein inhibiting virus replication directly, for example through its interaction with Z protein (9). The lack of effects due to overexpression suggests that the basal PML expression level is sufficient to mediate antiviral effects against LCMV and probably Lassa virus; elimination of basal expression would block the cascade. The latter may also explain why LCMV replicates to higher titers in PML−/− mice, and researchers have already speculated on the possibility that PML is an indirect mediator (7). Recent reports on rabies virus (6) and herpes simplex virus (14, 31), demonstrating positive effects on virus replication as a result of PML knockout but not negative effects due to PML overexpression, further support the hypothesis that PML functions as a mediator of the innate immune response in some virus infections.
Acknowledgments
We thank Michael Bruns for providing LCMV strains Armstrong and WE as well as LCMV NP-specific antibodies and Martin Pfeffer for providing VSV strain Indiana.
This study was supported in part by grants E/B31E/M0171/M5916 and E/B41G/1G309/1A403 from the Bundesamt für Wehrtechnik und Beschaffung. The Bernhard-Nocht-Institut is supported by the Bundesministerium für Gesundheit and the Freie und Hansestadt Hamburg.
REFERENCES
- 1.Armstrong, C., and R. D. Lillie. 1934. Experimental lymphocytic choriomeningitis of monkeys and mice produced by a virus encountered in studies of the 1933 St. Louis encephalitis epidemic. Public Health Rep. 49:1019-1027. [Google Scholar]
- 2.Asper, M., P. Hofmann, C. Osmann, J. Funk, C. Metzger, M. Bruns, F. J. Kaup, H. Schmitz, and S. Günther. 2001. First outbreak of callitrichid hepatitis in Germany: genetic characterization of the causative lymphocytic choriomeningitis virus strains. Virology 284:203-213. [DOI] [PubMed] [Google Scholar]
- 3.Baillie, J., D. A. Sahlender, and J. H. Sinclair. 2003. Human cytomegalovirus infection inhibits tumor necrosis factor alpha (TNF-alpha) signaling by targeting the 55-kilodalton TNF-alpha receptor. J. Virol. 77:7007-7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33:191-198. [DOI] [PubMed] [Google Scholar]
- 5.Biermer, M., R. Puro, and R. J. Schneider. 2003. Tumor necrosis factor alpha inhibition of hepatitis B virus replication involves disruption of capsid integrity through activation of NF-κB. J. Virol. 77:4033-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blondel, D., T. Regad, N. Poisson, B. Pavie, F. Harper, P. P. Pandolfi, H. De The, and M. K. Chelbi-Alix. 2002. Rabies virus P and small P products interact directly with PML and reorganize PML nuclear bodies. Oncogene 21:7957-7970. [DOI] [PubMed] [Google Scholar]
- 7.Bonilla, W. V., D. D. Pinschewer, P. Klenerman, V. Rousson, M. Gaboli, P. P. Pandolfi, R. M. Zinkernagel, M. S. Salvato, and H. Hengartner. 2002. Effects of promyelocytic leukemia protein on virus-host balance. J. Virol. 76:3810-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boom, R., C. J. Sol, M. M. Salimans, C. L. Jansen, P. M. Wertheim-van Dillen, and J. van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borden, K. L., E. J. Campbell Dwyer, and M. S. Salvato. 1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol. 72:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose, S., N. Kar, R. Maitra, J. A. DiDonato, and A. K. Banerjee. 2003. Temporal activation of NF-κB regulates an interferon-independent innate antiviral response against cytoplasmic RNA viruses. Proc. Natl. Acad. Sci. USA 100:10890-10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen, M. D., P. E. Rollin, T. G. Ksiazek, H. L. Hustad, D. G. Bausch, A. H. Demby, M. D. Bajani, C. J. Peters, and S. T. Nichol. 2000. Genetic diversity among Lassa virus strains. J. Virol. 74:6992-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bray, M., J. L. Raymond, T. Geisbert, and R. O. Baker. 2002. 3-Deazaneplanocin A induces massively increased interferon-alpha production in Ebola virus-infected mice. Antivir. Res. 55:151-159. [DOI] [PubMed] [Google Scholar]
- 13.Carrigan, D. R., and K. K. Knox. 1990. Identification of interferon-resistant subpopulations in several strains of measles virus: positive selection by growth of the virus in brain tissue. J. Virol. 64:1606-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 77:7101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chelbi-Alix, M. K., L. Pelicano, F. Quignon, M. H. Koken, L. Venturini, M. Stadler, J. Pavlovic, L. Degos, and H. de The. 1995. Induction of the PML protein by interferons in normal and APL cells. Leukemia 9:2027-2033. [PubMed] [Google Scholar]
- 16.Demby, A. H., J. Chamberlain, D. W. Brown, and C. S. Clegg. 1994. Early diagnosis of Lassa fever by reverse transcription-PCR. J. Clin. Microbiol. 32:2898-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djavani, M., J. Rodas, I. S. Lukashevich, D. Horejsh, P. P. Pandolfi, K. L. Borden, and M. S. Salvato. 2001. Role of the promyelocytic leukemia protein PML in the interferon sensitivity of lymphocytic choriomeningitis virus. J. Virol. 75:6204-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emeny, J. M., and M. J. Morgan. 1979. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 43:247-252. [DOI] [PubMed] [Google Scholar]
- 19.Friedman, J. M., and M. S. Horwitz. 2002. Inhibition of tumor necrosis factor alpha-induced NF-κB activation by the adenovirus E3-10.4/14.5K complex. J. Virol. 76:5515-5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geimonen, E., S. Neff, T. Raymond, S. S. Kocer, I. N. Gavrilovskaya, and E. R. Mackow. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. USA 99:13837-13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grötzinger, T., K. Jensen, and H. Will. 1996. The interferon (IFN)-stimulated gene Sp100 promoter contains an IFN-gamma activation site and an imperfect IFN-stimulated response element which mediate type I IFN inducibility. J. Biol. Chem. 271:25253-25260. [DOI] [PubMed] [Google Scholar]
- 22.Grötzinger, T., T. Sternsdorf, K. Jensen, and H. Will. 1996. Interferon-modulated expression of genes encoding the nuclear-dot-associated proteins Sp100 and promyelocytic leukemia protein (PML). Eur. J. Biochem. 238:554-560. [DOI] [PubMed] [Google Scholar]
- 23.Guidotti, L. G., P. Borrow, A. Brown, H. McClary, R. Koch, and F. V. Chisari. 1999. Noncytopathic clearance of lymphocytic choriomeningitis virus from the hepatocyte. J. Exp. Med. 189:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Günther, S., P. Emmerich, T. Laue, O. Kühle, M. Asper, A. Jung, T. Grewing, J. ter Meulen, and H. Schmitz. 2000. Imported Lassa fever in Germany: molecular characterization of a new Lassa virus strain. Emerg. Infect. Dis. 6:466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Günther, S., B. Weisner, A. Roth, T. Grewing, M. Asper, C. Drosten, P. Emmerich, J. Petersen, M. Wilczek, and H. Schmitz. 2001. Lassa fever encephalopathy: Lassa virus in cerebrospinal fluid but not in serum. J. Infect. Dis. 184:345-349. [DOI] [PubMed] [Google Scholar]
- 26.Hufert, F. T., W. Ludke, and H. Schmitz. 1989. Epitope mapping of the Lassa virus nucleoprotein using monoclonal anti-nucleocapsid antibodies. Arch. Virol. 106:201-212. [DOI] [PubMed] [Google Scholar]
- 27.Jahrling, P. B., J. D. Frame, S. B. Smith, and M. H. Monson. 1985. Endemic Lassa fever in Liberia. III. Characterization of Lassa virus isolates. Trans. R. Soc. Trop. Med. Hyg. 79:374-379. [DOI] [PubMed] [Google Scholar]
- 28.Jensen, K., C. Shiels, and P. S. Freemont. 2001. PML protein isoforms and the RBCC/TRIM motif. Oncogene 20:7223-7233. [DOI] [PubMed] [Google Scholar]
- 29.Khaiboullina, S. F., D. M. Netski, P. Krumpe, and S. C. St Jeor. 2000. Effects of tumor necrosis factor alpha on Sin Nombre virus infection in vitro. J. Virol. 74:11966-11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leyssen, P., C. Drosten, M. Paning, N. Charlier, J. Paeshuyse, E. De Clercq, and J. Neyts. 2003. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob. Agents Chemother. 47:777-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez, P., R. J. Jacob, and B. Roizman. 2002. Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins. J. Virol. 76:9355-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 33.Lucin, P., S. Jonjic, M. Messerle, B. Polic, H. Hengel, and U. H. Koszinowski. 1994. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumour necrosis factor. J. Gen. Virol. 75:101-110. [DOI] [PubMed] [Google Scholar]
- 34.McCormick, J. B., I. J. King, P. A. Webb, K. M. Johnson, R. O'Sullivan, E. S. Smith, S. Trippel, and T. C. Tong. 1987. A case-control study of the clinical diagnosis and course of Lassa fever. J. Infect. Dis. 155:445-455. [DOI] [PubMed] [Google Scholar]
- 35.McCormick, J. B., I. J. King, P. A. Webb, C. L. Scribner, R. B. Craven, K. M. Johnson, L. H. Elliott, and R. Belmont-Williams. 1986. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 314:20-26. [DOI] [PubMed] [Google Scholar]
- 36.McCormick, J. B., D. H. Walker, I. J. King, P. A. Webb, L. H. Elliott, S. G. Whitfield, and K. M. Johnson. 1986. Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am. J. Trop. Med. Hyg. 35:401-407. [DOI] [PubMed] [Google Scholar]
- 37.Moskophidis, D., M. Battegay, M. A. Bruendler, E. Laine, I. Gresser, and R. M. Zinkernagel. 1994. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J. Virol. 68:1951-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters, C. J., C. T. Liu, G. W. Anderson, J. C. Morrill, and P. B. Jahrling. 1989. Pathogenesis of viral hemorrhagic fevers: Rift Valley fever and Lassa fever contrasted. Rev. Infect. Dis. 11(Suppl. 4):S743-S749. [DOI] [PubMed] [Google Scholar]
- 40.Powell, P. P., L. K. Dixon, and R. M. Parkhouse. 1996. An IκB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J. Virol. 70:8527-8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz, H., B. Köhler, T. Laue, C. Drosten, P. J. Veldkamp, S. Günther, P. Emmerich, H. P. Geisen, K. Fleischer, M. F. Beersma, and A. Hoerauf. 2002. Monitoring of clinical and laboratory data in two cases of imported Lassa fever. Microbes Infect. 4:43-50. [DOI] [PubMed] [Google Scholar]
- 43.Scott, T. F. M., and T. M. Rivers. 1936. Meningitis in man caused by a filterable virus. I. Two cases and the method of obtaining a virus from their spinal fluids. J. Exp. Med. 63:397-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 45.Seo, S. H., and R. G. Webster. 2002. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol. 76:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spotts, D. R., R. M. Reich, M. A. Kalkhan, R. M. Kinney, and J. T. Roehrig. 1998. Resistance to alpha/beta interferons correlates with the epizootic and virulence potential of Venezuelan equine encephalitis viruses and is determined by the 5′ noncoding region and glycoproteins. J. Virol. 72:10286-10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sternsdorf, T., H. H. Guldner, C. Szostecki, T. Grötzinger, and H. Will. 1995. Two nuclear dot-associated proteins, PML and Sp100, are often co-autoimmunogenic in patients with primary biliary cirrhosis. Scand. J. Immunol. 42:257-268. [DOI] [PubMed] [Google Scholar]
- 48.Sternsdorf, T., K. Jensen, and H. Will. 1997. Evidence for covalent modification of the nuclear dot-associated proteins PML and Sp100 by PIC1/SUMO-1. J. Cell Biol. 139:1621-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su, Y. H., J. E. Oakes, and R. N. Lausch. 1990. Ocular avirulence of a herpes simplex virus type 1 strain is associated with heightened sensitivity to alpha/beta interferon. J. Virol. 64:2187-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker, D. H., J. B. McCormick, K. M. Johnson, P. A. Webb, G. Komba-Kono, L. H. Elliott, and J. J. Gardner. 1982. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107:349-356. [PMC free article] [PubMed] [Google Scholar]