Abstract
Cytotoxic T lymphocytes (CTLs) play an important role in the immune response against respiratory syncytial virus (RSV) infection. The cell surface molecule lymphocyte function-associated antigen 1 (LFA-1) is an important contributor to CTL activation, CTL-mediated direct cell lysis, and lymphocyte migration. In an attempt to determine the role of LFA-1 during RSV infection, we treated BALB/c mice with monoclonal antibodies to LFA-1 at days −1, +1, and +4 relative to primary RSV infection. Anti-LFA-1 treatment during primary RSV infection led to reduced illness and delayed clearance of virus-infected cells. CTLs from RSV-infected mice that were treated with anti-LFA-1 exhibited diminished cytolytic activity and reduced gamma interferon production. In addition, studies with BrdU (5-bromo-2′-deoxyuridine)- and CFSE [5-(and 6)-carboxyfluorescein diacetate succinimidyl ester]-labeled lymphocytes showed that anti-LFA-1 treatment led to delayed proliferation during RSV infection. These results indicate that LFA-1 plays a critical role in the initiation of the immune response to RSV infection by facilitating CTL activation. These results may prove useful in the development of new therapies to combat RSV infection or other inflammatory diseases.
Human respiratory syncytial virus (RSV) is a pneumovirus of the Paramyxoviridae family of viruses (14). The majority of infants and toddlers with RSV develop only a mild upper respiratory infection. However, 20 to 30% of infected children fall victim to dangerous lower respiratory tract infections and bronchiolitis, resulting in an excess of 130,000 hospitalizations annually in the United States alone (48). RSV infection among the institutionalized elderly is also associated with high rates of mortality (20). In immunocompromised patients, particularly bone marrow transplant recipients, RSV leads to acute respiratory failure with exceptionally high mortality rates (30). These data clearly make RSV infection a high priority for vaccine development. However, a formalin-inactivated, alum-precipitated virus (FI-RSV) produced in the 1960s caused more severe illness, increased rates of hospitalization, and some mortality (35). This history of vaccine-enhanced illness has stymied efforts to produce a safe and efficacious vaccine for RSV infection.
RSV-specific cytotoxic T lymphocytes (CTLs) have been isolated from humans and mice. In the murine model, primary RSV infection normally results in mild to moderate disease and histopathology dominated by a lymphocytic infiltrate (22, 23, 25). While there is support for the concept that the FI-RSV vaccine-enhanced illness is mediated by a Th2-dominated T-cell response (24, 26), the pathogenesis of primary RSV infection is quite different. In mice, depletion of CD4+ and CD8+ T cells results in an extended period of virus replication that is accompanied by a lack of visible illness. When mice are depleted of CD8+ T cells, virus clearance is delayed but the moderate illness observed during primary infection is abolished (23). Conversely, illness is more severe when CD8+ T cells are present in excess (12). These data indicate that T lymphocytes not only shoulder the burden of RSV clearance during primary infection but are also major contributors to the observed illness. Recent data from RSV-infected infants suggest that in primary infection, disease severity correlates with gamma interferon (IFN-γ) levels, and this finding is consistent with immunopathology mediated by an overly exuberant CD8+-CTL response (9).
Lymphocyte function-associated antigen 1 (LFA-1) is an integrin composed of noncovalently associated CD11a and CD18 chains (50). It has been well documented that LFA-1 is of paramount importance in multiple cellular processes, including activation, migration, and CTL effector functions (6, 10, 11, 15, 19, 29, 32, 49, 55). Through its role as an adhesion molecule, LFA-1 helps define the immunological synapse (16). Briefly, LFA-1, along with CD2, constitutes a peripheral supramolecular activation complex, which surrounds a central supramolecular activation complex comprising the T-cell receptor and CD28. The immunological synapse is the site of T-cell activation, which is governed by a complex series of signaling events and cytoskeletal rearrangements (17-19, 38). The primary ligand for LFA-1 is intercellular adhesion molecule 1 (ICAM-1) (39, 46). Past studies have identified ICAM-1 as the receptor for the major groups of human rhinoviruses (27, 53, 54).
The characteristics of typical RSV infection and the importance of LFA-1 in the immune response led us to hypothesize that LFA-1 may play a major role in RSV-induced illness. Other work has demonstrated that treatment with anti-LFA-1 monoclonal antibody can assist in neutralizing human immunodeficiency virus infection in vitro (31) and blocks the induction of experimental autoimmune encephalomyelitis in a murine model (21). We therefore examined the effect of anti-LFA-1 treatment during primary RSV infection. Our results demonstrate that treatment with anti-LFA-1 during primary RSV infection delayed viral clearance and diminished illness. This was associated with diminished CTL activation and migration to the lungs. However, antibody responses were unaltered, resulting in sufficient memory immune responses to protect mice from subsequent RSV challenge. We conclude that anti-LFA-1 treatment during primary RSV infection in mice leads to delayed T-cell trafficking and activation, resulting in a different balance of responses used to clear virus, with the consequence of reduced immunopathology.
MATERIALS AND METHODS
Mice.
Pathogen-free, BALB/c female mice between the ages of 8 and 10 weeks were purchased from Harlan Industries (Indianapolis, Ind.) or Charles River Laboratories (Raleigh, N.C.). The mice were cared for in accordance with the Guide for the Care and Use of Laboratory Animals (42), as described previously (25). Experiments were performed with age-matched groups.
Cell lines and antibodies.
HEp-2 cells, used to determine titers of RSV in lungs, were maintained in Eagle's minimal essential medium containing 10% fetal bovine serum (10% EMEM). CTL cytolytic activity was measured using persistently RSV-infected BCH4 cells, with BC cells as uninfected controls. Both cell lines were also maintained in 10% EMEM. Hybridoma cell lines producing a monoclonal antibody directed against the CD11a subunit of murine LFA-1 (clone number M17/5.2) (47) and HLA-DR5 (HB151), an isotype control monoclonal antibody, were purchased from the American Type Culture Collection (Rockville, Md.). Monoclonal antibodies were prepared as clarified ascites fluid from hybridoma-inoculated, pristane-primed BALB/c nu/nu mice. Total protein and albumin concentrations were quantitated using a Multistat III microcentrifugal analyzer (Instrumentation Laboratory, Lexington, Mass.). Protein electrophoresis was performed with a Titan Gel high-resolution REP SP-30 kit, and the gamma globulin fraction was determined by densitometry with an electrophoresis data center (Helena Laboratories, Beaumont, Tex.). Ascites fluid was diluted in phosphate-buffered saline to 1 μg of immunoglobulin/μl before injection. All cell lines were supplemented with 2 mM glutamine, 10 U of penicillin G per ml, and 10 μg of streptomycin sulfate per ml and were determined to be free of mycoplasma contamination by analysis with the PCR (American Type Culture Collection).
Virus infection.
The RSV challenge stock was derived from the A2 strain of RSV by sonication of HEp-2 monolayers as previously described (25). Mice were anesthetized intramuscularly with ketamine (40 μg/g of body weight) and xylazine (6 μg/g of body weight) prior to intranasal inoculation with 107 PFU of live RSV in 100 μl of 10% EMEM. In most experiments, mice received intraperitoneal injections of 200 μg of either isotype control or anti-LFA-1 on days −1, +1, and +4 relative to RSV infection. In experiments to examine the kinetics of the CTL response, another group of mice received an additional anti-LFA-1 injection on day 7 postinfection. Treatment was administered on days 5, 7, and 10 postinfection in experiments designed to examine the effect of a late treatment regimen during primary RSV infection. Mice were weighed daily after infection, and clinical illness was graded blindly each day. Clinical illness was scored as follows: 0, mice showed no apparent illness; 1, mice had slightly ruffled fur; 2, mice had ruffled fur but were active; 3, mice had ruffled fur and were inactive; 4, mice had ruffled fur and hunched postures and were inactive and gaunt; 5, mice were dead.
Plaque assays.
Mice were sacrificed and lung tissue was removed and quick-frozen in 10% EMEM. Thawed tissues were kept chilled while individually ground. Dilutions of clarified supernatant were inoculated onto 80% confluent HEp-2 cell monolayers in triplicate and overlaid with 0.75% methylcellulose in 10% EMEM. After incubation for 4 days at 37°C, the monolayers were fixed with 10% buffered formalin and stained with hematoxylin and eosin. Plaques were counted and expressed as log10 PFU per gram of tissue.
Synthetic peptides.
Peptides synthesized by Biosynthesis (Lewisville, Tex.) included RSV amino acids (aa) 82 to 90 (SYIGSINNI), derived from the M2 protein of the RSV A2 strain, and influenza virus nucleoprotein aa 147 to 155 (TYQRTRALV), derived from the influenza virus A/Puerto Rico/8/34 nucleoprotein (36). Both peptides are H-2Kd restricted.
Cytotoxicity assays.
Mice were sacrificed and lungs were harvested on day 8 postinfection. Lymphocytes were manually isolated by mashing lung tissue between the frosted ends of two sterile glass microscope slides in RPMI medium containing 10% fetal bovine serum. Lymphocytes were isolated by centrifugation on a cushion of Ficoll-Hypaque at room temperature, washed twice, and resuspended in 10% RPMI medium. BC and BCH4 cells were used as target cells, and the assay was performed as previously described (4). Specific release of 51Cr from target cells was defined as follows: 100 × (sample cpm − background cpm)/(total cpm − background cpm), where cpm is number of counts per minute.
ICS and flow cytometry.
Mice were sacrificed and lungs were harvested at days 6, 8, 10, 12, and 14 postinfection. Lymphocytes were isolated as described for cytotoxicity assays, and intracellular cytokine staining (ICS) was performed as previously described (5). Cells were stained with fluorescein isothiocyanate-anti-mouse CD4 (clone GK1.5), allophycocyanin-anti-mouse CD8 (clone 53-6.7), PerCP-anti-mouse CD3 (clone 145-2C11), and phycoerythrin-anti-mouse IFN-γ (clone XMG1.2) (all monoclonal antibodies from BD Pharmingen, San Diego, Calif.) for 30 min at 4°C. Flow cytometry was performed with a FACSCaliber (Becton Dickinson, San Jose, Calif.) argon ion laser at 15 mW and 488 nm. Data were analyzed by using FlowJo version 3.6.1 (Tree Star, San Carlos, Calif.).
BrdU experiments.
RSV-infected mice were treated with 200 μg of isotype control or anti-LFA-1 antibody on days −1, +1, and +4 relative to the time of infection. Lungs were isolated from RSV-infected mice at days 8 and 12 postinfection, and lymphocytes were isolated as described for cytotoxicity assays. The procedure was performed with a 5-bromo-2′-deoxyuridine (BrdU) flow kit according to the protocol of the manufacturer (BD Pharmingen). Lymphocytes were stained with fluorescein isothiocyanate-anti-BrdU, PerCP-anti-mouse CD3, and allophycocyanin-anti-mouse CD8.
Proliferation experiments.
Spleens were harvested from mice that had been previously immunized with RSV. Lymphocytes were isolated on a Ficoll gradient as described above. Lymphocytes were then labeled with 0.5 μM 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, Oreg.) at 37°C for 15 min. After the incubation, the lymphocytes were washed twice in phosphate-buffered saline containing 5% fetal bovine serum to sequester any free CFSE that had failed to diffuse into the cells. The lymphocytes were then resuspended in 10% RPMI medium in six-well plates and supplemented with 1 mg of anti-CD28 and anti-CD49d antibodies/ml, 10 ng of interleukin-2 (IL-2), which was replenished late during day 2, and 4 μg of the RSV M2 peptide (aa 82 to 90). One group of cells was treated with 500 μg of isotype control antibody, whereas the second group was treated with 500 μg of anti-LFA-1. Cells were incubated at 37°C for 5 days and were then analyzed by flow cytometry. Cells were stimulated with the aforementioned flu virus peptide (nucleoprotein aa 147 to 155) as a negative control.
Lung histopathology.
Mice were sacrificed 8 days postinfection, and the left lungs were inflated with 0.2 to 0.3 ml of 10% formalin. The formalin-fixed lungs were paraffin embedded, and thin sections were cut and stained with hematoxylin and eosin. Slides were viewed with a Zeiss Axioplan light microscope at a magnification of ×40 under oil immersion.
RPA for detection and quantitation of mRNA species.
Mice were sacrificed 4 days after infection, and lungs were quick-frozen in liquid nitrogen. Total RNA was isolated, and RNase protection assays (RPAs) were performed as previously described (33) using the Pharmingen RiboQuant mCK-1 and mCK-2b template sets. These templates include IL-1α, IL-1β, IL-1 receptor antagonist, IL-6, IL-10, IL-12p35, IL-12p40, IFN-γ, and IFN-γ-inducing factor.
ELISAs.
IFN-γ production was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R & D Systems, Minneapolis, Minn.). Briefly, 50 μl of supernatant from ground lungs of RSV-infected mice was thawed and added to precoated 96-well microtiter plates. Peroxidase-labeled anti-cytokine antibody was added to detect bound cytokine, and the plates were developed by addition of tetramethylbenzidene substrate. Separate ELISAs were performed to quantitate the isotypes and titers of RSV F protein-specific antibodies. Wells were coated with purified RSV F protein (a gift from Wyeth-Lederle-Praxis, Pearl River, N.Y.), and the assay was performed as previously described for RSV G protein-specific antibodies (34).
Statistical analysis.
Data from individual mouse experiments were maintained in a Paradox database. Statistical analysis was performed by transferring data from the database into the SAS (Chapel Hill, N.C.) statistical software program and performing analysis of variance by using Kruskal-Wallis and Wilcoxon rank serum tests. Values of P of <0.05 were considered statistically significant.
RESULTS
Illness is reduced in mice treated with anti-LFA-1.
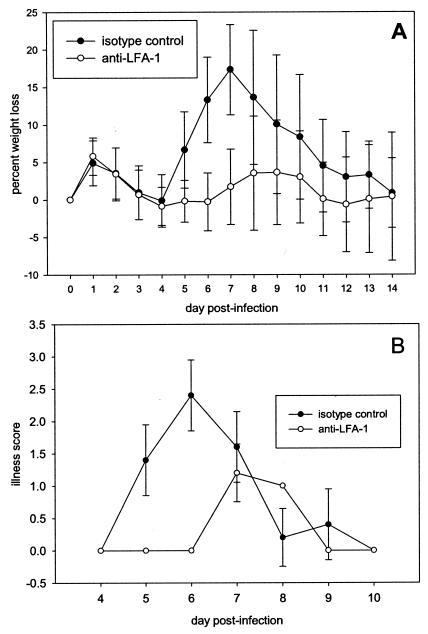
BALB/c mice received 200 μg of an isotype control antibody or anti-LFA-1 antibody intraperitoneally at days −1, +1, and +4 relative to the time of RSV infection. On day 0, mice were infected intranasally with 107 PFU of RSV in 100 μl of 10% EMEM. Mice treated with the isotype control exhibited a typical pattern of RSV-induced illness. Peak weight loss was about 18% of original weight on day 7 postinfection (Fig. 1A). In contrast, peak weight loss in mice that received anti-LFA-1 treatment was only 4% of original weight on days 8 to 9 postinfection. Clinical illness score patterns paralleled the weight loss data in both groups (Fig. 1B). The peak illness occurred earlier in isotype control-treated mice and was more severe. These illness profiles suggest that anti-LFA-1 treatment offers some protection against RSV-induced illness.
FIG. 1.
Illness profiles of mice treated with anti-LFA-1 during primary RSV infection. Mice were infected intranasally with 107 PFU of RSV on day 0. On days −1, +1, and +4 relative to infection, 200 μg of isotype control antibody or anti-LFA-1 antibody was administered to mice intraperitoneally. (A) Combined data representing the mean percentage of weight loss ± the standard deviation for each group from five experiments (n, 30 through day 10 and 15 through day 14). (B) Illness scores for the same mice.
Viral clearance from the lungs is delayed during primary RSV infection in anti-LFA-1-treated mice.
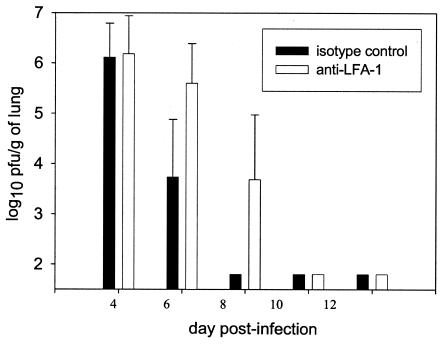
RSV titers were measured in the lungs on days 4, 6, 8, 10, and 12 postinfection (Fig. 2). In the lungs, viral titers on day 4 showed no significant difference between the control group and the anti-LFA-1-treated group (P > 0.05). On days 6 and 8 postinfection, mice treated with anti-LFA-1 retained significantly more virus than the isotype control-treated mice (P < 0.05). By day 8, RSV could not be detected in the lungs of isotype control-treated mice but was still present in the lungs of anti-LFA-1-treated mice. By day 10 postinfection, virus had been cleared in both groups. These data indicate that treatment with anti-LFA-1 results in a delay in viral clearance.
FIG. 2.
RSV titers in lungs of anti-LFA-1-treated mice. RSV-infected mice were treated with isotype control or anti-LFA-1 antibody as previously described. On days 4, 6, 8, 10, and 12 postinfection, lungs were harvested from RSV-infected mice and viral titers were measured by standard plaque assay on HEp-2 monolayers at 80% confluency. The data are a combination of results from five experiments and are expressed as the log10 PFU per gram of lung ± the standard deviation. Fifteen mice are represented at each time point. The limit of detection is 1.8 log10 PFU/g. For days 6 and 8, P is <0.05.
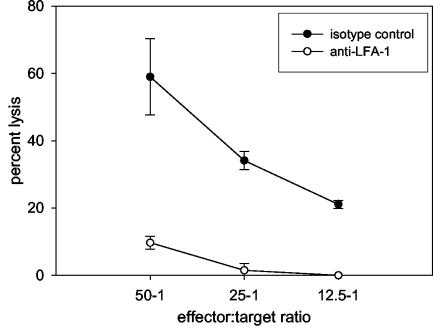
Anti-LFA-1 therapy impairs CTL-mediated killing.
Since anti-LFA-1 treatment caused a delay in viral clearance, we reasoned that the cytolytic activity of CTLs was impaired in these mice. To this end, lungs were harvested on day 8 postinfection from RSV-infected mice that received 200 μg of either isotype control or anti-LFA-1 antibody at days −1, +1, and +4 relative to the time of RSV infection. We chose to examine the cytolytic effector function of virus-specific CTLs on day 8 because that is the day of peak CD8+ T cell-mediated cytolytic activity in response to primary infection. Cytolytic activity of these lymphocytes was measured by incubation with a persistently RSV-infected mouse fibroblast cell line, BCH4, in a direct 51Cr release assay (Fig. 3). A high percentage of RSV-specific CTL activity was observed in isotype control-treated mice. In contrast, lymphocytes from mice treated with anti-LFA-1 demonstrated a sixfold lower effector activity at the same dilution. No specific lysis was observed when CTLs from either group were incubated with an uninfected fibroblast cell line. From this result, we conclude that anti-LFA-1 treatment significantly impairs the lytic activity of RSV-specific CTLs.
FIG. 3.
RSV-specific cytolytic activity. RSV-infected mice were treated with isotype control or anti-LFA-1 antibody as previously described. On day 8 postinfection, lungs were harvested to determine the cytolytic activity of lung lymphocytes by the 51Cr release assay. The data are from a single experiment and are representative of results from two experiments.
Anti-LFA-1 treatment delays the kinetics of the CTL response during primary RSV infection.
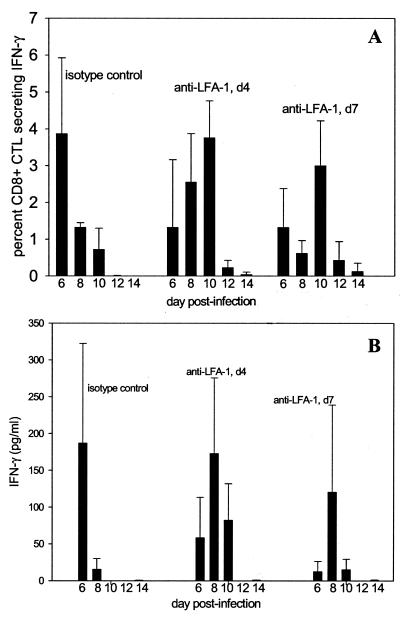
The binding of LFA-1 to ICAM-1 facilitates lymphocyte migration, as well as adhesion and costimulation during antigen presentation. Since our previous data indicated a delay in the immune response during anti-LFA-1 treatment, we hypothesized that extending the treatment with anti-LFA-1 would further delay the RSV-specific immune response. To test this, we produced a primary RSV infection in three additional groups of mice. Two independent groups of mice were treated with either the isotype control or anti-LFA-1 at days −1, +1, and +4, exactly as in our previous experiments. In addition, we administered anti-LFA-1 antibody to a third group of mice on days −1, +1, +4, and +7. Mice were sacrificed at days 6, 8, 10, and 12, and the right lungs were used in ICS for IFN-γ to define the effect of LFA-1 on CTL function during primary RSV infection.
Prolonged treatment with anti-LFA-1 further delayed detection of functional CTLs at the site of infection, as evidenced by a reduction in the percentage of IFN-γ-producing CD8+ T cells (Fig. 4A). In isotype control-treated mice, peak CTL activation was observed on day 6 postinfection, when approximately 5% of CD8+ T cells produced IFN-γ. IFN-γ production by CD8+ T cells then steadily declined for the duration of the experiment, indicative of a normal pattern of CTL activation during RSV infection. In mice that received anti-LFA-1 through day 4, CTL production of IFN-γ was delayed, steadily increasing until it peaked on day 10. A similar pattern of delayed IFN-γ production was seen in mice that received anti-LFA-1 through day 7. When we examined IFN-γ production by cytokine ELISA of lung supernatants from these mice, we observed the same trends (Fig. 4B). The peaks of IFN-γ production were therefore not only delayed but also reduced in both anti-LFA-1-treated groups, with production slightly more depressed in the mice that received prolonged anti-LFA-1 treatment. These results suggest that anti-LFA-1 treatment delays the appearance of functionally active CD8+ T cells responding to the viral infection.
FIG. 4.
Kinetics of IFN-γ production by CD8+ T cells in anti-LFA-treated mice. Mice were treated with isotype control antibody or anti-LFA-1 antibody on days −1, +1, and +4 (d4) relative to RSV infection. A third group of mice received an additional anti-LFA-1 injection on day 7 postinfection (d7). (A) Intracellular IFN-γ staining was used to quantitate the percentage of CD8+ T cells secreting IFN-γ. Uninfected mice exhibited no significant levels of IFN-γ production. (B) ELISAs were performed using lung supernatants to determine the concentration of total IFN-γ in the lungs of RSV-infected mice. The results shown are from a single experiment and are representative of results from five experiments.
Anti-LFA-1 treatment impairs lymphocyte activation.
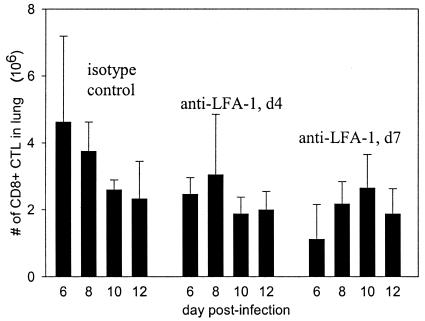
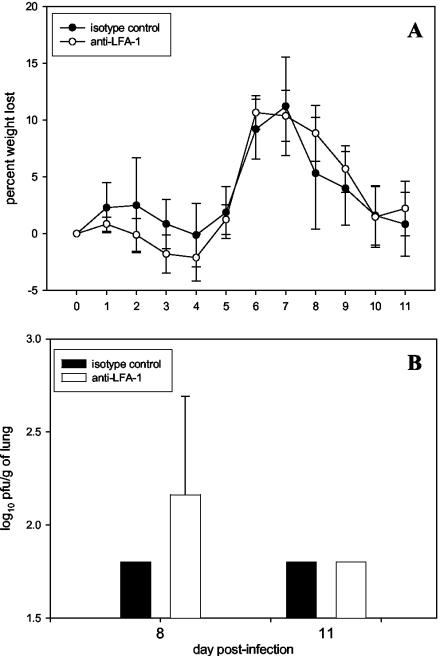
LFA-1 is known to contribute to lymphocyte migration as well as play a potential role in activation. For this reason, we chose to examine the effect of anti-LFA-1 treatment on the pulmonary lymphocytic infiltrate during RSV infection. The absolute number of CD8+ T cells was lower in the lungs of anti-LFA-1-treated mice at the earliest time points (Fig. 5), but by the conclusion of the experiment, CD8+-T-cell numbers were equivalent to those in isotype control-treated mice. These data suggested that anti-LFA-1 treatment might also be inhibiting CD8+-T-cell migration to the lungs. However, delayed activation of CD8+ T cells in the lymph nodes would also preclude their migration into the periphery in search of infected target cells. Consequently, a significant impairment in activation would similarly result in reduced lymphocyte numbers in the lungs. In addition, a late schedule of anti-LFA-1 treatment on days 5, 7, and 10 postinfection had no effect on illness, viral clearance (Fig. 6), or cytolytic activity. In addition, no significant difference in lung pathology or degree of inflammatory infiltrate was noted in mice starting treatment on day 5 (data not shown). Treatment on day 5 begins after activation has been initiated but before activated CTLs begin to infiltrate the lungs. The results of this experiment strongly argue against anti-LFA-1 treatment causing a significant effect on lymphocyte migration. We therefore focused on the role of impaired activation as the potential mechanism for the effect.
FIG. 5.
Lymphocyte numbers in lungs of anti-LFA-1-treated mice. The absolute number of CD8+ T lymphocytes in the lungs of the mice used for the experiments described in the legend to Fig. 4 was determined by flow cytometry. The results shown are from a single experiment and are representative of results from five experiments.
FIG. 6.
Illness and virus clearance in mice with delayed anti-LFA-1 treatment during primary RSV infection. Mice were infected as previously described. On days +5, +7, and +10 relative to infection, 200 μg of isotype control antibody or anti-LFA-1 antibody was administered to mice intraperitoneally. (A) Data representing the mean percent weight loss ± the standard deviation for six mice. (B) Virus titers are represented as the log10 PFU per gram of lung ± the standard deviation for mice that were subjected to the same treatment regimen as those for which results are shown in panel A. Five mice are represented at each time point. The limit of detection is 1.8 log10 PFU/g. For day 8, P is >0.05.
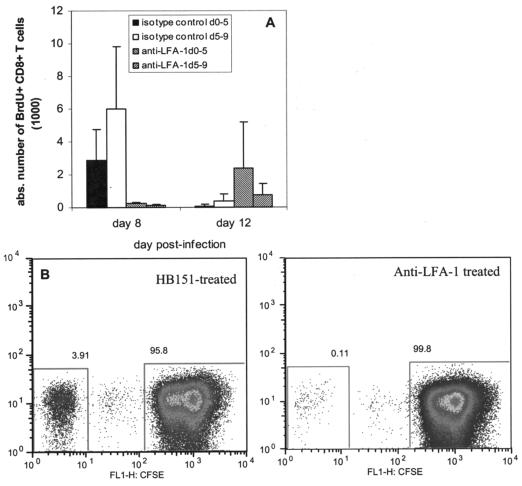
The impact of anti-LFA-1 treatment on lymphocyte proliferation was evaluated both in vivo and in vitro. First, we treated mice with BrdU to examine lymphocyte proliferation during anti-LFA-1 treatment in the context of primary RSV infection. We used two groups each of isotype control-treated mice and anti-LFA-1-treated mice. The first pair of groups received a single daily intraperitoneal injection of 100 μg of BrdU on days 0 to 5 postinfection, and the second pair of groups received BrdU on days 5 to 9. The staggering of the BrdU treatments was performed to determine the timing of CTL activation and expansion. All mice received 200 μg of either isotype control or anti-LFA-1 antibody at days −1, +1, and +4 relative to the time of RSV infection. We observed that proliferation of CD8+ T cells occurred earlier in the isotype control-treated mice (Fig. 7A). Isotype control-treated mice were characterized by a dramatic reduction in proliferation between days 8 and 12. In contrast, proliferation of CD8+ T cells increased in anti-LFA-1-treated mice between days 8 and 12. Moreover, the level of proliferation in anti-LFA-1-treated mice was not as robust as that observed in isotype control-treated mice. These data indicate that the administration of anti-LFA-1 to RSV-infected mice resulted in a delayed and inefficient activation and expansion of CD8+ T lymphocytes.
FIG. 7.
Lymphocyte proliferation during anti-LFA-1 treatment. (A) RSV-infected mice were treated with either isotype control antibody or anti-LFA-1 antibody as previously described. One set of six mice from each group was then treated intraperitoneally with 100 μg of BrdU on days 0 to 5 postinfection. A second set of six mice received BrdU at days 5 to 9. Lungs were harvested on days 8 and 12 postinfection from three mice in each set. The absolute (abs.) numbers of BrdU-labeled CD8+ T cells in the lungs on both days were determined by flow cytometry. (B) Lymphocytes were isolated from the spleens of RSV-immune BALB/c mice. The cells were labeled with 0.5 μM CFSE and treated with 500 μg of either isotype control antibody or anti-LFA-1 antibody. The cells were incubated for 5 days at 37°C in the presence of costimulatory antibodies, IL-2, and the RSV M2 peptide comprising aa 82 to 90. The data are representative of results from two experiments (n, six in each group). Maximum proliferation was 4.13% ± 2.26% in isotype control-treated mice and 0.07% ± 0.09% in anti-LFA-1-treated mice (P = 0.0013). FL1-H, fluorescence intensity of CFSE.
To better understand the timing of the anti-LFA-1 effect, we performed additional in vitro studies. We isolated lymphocytes from the spleens of RSV-immune BALB/c mice, labeled them with CFSE, and incubated them in the presence of 500 μg of either the isotype control antibody or anti-LFA-1. The lymphocytes were also supplemented with costimulatory antibodies, IL-2, and the immunodominant RSV M2 epitope for 5 days at 37°C. When we examined the cells by flow cytometry, it was very clear that anti-LFA-1 compromised lymphocyte proliferation (Fig. 7B). Isotype control-treated lymphocytes that were stimulated with the RSV peptide exhibited a maximum proliferation of 4.13% ± 2.26%, as determined by CFSE dilution. In contrast, proliferation of anti-LFA-1-treated lymphocytes was only 0.07% ± 0.09%. The cumulative results of these experiments confirm that anti-LFA-1 treatment impairs lymphocyte activation by interfering with an early step of the antigen presentation process.
Lung pathology in mice treated with anti-LFA-1 is similar to that in control mice.
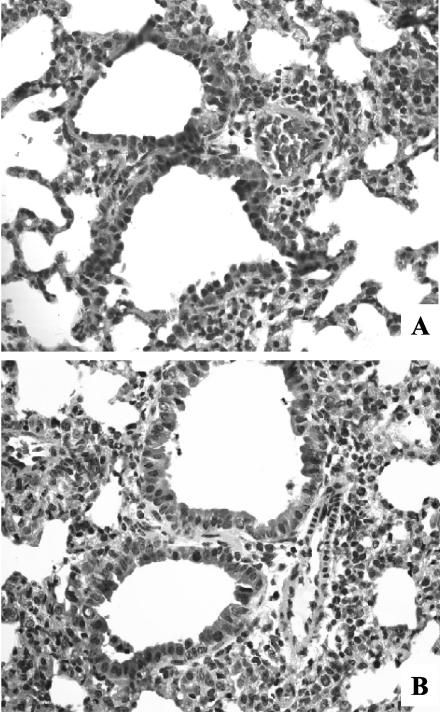
Since a significant portion of the illness observed during primary RSV infection is immune mediated, anti-LFA-1 has the effect of reducing illness, as shown in Fig. 1. However, it also leads to delayed clearance of the virus. We therefore examined pathology in the lungs of isotype control (Fig. 8A) - and anti-LFA-1 (Fig. 8B)-treated mice on day 8 postinfection, the day of peak cytolytic activity. The overall level of perivascular and peribronchiolar infiltration was similar in anti-LFA-1-treated mice and isotype-treated controls. However, the anti-LFA-1-treated mice had a more diverse cell population in the infiltrate that included a higher frequency of macrophages and polymorphonuclear leukocytes. The composition of the infiltrate in the isotype-treated controls was more uniform, with a higher frequency of lymphocytes. This difference in the composition of the infiltrate may reflect a compensatory response to the lack of effective RSV-specific CTLs in the lungs of anti-LFA-1-treated mice.
FIG. 8.
Lung histopathology of anti-LFA-1-treated mice. RSV-infected mice received 200 μg of either isotype control or anti-LFA-1 antibody on days −1, +1, and +4 relative to the time of primary RSV infection. On day 8 postinfection, the left lung was harvested and lung histopathology was evaluated by staining formalin-fixed lung sections with hematoxylin and eosin. Photographs were taken at a magnification of ×40 under oil immersion. (A) Single lung representative of four from isotype control-treated mice. (B) Single lung representative of five from anti-LFA-1-treated mice.
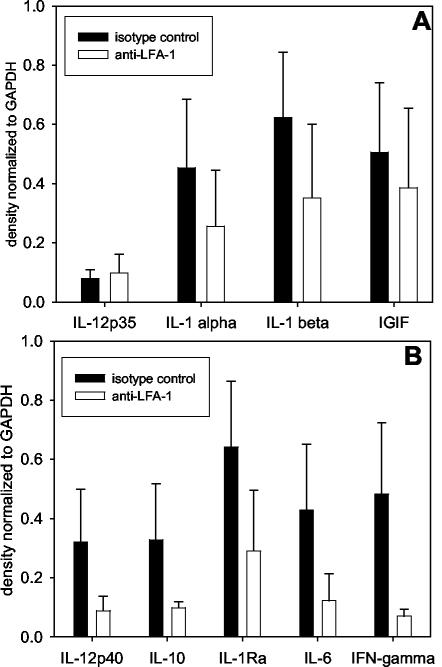
Anti-LFA-1 treatment leads to reduced cytokine mRNA levels in the lungs of RSV-infected mice.
The kinetics of viral clearance and CTL induction may be influenced by cytokine expression. For this reason, we performed RPAs to determine cytokine mRNA levels in the lungs of isotype control- and anti-LFA-1-treated mice following primary RSV infection. We examined mRNA production of the cytokines included in the RiboQuant mCK-1 and mCK-2b template sets from BD Pharmingen. No significant differences (P > 0.05) between the groups were detected in levels of IL-1α, IL-1β, IL-12p35, and IFN-γ-inducing factor (Fig. 9A). However, administration of anti-LFA-1 to RSV-infected mice did result in significant reductions (P < 0.05) in levels of IL-1 receptor antagonist, IL-6, IL-10, IL-12p40, and IFN-γ mRNA (Fig. 9B). Interestingly, the significant reductions were seen in cytokines that are largely produced by antigen-presenting cells (APC) and lymphocytes after their reciprocal activation following interaction through the immunological synapse. This observation supports the concept that blocking LFA-1 function negatively impacts T lymphocytes by inhibiting the initial APC-T lymphocyte interaction required for activating the expansion and cytolytic function of CD8+ CTLs.
FIG. 9.
Cytokine mRNA levels after RSV infection of anti-LFA-1-treated mice. RSV-infected mice were treated with isotype control or anti-LFA-1 antibody as previously described. Four days postinfection, induction of cytokine mRNA was examined by RPA by using radiolabeled riboprobes. The data are represented as results of densitometric analysis of RPA radiographs, with cytokine mRNA levels normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) levels (mean ± standard error of the mean). Five mice per group were used. (A) P, >0.05 for all mRNA levels shown. IGIF, IFN-γ-inducing factor. (B) P, <0.05 for all mRNA levels shown.
The RSV-specific antibody response is unaltered in anti-LFA-1-treated mice.
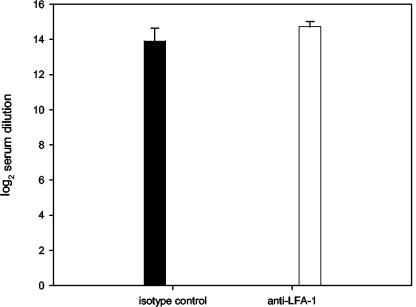
We next examined the RSV-specific antibody response to primary RSV infection in mice treated with anti-LFA-1. To accomplish this, sera of mice obtained 4 weeks after primary infection were evaluated by measuring the total immunoglobulin to the fusion protein of RSV (F). The titers of F-specific immunoglobulin G antibody after primary infection were similar in both groups (Fig. 10). In control mice, the titer was measured as a 14.7 ± 0.29 log2 reciprocal serum dilution, whereas the titer of mice treated with anti-LFA-1 was a 13.9 ± 0.74 log2 reciprocal serum dilution. The similarities in antibody titers after infection indicate that anti-LFA-1 treatment does not affect the humoral immune response to primary RSV infection. Furthermore, when these findings are combined with our other data, it appears that LFA-1 is more important for major histocompatibility complex class I costimulation than for major histocompatibility complex class II stimulation during primary RSV infection and has a more profound effect on CD8+ T cell-mediated events than on CD4+-T-cell function.
FIG. 10.
RSV-specific antibody titers. RSV-infected mice were treated with isotype control or anti-LFA-1 antibody as previously described. Serum samples were collected from RSV-infected mice on day 28 postinfection. The total amount of antibody specific for the fusion protein of RSV was determined by ELISA. Data are represented as means ± standard errors of the means of the log2 reciprocal serum dilution producing an optical density at 450 nm of 0.1 for each group. Five mice per group were used, and P was 0.768.
DISCUSSION
With this report, we provide evidence that the role of LFA-1 is important in RSV pathogenesis and that interference with LFA-1 interactions during the initial phases of virus infection can delay the CD8+-T-cell response. It has been shown in the murine model that RSV disease is mediated by CD8+ T cells (22, 23, 25). For this reason, we concentrated our efforts on the CD8+-T-cell response, although many different cells express LFA-1. Our present study shows that RSV disease was significantly reduced when infected mice were treated with anti-LFA-1 (Fig. 1). Anti-LFA-1 treatment also caused a delay in viral clearance (Fig. 2) during RSV infection. An impaired CD8+-CTL response was demonstrated by weakened killing activity as measured in 51Cr release assays (Fig. 3), diminished IFN-γ production (Fig. 4), and reduced proliferation both in vivo and in vitro (Fig. 7). Furthermore, administration of anti-LFA-1 on days 5, 7, and 10 postinfection, a regimen which began prior to the time of CTL infiltration into the lungs but well after the period of initial T-cell activation, had no statistically significant impact on illness or viral clearance (Fig. 6). Nevertheless, anti-LFA-1-treated mice were still able to establish protective immunity, as they were immune to RSV infection upon challenge (data not shown). These data lead us to conclude that anti-LFA-1 treatment interferes primarily with the process of CTL activation.
The interaction of LFA-1 with ICAM-1 is a critical event in the process of T-cell activation and effector function. Over the last decade, a significant amount of research has appeared in the literature supporting the idea that lymphocyte activation and effector function are triggered at the immunological synapse. One recent report has suggested that the formation of the immunological synapse does not occur in vivo (28). This study showed that naïve T cells and dendritic cells fail to form immunological synapses in vitro in collagen gels, which were intended to recreate the environment of the peripheral organs. However, Dustin and de Fougerolles argue that since activation of naïve T cells by APC occurs in the secondary lymphoid tissues, where collagen fibers are sequestered in reticular fibers, this interference with immunological synapse formation is not a significant problem (19). Furthermore, a recent study has provided high-resolution microscopic evidence that synapse formation does occur in vivo (41). While our data do not address the issue of the existence of immunological synapse formation in vivo, they demonstrate that disruption of LFA-1 function delayed T-cell activation in a well-established murine model of viral infection and support the validity of an immunological synapse in vivo.
A large body of literature addresses adhesion molecule function during RSV infection. This work has clearly demonstrated that a consequence of RSV infection is an increase in ICAM-1 mRNA levels and surface expression on epithelial cells and neutrophils (2, 3, 40, 44, 52, 56, 57). Another report has further shown that ICAM-1 mRNA levels are increased in RSV-infected epithelial cells via NF-κB and C/EBP activation (13). In addition, one study has provided evidence that the increased ICAM-1 expression is the result of an autocrine mechanism of IL-1α secretion by RSV-infected epithelial cells (45). Production of IL-1α/β and tumor necrosis factor alpha leads to a subsequent release of IL-8 during the inflammatory immune response, and increases in IL-8 production by epithelial cells and mononuclear phagocytes have been demonstrated during RSV infection (1, 2, 8, 43). These consequences of the inflammatory response to RSV are likely to facilitate T-cell chemotaxis and activation via the upregulation of adhesion molecules such as ICAM-1, which is the primary ligand for LFA-1 (7, 37, 51). These facts indicate the LFA-1 is an important component of T-cell activity at multiple points over the course of an immune response. We recognize that anti-LFA-1 treatment may, therefore, influence T-cell activity at multiple stages of the immune response and that these effects may not occur independently of one another. However, when we administered a late treatment regimen of anti-LFA-1 at days 5, 7, and 10 post-RSV infection, no statistically significant impact on virus clearance, illness (Fig. 6), or pathology was observed. It is important to note that in this scenario, anti-LFA-1 treatment begins after CTL activation has been initiated but prior to CTL infiltration into the lungs. Our data suggest that the primary effect of anti-LFA-1 treatment is interference with lymphocyte activation, but a minor impact on lymphocyte migration cannot be excluded.
The findings presented in this report demonstrate that the treatment of mice with neutralizing antibodies to LFA-1 during primary RSV infection resulted in diminished illness and delayed viral clearance. We hypothesize that our observations stem from a disturbance of the early immune response to RSV. Specifically, we propose that neutralization of LFA-1 function inhibits the activation of CTLs and the induction of other cytolytic functions at the level of antigen presentation and CD8+-T-cell activation. This work has clear implications for the development of immunotherapeutic strategies that could be combined with new antivirals for the treatment of RSV-induced disease.
Acknowledgments
We thank Joyce Johnson and Lewis McCurdy for assistance with the histopathology preparation and analysis and Amanda K. Johnson and Nancy Barrett for technical assistance.
This work was supported in part by NIH grant RO1-AI-33933.
REFERENCES
- 1.Arnold, R., B. Humbert, H. Werchau, H. Gallati, and W. Konig. 1994. Interleukin-8, interleukin-6, and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology 82:126-133. [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold, R., and W. Konig. 1996. ICAM-1 expression and low-molecular-weight G-protein activation of human bronchial epithelial cells (A549) infected with RSV. J. Leukoc. Biol. 60:766-771. [DOI] [PubMed] [Google Scholar]
- 3.Arnold, R., H. Werchau, and W. Konig. 1995. Expression of adhesion molecules (ICAM-1, LFA-3) on human epithelial cells (A549) after respiratory syncytial virus infection. Int. Arch. Allergy Immunol. 107:392-393. [DOI] [PubMed] [Google Scholar]
- 4.Aung, S., J. A. Rutigliano, and B. S. Graham. 2001. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease. J. Virol. 75:9918-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aung, S., Y. W. Tang, and B. S. Graham. 1999. Interleukin-4 diminishes CD8+ respiratory syncytial virus-specific cytotoxic T-lymphocyte activity in vivo. J. Virol. 73:8944-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmann, M. F., K. McKall-Faienza, R. Schmits, D. Bouchard, J. Beach, D. E. Speiser, T. W. Mak, and P. S. Ohashi. 1997. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity 7:549-557. [DOI] [PubMed] [Google Scholar]
- 7.Baggiolini, M., and I. Clark-Lewis. 1992. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 307:97-101. [DOI] [PubMed] [Google Scholar]
- 8.Becker, S., J. Quay, and J. Soukup. 1991. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol. 147:4307-4312. [PubMed] [Google Scholar]
- 9.Brandenburg, A. H., A. KleinJan, L. B. van Het, H. A. Moll, H. H. Timmerman, R. L. de Swart, H. J. Neijens, W. Fokkens, and A. D. Osterhaus. 2000. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J. Med. Virol. 62:267-277. [PubMed] [Google Scholar]
- 10.Cai, Z., A. Brunmark, M. R. Jackson, D. Loh, P. A. Peterson, and J. Sprent. 1996. Transfected Drosophila cells as a probe for defining the minimal requirements for stimulating unprimed CD8+ T cells. Proc. Natl. Acad. Sci. USA 93:14736-14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai, Z., H. Kishimoto, A. Brunmark, M. R. Jackson, P. A. Peterson, and J. Sprent. 1997. Requirements for peptide-induced T cell receptor downregulation on naive CD8+ T cells. J. Exp. Med. 185:641-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon, M. J., P. J. Openshaw, and B. A. Askonas. 1988. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 168:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chini, B. A., M. A. Fiedler, L. Milligan, T. Hopkins, and J. M. Stark. 1998. Essential roles of NF-kappaB and C/EBP in the regulation of intercellular adhesion molecule-1 after respiratory syncytial virus infection of human respiratory epithelial cell cultures. J. Virol. 72:1623-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1486. In D. M. Knipe, P. M. Howley, and D. Griffin (ed.), Fields virology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 15.Davignon, D., E. Martz, T. Reynolds, K. Kurzinger, and T. A. Springer. 1981. Monoclonal antibody to a novel lymphocyte function-associated antigen (LFA-1): mechanism of blockade of T lymphocyte-mediated killing and effects on other T and B lymphocyte functions. J. Immunol. 127:590-595. [PubMed] [Google Scholar]
- 16.Dustin, M. L. 2002. Membrane domains and the immunological synapse: keeping T cells resting and ready. J. Clin. Investig. 109:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dustin, M. L., and A. C. Chan. 2000. Signaling takes shape in the immune system. Cell 103:283-294. [DOI] [PubMed] [Google Scholar]
- 18.Dustin, M. L., and J. A. Cooper. 2000. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1:23-29. [DOI] [PubMed] [Google Scholar]
- 19.Dustin, M. L., and A. R. de Fougerolles. 2001. Reprogramming T cells: the role of extracellular matrix in coordination of T cell activation and migration. Curr. Opin. Immunol. 13:286-290. [DOI] [PubMed] [Google Scholar]
- 20.Falsey, A. R., and E. E. Walsh. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13:371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon, E. J., K. J. Myers, J. P. Dougherty, H. Rosen, and Y. Ron. 1995. Both anti-CD11a (LFA-1) and anti-CD11b (MAC-1) therapy delay the onset and diminish the severity of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 62:153-160. [DOI] [PubMed] [Google Scholar]
- 22.Graham, B. S. 1996. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 4:290-293. [DOI] [PubMed] [Google Scholar]
- 23.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham, B. S., G. S. Henderson, Y. W. Tang, X. Lu, K. M. Neuzil, and D. G. Colley. 1993. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J. Immunol. 151:2032-2040. [PubMed] [Google Scholar]
- 25.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 26.Graham, B. S., J. A. Rutigliano, and T. R. Johnson. 2002. Respiratory syncytial virus immunobiology and pathogenesis. Virology 297:1-7. [DOI] [PubMed] [Google Scholar]
- 27.Greve, J. M., G. Davis, A. M. Meyer, C. P. Forte, S. C. Yost, C. W. Marlor, M. E. Kamarck, and A. McClelland. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839-847. [DOI] [PubMed] [Google Scholar]
- 28.Gunzer, M., A. Schafer, S. Borgmann, S. Grabbe, K. S. Zanker, E. B. Brocker, E. Kampgen, and P. Friedl. 2000. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity 13:323-332. [DOI] [PubMed] [Google Scholar]
- 29.Hamann, A., D. Jablonski-Westrich, A. Duijvestijn, E. C. Butcher, H. Baisch, R. Harder, and H. G. Thiele. 1988. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J. Immunol. 140:693-699. [PubMed] [Google Scholar]
- 30.Hertz, M. I., J. A. Englund, D. Snover, P. B. Bitterman, and P. B. McGlave. 1989. Respiratory syncytial virus-induced acute lung injury in adult patients with bone marrow transplants: a clinical approach and review of the literature. Medicine (Baltimore) 68:269-281. [DOI] [PubMed] [Google Scholar]
- 31.Hildreth, J. E., and R. J. Orentas. 1989. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science 244:1075-1078. [DOI] [PubMed] [Google Scholar]
- 32.Issekutz, A. C., and T. B. Issekutz. 1995. Monocyte migration to arthritis in the rat utilizes both CD11/CD18 and very late activation antigen 4 integrin mechanisms. J. Exp. Med. 181:1197-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, T. R., and B. S. Graham. 1999. Secreted respiratory syncytial virus G glycoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-independent mechanism. J. Virol. 73:8485-8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson, T. R., J. E. Johnson, S. R. Roberts, G. W. Wertz, R. A. Parker, and B. S. Graham. 1998. Priming with secreted glycoprotein G of respiratory syncytial virus (RSV) augments interleukin-5 production and tissue eosinophilia after RSV challenge. J. Virol. 72:2871-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89:422-434. [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni, A. B., H. C. Morse III, J. R. Bennick, J. W. Yewdell, and B. R. Murphy. 1993. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ T cells. J. Virol. 67:4086-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard, E. J., and T. Yoshimura. 1990. Neutrophil attractant/activation protein-1 (NAP-1 [interleukin-8]). Am. J. Respir. Cell Mol. Biol. 2:479-486. [DOI] [PubMed] [Google Scholar]
- 38.Lub, M., Y. van Kooyk, and C. G. Figdor. 1995. Ins and outs of LFA-1. Immunol. Today 16:479-483. [DOI] [PubMed] [Google Scholar]
- 39.Marlin, S. D., and T. A. Springer. 1987. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell 51:813-819. [DOI] [PubMed] [Google Scholar]
- 40.Matsuzaki, Z., Y. Okamoto, N. Sarashina, E. Ito, K. Togawa, and I. Saito. 1996. Induction of intercellular adhesion molecule-1 in human nasal epithelial cells during respiratory syncytial virus infection. Immunology 88:565-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGavern, D. B., U. Christen, and M. B. A. Oldstone. 2002. Molecular anatomy of antigen-specific CD8+ T cell engagement and synapse formation in vivo. Nat. Immunol. 3:918-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Academy Press. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 43.Noah, T. L., and S. Becker. 1993. Respiratory syncytial virus-induced cytokine production by a human bronchial epithelial cell line. Am. J. Physiol. 265:L472-L478. [DOI] [PubMed] [Google Scholar]
- 44.Olszewska-Pazdrak, B., K. Pazdrak, P. L. Ogra, and R. P. Garofalo. 1998. Respiratory syncytial virus-infected pulmonary epithelial cells induce eosinophil degranulation by a CD18-mediated mechanism. J. Immunol. 160:4889-4895. [PubMed] [Google Scholar]
- 45.Patel, J. A., M. Kunimoto, T. C. Sim, R. Garofalo, T. Eliott, S. Baron, O. Ruuskanen, T. Chonmaitree, P. L. Ogra, and F. Schmalstieg. 1995. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 13:602-609. [DOI] [PubMed] [Google Scholar]
- 46.Rothlein, R., M. L. Dustin, S. D. Marlin, and T. A. Springer. 1986. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J. Immunol. 137:1270-1274. [PubMed] [Google Scholar]
- 47.Sanchez-Madrid, F., D. Davignon, E. Martz, and T. A. Springer. 1982. Antigens involved in mouse cytolytic T-lymphocyte (CTL)-mediated killing: functional screening and topographic relationship. Cell. Immunol. 73:1-11. [DOI] [PubMed] [Google Scholar]
- 48.Shay, D. K., R. C. Holman, R. D. Newman, L. L. Liu, J. W. Stout, and L. J. Anderson. 1999. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 282:1440-1446. [DOI] [PubMed] [Google Scholar]
- 49.Sligh, J. E., Jr., C. M. Ballantyne, S. S. Rich, H. K. Hawkins, C. W. Smith, A. Bradley, and A. L. Beaudet. 1993. Inflammatory and immune responses are impaired in mice deficient in intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 90:8529-8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Springer, T. A. 1990. Adhesion receptors of the immune system. Nature 346:425-434. [DOI] [PubMed] [Google Scholar]
- 51.Standiford, T. J., S. L. Kunkel, M. A. Basha, S. W. Chensue, J. P. Lynch III, G. B. Toews, J. Westwick, and R. M. Strieter. 1990. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J. Clin. Investig. 86:1945-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stark, J. M., V. Godding, J. B. Sedgwick, and W. W. Busse. 1996. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells. Roles of CD18 and intercellular adhesion molecule-1. J. Immunol. 156:4774-4782. [PubMed] [Google Scholar]
- 53.Staunton, D. E., V. J. Merluzzi, R. Rothlein, R. Barton, S. D. Marlin, and T. A. Springer. 1989. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 56:849-853. [DOI] [PubMed] [Google Scholar]
- 54.Tomassini, J. E., D. Graham, C. M. DeWitt, D. W. Lineberger, J. A. Rodkey, and R. J. Colonno. 1989. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 86:4907-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Seventer, G. A., Y. Shimizu, K. J. Horgan, and S. Shaw. 1990. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J. Immunol. 144:4579-4586. [PubMed] [Google Scholar]
- 56.Wang, S. Z., P. G. Hallsworth, K. D. Dowling, J. H. Alpers, J. J. Bowden, and K. D. Forsyth. 2000. Adhesion molecule expression on epithelial cells infected with respiratory syncytial virus. Eur. Respir. J. 15:358-366. [DOI] [PubMed] [Google Scholar]
- 57.Wang, S. Z., P. K. Smith, M. Lovejoy, J. J. Bowden, J. H. Alpers, and K. D. Forsyth. 1998. Shedding of L-selectin and PECAM-1 and upregulation of Mac-1 and ICAM-1 on neutrophils in RSV bronchiolitis. Am. J. Physiol. 275:L983-L989. [DOI] [PubMed] [Google Scholar]