Abstract
Animals constantly integrate external stimuli with their own internal physiological state to make appropriate behavioural decisions. Little is known, however, about where in the brain the salience of these signals is evaluated, or which neural and transcriptional mechanisms link this integration to adaptive behaviours. We used an African cichlid fish Astatotilapia burtoni to test the hypothesis that a new social opportunity activates the conserved ‘social behaviour network’ (SBN), a collection of brain nuclei known to regulate social behaviours across vertebrates. We measured mRNA levels of immediate early genes (IEGs) in microdissected brain regions as a proxy for neuronal activation, and discovered that IEGs were higher in all SBN nuclei in males that were given an opportunity to rise in social rank compared to control stable subordinate and dominant individuals. Further, since the presence of sex-steroid receptors is one defining criteria of SBN nuclei, we also tested whether social opportunity or status influenced androgen and oestrogen receptor mRNA levels within these same regions. There were several rapid region-specific changes in receptor mRNA levels induced by social opportunity, most notably in oestrogen receptor subtypes in areas that regulate social aggression and reproduction, suggesting that oestrogenic signalling pathways play an important role in regulating male status. Several receptor mRNA changes occurred in regions with putative homologies to the mammalian septum and extended amygdala, two regions shared by SBN and reward circuits, suggesting an important role in integration of social salience, stressors, hormonal state, and adaptive behaviours. We also show increases in plasma sex- and stress-steroids at 30-min after a rise in social rank. This rapid endocrine and transcriptional response suggests that the SBN is involved in the integration of social inputs with internal hormonal state to facilitate the transition to dominant status, which ultimately leads to improved fitness for the previously reproductively-suppressed individual.
Keywords: Astatotilapia burtoni, dominance, immediate early gene, SBN, steroid receptor, teleost
Introduction
Animals must continually integrate signals from the environment, social interactions, and their own internal physiological state to make appropriate behavioural decisions that will improve their chances for survival and reproduction. While these decisions are all mediated by the brain, little is known about the neural and transcriptional mechanisms that link the reception of sensory inputs to specific behavioural actions (1, 2). In social species that form dominance hierarchies, where only the dominant individuals typically reproduce, there are often opportunities for lower-ranking individuals to challenge higher-ranking ones and become dominant (3–6). The perception of this opportunity and acquisition of the higher status likely involves complex neural plasticity, networks and gene modules that interact collectively to produce changes that facilitate transition to the new social position (7, 8), which often includes dramatic changes in behavioural repertoire and reproductive physiology. The brain regions involved in such social transitions are not well understood, but are hypothesised to include core neural networks such as the ‘social behaviour network’ and ‘mesolimbic reward system’ that are conserved among vertebrates, which may form a larger ‘social decision-making network’ that regulates adaptive behaviour (9).
Social behaviours are thought to be coordinated by conserved neural circuits that continuously evaluate the salience of observed inputs and contexts to produce appropriate behaviours. One such network, the ‘social behaviour network’ (SBN), is a collection of six brain nuclei, or nodes (lateral septum, medial extended amygdala/bed nucleus of the stria terminalis, preoptic area, anterior hypothalamus, ventromedial hypothalamus, midbrain periaqueductal grey/tegmentum), that are all reciprocally connected and implicated in the regulation of many social behaviours including aggression, stress coping, parental care, mating and sexual behaviours, and communication (10, 11). The SBN was originally described in mammals (10), but has since been applied to reptiles and amphibians (11, 12), fishes (11, 13), and birds (11), and therefore provides an important evolutionary framework for studying the neural basis of social behaviours (5, 9, 14). Importantly, the nuclei of the SBN all express sex steroid receptors (10), suggesting that these nodes are also crucial neural substrates for integration of social signals with an animal’s own internal hormonal state. Few studies, however, have examined how social information, and/or social opportunity, might influence the expression of sex steroid receptors in these nuclei.
Many animals face different types of opportunities (e.g., rising in social rank, finding food, shelter, a territory, or a mate) throughout their lifetimes that can influence their survival and reproductive fitness (5), but how is the perception of a social opportunity translated into functional activation of appropriate neural circuitry in the brain? Immediate early genes (IEGs), such as the transcription factors egr-1 and cfos, have become useful tools for examining region-specific activation of brain nuclei in response to novel or changing social stimuli (2, 14–18). Importantly, activation of this IEG response, or ‘genomic action potential’, occurs rapidly (within minutes) and is often indicative of changes in relative neuronal activity that leads to downstream transcriptional modifications (19–21). Further, social interactions also activate the neuroendocrine system, producing a ‘neuroendocrine action potential’, which is often preceded by IEG induction and involves signalling via steroid hormones, neuropeptides, and biogenic amines to influence neural circuits, organ systems, and behaviours (22). Thus, measuring IEG expression in response to specific novel social stimuli can provide important information on which brain regions are involved in the neural circuitry that ultimately leads to behavioural decisions and adaptive phenotypic change.
The African cichlid fish Astatotilapia burtoni is an ideal model system for assessing how social opportunity activates the nodes of the SBN because males of this species have evolved two distinct and reversible phenotypes: dominant males that comprise ~10–30% of the population, are brightly-colored, defend territories, and actively court and spawn with females, and subordinate males that are more drab-colored similar to females, do not hold territories, and are reproductively suppressed (4). Importantly, there are frequent opportunities for subordinate individuals to rise in social rank and acquire a vacant territory (6, 23), and this ascent can be experimentally induced by manipulating the social environment to test specific hypotheses on the changes that occur during natural social transition. For example, a previous study in A. burtoni used in situ hybridization to show that egr-1 mRNA levels in the preoptic area, one node in the SBN, were higher in males that perceived an opportunity to ascend in social status compared to stable subordinate and dominant states (18). This response is likely due to the recognition of the social opportunity because it is not elicited in males who are already dominant and performing similar dominance behaviours. Social ascent is also associated with rapid and dramatic changes in behaviour and reproductive physiology to prepare this previously suppressed male for his new role as a reproductively-active territory holder (18, 24–28), but whether this transition also induces IEG expression in other relevant social centers of the brain, such as the SBN, is unknown. The pattern of activity in these brain regions will extend our understanding of how the brain coordinates social behaviours.
The goal of this study was to test the hypothesis that brain nuclei within the SBN are activated by a novel social opportunity, using IEG expression as a proxy for transcriptional activation. Since abundant expression of steroid receptors is one of the defining criteria of the SBN nuclei, we also tested whether mRNA expression of androgen and oestrogen receptors were influenced by social opportunity or by stable subordinate and dominant social status. We demonstrate a rapid and widespread IEG mRNA response throughout the SBN in males that were given an opportunity to rise in social rank and become dominant. We also show rapid increases in circulating sex- and stress-steroids, and region-specific differences in sex-steroid receptor subtype mRNAs associated with both perception of social opportunity and stable status. These results highlight the dramatic and rapid transcriptional and endocrine changes that can be induced solely by the perception of social opportunity. Such changes have important adaptive consequences for survival and reproductive fitness in this and presumably other vertebrate species that maintain dominance hierarchies with concomitant status transitions.
Materials and methods
Animals
Adult cichlid fish, Astatotilapia burtoni, were derived from wild-caught stock from Lake Tanganyika, Africa, and laboratory-bred in aquaria under environmental conditions that mimic their natural equatorial habitat (28°C; pH 8.0; 12h light:12h dark with full spectrum illumination; constant aeration). Aquaria contained gravel-covered bottoms with terra cotta pots cut in half to serve as shelters and spawning territories. Fish were fed cichlid pellets and flakes (AquaDine, Healdsburg, CA, USA) each morning. All experimental procedures were approved by the Stanford Administrative Panel for Laboratory Animal Care.
Social manipulation
To create an opportunity for social ascent and to generate stable subordinate and dominant animals, we used a previously described experimental paradigm (24–26). Briefly, to establish socially-suppressed fish, dominant males were placed into aquaria for 4–5 weeks with several larger dominant suppressor males, females, and subordinate males. At the end of the suppression period, subjects were moved into the central compartment of an experimental tank that contained one larger resident dominant male and 3–4 females. This central compartment was separated with transparent acrylic barriers from community tanks on either side that contained smaller males (dominant and subordinate) and females of various reproductive states so that fish could interact visually but not physically with community animals.
Suppressed subject males remained in the abovementioned experimental tank for 2 days and were verified by behavioural observations to remain subordinate to the larger resident male. On the morning of ascent, the larger resident suppressor male was removed with a net 1 hr prior to light onset using infrared night vision goggles (Bushnell night vision, Model 26–1020), providing an opportunity for the suppressed subject male to occupy the vacant territory and rise to dominant status at light onset. These ascending males (standard length, SL: 58.8±2.9 mm; body mass, BM: 5.3±0.7 g; n=12) were then killed at 30 min after ascent. Time of ascent was defined as the time between light onset and the time when subject males performed dominance behaviours (combined territorial and reproductive) at a rate of 3 behaviours min−1 as described previously (24). Mean latency to ascend was 13.5±1.5 min, thus ascending males were killed at an average of 43.5 min after light onset.
Stable dominant (SL, 58.3±4.3 mm; BM, 5.8±1.4 g; n=12) and stable subordinate (SL, 57.5±3.1 mm; BM, 5.2±1.3 g; n=12) males were used as controls in comparison to males ascending in social status. Stable subordinate males were suppressed in community tanks for 4–5 weeks and transferred to the same experimental tank as described above. On the day of ascent, however, removal of the suppressor dominant male was only simulated by dipping a net into the tank prior to light onset without removal of the dominant resident. Stable dominants were dominant males that maintained their status in community tanks for 4–5 weeks, and were then placed in the experimental tank with females but no larger resident male, which maintains their dominance status. On the stimulus day, a net was dipped into the water prior to light onset to simulate resident male removal. Stable dominant animals were all killed on the simulated stimulus day at 30 min after they displayed 3 dominance behaviours min−1 as described above for ascending males (mean latency, 6.3±1.1 min; mean sample time, 36.3 min after light onset), while stable subordinates were sampled at a time after light onset that was matched to the sampling times of ascending and stable dominant animals. Thus, animals from all three social groups were sampled during an equivalent time window after light onset (~35–45 min), ensuring that all groups were swimming around and socializing for a similar amount of time prior to collection.
Tissue preparation
Fish were anaesthetised in ice-cold tank water and standard length (SL) and total body mass (BM) were measured. Immediately before being killed by swift cervical transection, blood samples were collected with capillary tubes from the caudal vein, centrifuged for 10 min at 8000 rpm, and the plasma was removed and stored at −80°C until assayed. Brains were removed, embedded in mounting media (Neg50, Thermo Scientific, Inc.), rapidly frozen on dry ice, and stored at −80°C until sectioning. Pituitaries were also removed, frozen, and stored at −80°C. Testes were removed and weighed to calculate the gonadosomatic index [GSI = (gonad mass/body mass) × 100].
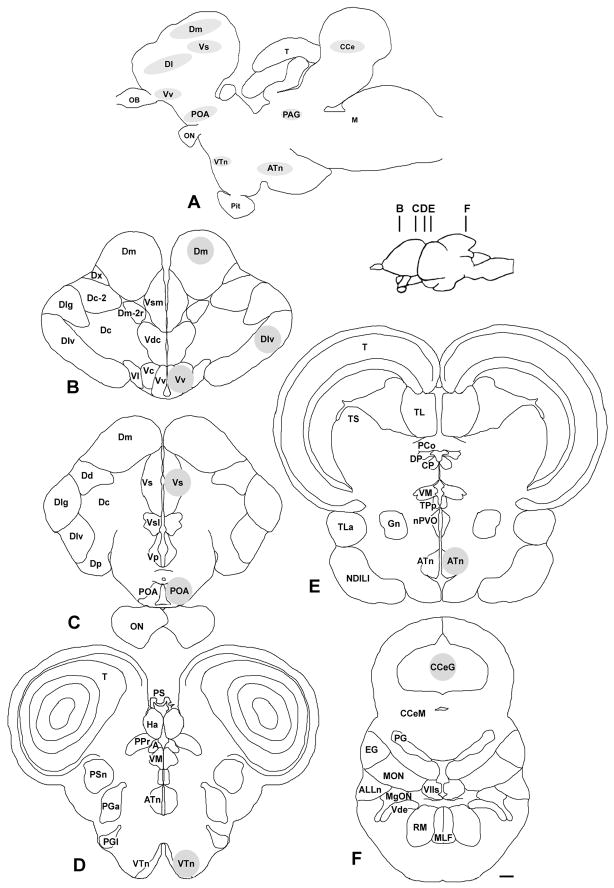
Brains were sectioned in the coronal plane at 300 μm on a cryostat (Microm HM 550) and sections were collected serially onto charged slides (Superfrost Plus, VWR) and stored at −80°C until microdissection. To identify and microdissect specific brain regions, slides were placed on a frozen stage (BFS-30MP, Physitemp) and viewed under a dissection microscope. Tissue was collected with a modified 23G needle (internal diam. ~390 μm) attached to a syringe. To reduce cross-contamination and prevent RNA degradation, the needle was completely cleaned sequentially with RNase away (Invitrogen, Inc.), ethanol, and RNase-free water between each sample. Brain atlases from A. burtoni (29–31) and other fish species (32–34) were used to target brain regions that were centered on the following nuclei (proposed putative mammalian homologues are given in parentheses based primarily on consensus from the following reviews (9, 11, 35)): lateral part of the dorsal telencephalon (Dl; sampled in Dlv region) (medial pallium, hippocampus in part); medial part of the dorsal telencephalon (Dm) (pallial amygdala, dorsal pallium); ventral nucleus of the ventral telencephalon (Vv) (septal formation in part, lateral septum); supracommissural nucleus of the ventral telencephalon (Vs; sampled in Vsm region) (extended amygdala: medial amygdala and bed nucleus of the stria terminalis); preoptic area (POA; see description below for sampled nuclei) (preoptic area); ventral tuberal nucleus (VTn) (anterior hypothalamus in part); anterior tuberal nucleus (ATn) (ventromedial hypothalamus in part); and corpus cerebellum (Ce) (sampled in the granule cell layer, CCeG) (see Fig. 1). The periaqueductal grey (PAG), a mesencephalic nucleus within the SBN, was not collected in this study because it is small and difficult to locate with our microdissection technique. The Dl, while not part of the SBN, was included here because we hypothesised that the social transition in males that were previously dominant might also involve memory or reward circuits, which includes this putative hippocampal/medial pallium homologue. The Ce was used as a control region outside of the SBN.
Fig. 1.
The social behaviour network and locations of microdissected nuclei in the brain of the African cichlid Astatotilapia burtoni. A) Schematic sagittal diagram of A. burtoni brain shows the approximate locations of sampled nuclei, including those within the social behaviour network. B–F) Representative coronal sections illustrate the locations of the microdissection samples that were centered on the following brain regions (grey circles): the lateral zone of the dorsal telencephalon (Dl), medial zone of the dorsal telencephalon (Dm), supracommissural nucleus of the ventral telencephalon (Vs), ventral nucleus of the ventral telencephalon (Vv), preoptic area (POA), ventral tuberal nucleus (VTn), anterior tuberal nucleus (ATn), and the granule zone of the corpus cerebellum (CCeG) as indicated. Inset shows the approximate locations of each coronal section (B–F). The periaqueductal grey (PAG) was not sampled in this study. See list for other abbreviations and Methods section for further details. Scale bar, 200 μm.
It is important to note that the putative mammalian homologies in the teleost brain are not yet fully resolved, and we therefore have tried to more specifically describe the regions we sampled to aid in interpretation of our data, as well as for comparative purposes across taxa, until more definitive homologies are established. Further, due to limitations of the microdissection technique, tissue collection was centered on the abovementioned nuclei, but some sampled nuclei likely also contained portions of surrounding regions. For example, Vv likely also contained portions of Vl (another putative septal formation homolog) and Vc (putative striatal formation homolog), and due to their smaller size, VTn and ATn likely contained portions of other surrounding hypothalamic nuclei. While we attempted to collect the majority of the POA (periventricular preoptic nucleus, parvocellular preoptic nucleus, magnocellular preoptic nucleus, suprachiasmatic nucleus), samples likely did not include the more caudally positioned gigantocellular preoptic nucleus. It is also relevant to recognize that the POA of fishes and tetrapods such as mammals are both complex heterogeneous regions that include multiple nuclei and subdivisions, some of which have established homologies, but they also show distinct differences in location and inclusion of certain peptidergic cell groups (9, 36). Based on the size and extent of the Dm, Dlv, Ce, and Vs relative to our section thickness and microdissection tool diameter, there was likely minimal inclusion of any other regions for samples taken within these nuclei. The amount of tissue collected from each region was standardised across all individuals, and included both hemispheres (except Ce), but differed among brain regions (range=2–6 300-μm microdissected samples per region) according to the relative size and extent of each nucleus. Tissue was collected directly into lysis buffer (RNeasy Micro kit, Qiagen), frozen on dry ice, and stored at −80°C until RNA isolation.
Total RNA was isolated from homogenised pituitaries and microdissected brain tissue following standard methods (RNeasy Micro kit, Qiagen) described previously (26, 37). RNA was treated with DNase (RNase-free DNase set, Qiagen) during the isolation procedure according to kit instructions to remove contaminating genomic DNA. RNA from each sample was then reverse transcribed to cDNA (iScript cDNA synthesis kit, Bio-Rad) and diluted 1:3 prior to use as a template for quantitative RT-PCR reactions.
Quantitative reverse transcription PCR (qRT-PCR)
qRT-PCR was used to measure the mRNA levels of two immediate early genes (egr-1; cfos), two androgen receptors (ARα; ARβ), three oestrogen receptors (ERα; ERβa; ERβb), and brain aromatase (enzyme that converts testosterone to oestradiol). Primer pairs for all steroid receptors, egr-1, and the reference genes, g3pdh and 18s rRNA, were commercially synthesised (Invitrogen) and identical to those used in previous studies (14, 37–41). Primers for cfos and brain aromatase were: cfos (Accession #HQ232413.1) forward, 5′-GAGGAATAAGCAGGCAGCAGCAAA-3′, reverse, 5′-TCTCCTTCAGCAGGTTGG CGATA-3′ (133 bp product); cytochrome P450 brain aromatase (Accession #FJ605734.1) forward, 5′-TCATGAAGCCGAGAAACTGGACGA-3′, reverse, 5′-TCTCTAGCACACACTGCCTGACAT-3′ (110 bp product). qRT-PCR was performed using a previously described protocol (37) in 20 μl duplicate reactions (SSoFast EvaGreen Supermix, Bio-Rad) that were run on a CFX96 (Bio-Rad) qPCR machine.
PCR Miner software (38) was used to calculate reaction efficiencies and cycle thresholds (CT) from the fluorescence readings of individual wells during the reaction, and the relative amount of mRNA in each sample was normalised to the geometric mean of the two reference genes (g3pdh, 18s) as previously described (26, 37, 38). Mean values for g3pdh and 18s did not differ among the three male groups for any brain region (ANOVAs, P>0.05), indicating they are appropriate reference genes for comparing mRNA levels in this study. Each primer pair produced a single melting curve peak in the presence of cDNA template, and showed no or late amplification (>cycle 40) when water was used as a template in the reaction mix, or when reverse transcriptase was omitted from the cDNA synthesis reaction (negative controls). Individual samples that did not amplify correctly (i.e., produced errors in PCR Miner) were eliminated from analyses, resulting in sample sizes that ranged from 9–12 for each gene of interest within each brain region.
Steroid hormone assays
Plasma testosterone (T), 11-ketotestosterone (11-KT), 17β-oestradiol (E2), and cortisol levels were measured using commercially available Enzyme ImmunoAssay (EIA) kits (Cayman Chemical, Inc.) as previously described and validated for this species (37). Briefly, for T, 11-KT and E2 assays, a 10μl sample of plasma from each subject was extracted three times using 200 μl of ethyl ether (extraction efficiency 87–91%) and evaporated under a fume hood prior to re-constitution in EIA assay buffer (1:40–1:50 dilution). Plasma used for the cortisol assay was not extracted prior to dilution (1:55). EIA kit protocols were then strictly followed, plates were read at 405 nm using a microplate reader (UVmax Microplate Reader, Molecular Devices), and steroid concentrations were determined based on standard curves. All samples were assayed in duplicate and intra-assay coefficients of variation (CV) were: T (15.1%); 11-KT (10.4%); E2 (11.7%); cortisol (5.8%).
Statistical analysis
Comparisons of GSI among social groups were made with one-way ANOVA followed by Student-Newman-Keuls (SNK) tests for multiple comparisons. Circulating steroid levels were compared with generalized linear mixed model repeated measures ANOVA (GLZM), with steroid hormones (T, 11-KT, E2) as repeated within-subject factors and social status as between-subject factors, followed by pair-wise comparisons. All data are plotted as mean ± SEM. To determine which genes in different brain nuclei might be the best predictors of social status (subordinate, ascending, dominant), we used linear discriminant function analyses (LDA) on: 1) egr-1 and cfos mRNA levels in all brain regions, and 2) all steroid receptors and aromatase mRNA in all brain regions. LDA analysis combines (weighs) all of the input variables to produce a single new composite variable, or discriminant score, which identifies the variables that contribute most to differentiating the social groups. Factor loadings above 0.30 were considered important for interpreting group membership. GLZM ANOVAs for mRNA levels of each receptor type were then used to test for differences in group means, with brain region as repeated within-subject factors and social status as between-subject factors, followed by pairwise comparisons. To test for correlations among mRNA levels and plasma steroid levels (T, 11-KT, E2) within each brain region among all males together, we used Pearson Product Moment tests adjusted for multiple comparisons (Bonferroni method; α/n). Statistical analyses were performed with SPSS 19 (IBM, Inc.).
Results
GSI and circulating steroid levels
Gonadosomatic index was higher in stable dominant males compared to both stable subordinate and socially transitioning males at 30 min after ascent (ANOVA, F(2,33)=56.52, P<0.001; SNK, P<0.001) (Fig. 2). Circulating levels of T, 11-KT and E2 were all higher in ascending males sampled at 30 min after social opportunity compared to stable subordinate males (GLZM, between-subjects factor, F(2,33)=7.28, P=0.002; T, P=0.006; 11-KT, P=0.008; E2, P<0.001), but ascending male levels did not differ from stable dominant male levels (Fig. 2). Cortisol levels differed among all three groups (sub < dom < ascend) (ANOVA, F(2,33)=16.06, P<0.001), with ~5-fold higher levels found in ascending males compared to subordinate males (Fig. 2).
Fig. 2.
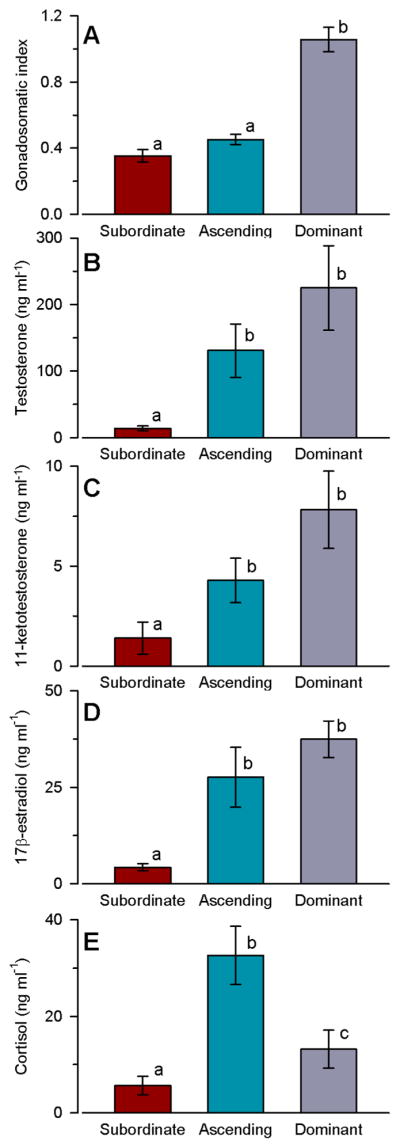
Gonadosomatic index and circulating steroid levels differ among social groups in male A. burtoni. A) Gonadosomatic index was higher in dominant males compared to both subordinate and ascending males. B) Circulating testosterone levels were higher in ascending and dominant males compared to subordinate males. C) Plasma levels of 11-ketotestosterone were also higher in ascending and dominant males compared to subordinate males. D) Plasma levels of 17β-oestradiol were higher in ascending and dominant males compared to subordinate males. E) Plasma cortisol levels differed among all three social groups. n=12 fish per social group. Different letters indicate statistical differences among groups at P<0.05.
IEG expression among social groups
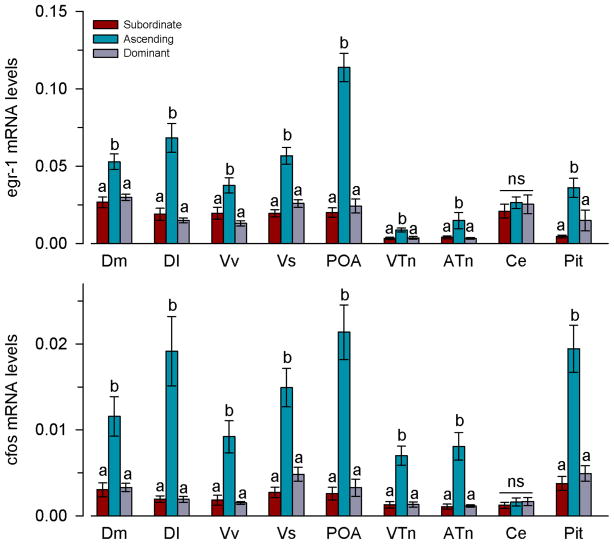
Ascending males had higher mRNA levels of both egr-1 (GLZM between-subjects factor, F(2,33)=20.23, P<0.001) and cfos (F(2,33)=32.23, P<0.001) in the pituitary and all brain regions examined, with the exception of the cerebellum (egr-1: P=0.413; cfos: P=0.741), compared to stable subordinate and dominant states (Fig. 3). Within each brain region, there was also a positive correlation between egr-1 and cfos mRNA levels (Pearson, r=0.42–0.88; P=0.008-<0.001).
Fig. 3.
Social opportunity rapidly increases immediate early gene mRNA expression in nuclei of the social behaviour network in male A. burtoni. Relative mRNA levels of both egr-1 and cfos were higher in ascending males compared to the stable subordinate and dominant states in all brain regions and the pituitary with the exception of the cerebellum. mRNA levels are normalised to the geometric mean of the reference genes 18s and g3pdh. n=12 fish per social group. Different letters indicate significant differences among social groups at P<0.05. ns, not significant. See list for abbreviations.
Linear discriminant function analysis of both IEGs in all regions revealed a single significant function (function 1, X2=107.13, P<0.001) that explained 98% of the variance (Fig. 4). This function was most heavily loaded by egr-1 and cfos in the preoptic area, suggesting that IEG levels in the POA is the best predictor for distinguishing ascending males (group centroid=8.73) from stable subordinate (−5.03) and dominant (−3.70) states. This LDA correctly classified 100% of ascending males, 91.7% of subordinate males, and 83.3% of dominant males (overall 91.7%), all of which greatly exceed the value for group classification based on chance (33.3%).
Fig. 4.
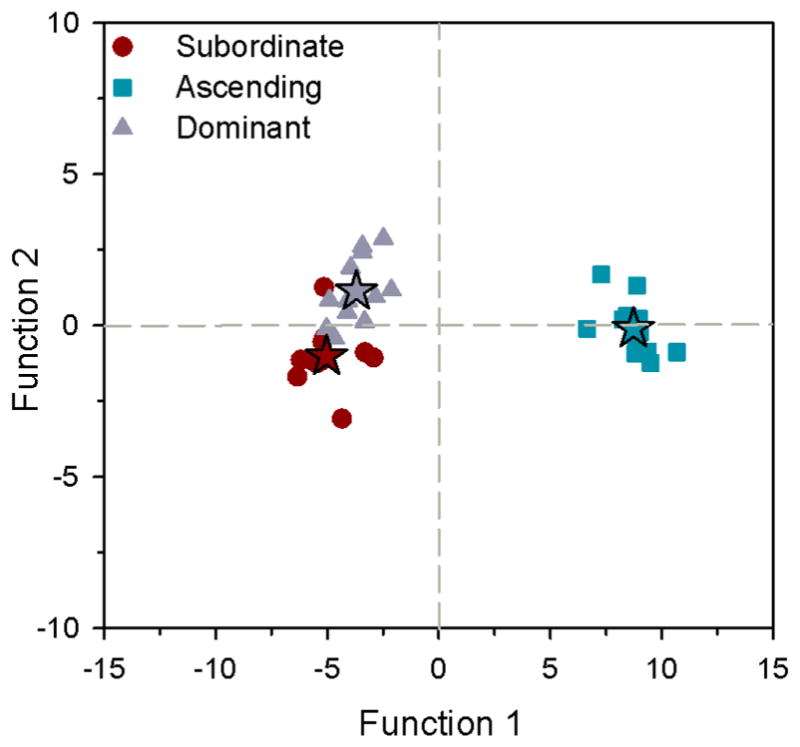
Linear discriminant function analysis of immediate early gene mRNA levels in microdissected brain regions of male A. burtoni. Function 1 was most heavily loaded by both egr-1 and cfos mRNA levels in the preoptic area, which best discriminates ascending males (squares) from the stable subordinate (circles) and dominant (triangles) states. Discriminant scores are plotted and stars represent the centroid of each social group. n=12 fish per social group.
Steroid receptor and aromatase mRNA levels among social groups
Androgen receptors
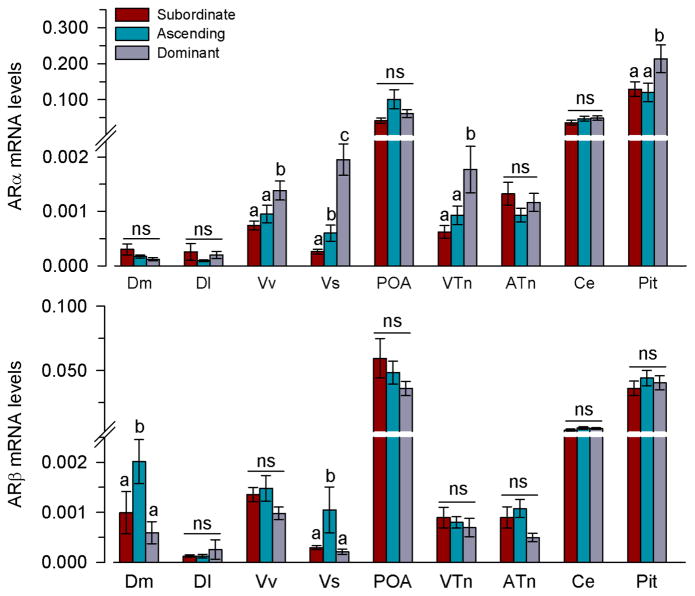
Dominant males had higher mRNA levels of ARα in Vv (P=0.004), VTn (P=0.005), and the pituitary (P=0.031) compared to both ascending and subordinate males (GLZM between-subjects factor, F(2,33)=4.0, P=0.031) (Fig. 5). In Vs, ascending males had higher mRNA levels of ARα compared to subordinate males, and dominant males had higher levels than both ascending and subordinate animals (P=0.025). There was no difference in ARα mRNA levels among social groups in Dm (P=0.282), Dl (P=0.608), POA (P=0.342), ATn (P=0.298), or Ce (P=0.712).
Fig. 5.
Relative mRNA levels of androgen receptors in the brain and pituitary of male A. burtoni. mRNA levels are normalised to the geometric mean of the reference genes 18s and g3pdh. n=9–12 fish per social group. Different letters indicate significant differences among social groups at P<0.05. ns, not significant. See list for abbreviations.
Ascending males had higher mRNA levels of ARβ compared to both subordinate and dominant males in the Dm (P=0.008) and Vs (P=0.037), both homologous in part to different regions of the amygdala of mammals (GLZM between-subjects factor, F(2,33)=4.13, P=0.028) (Fig. 5). There was no difference in ARβ mRNA levels among social groups in Dl (P=0.683), Vv (P=0.147), POA (P=0.274), VTn (P=0.710), ATn (P=0.073), Ce (P=0.453), or the pituitary (P=0.693).
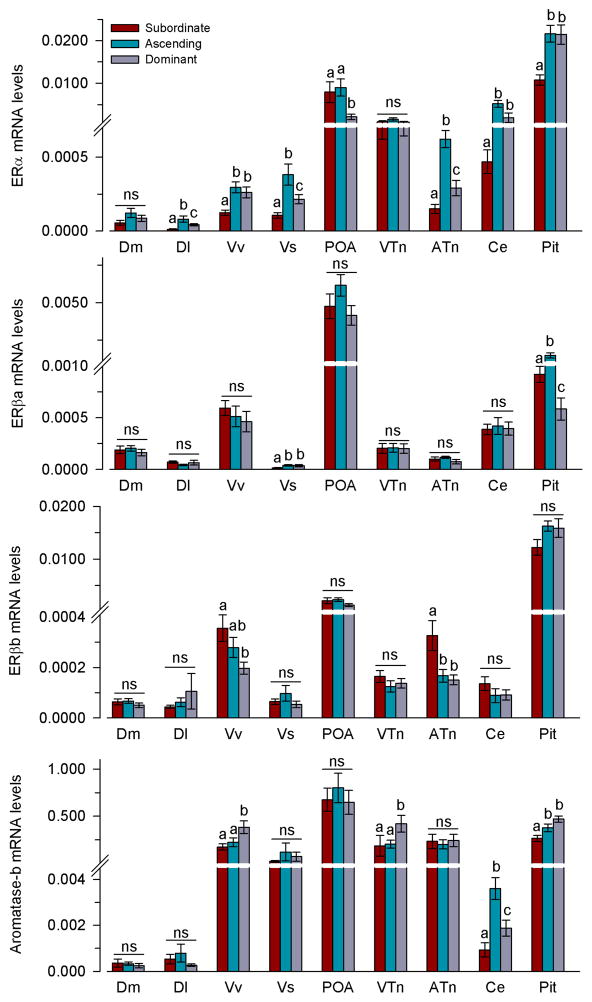
Oestrogen receptors and aromatase
The majority of mRNA differences in the oestrogenic pathway among social states were seen in ERα levels (Fig. 6). ERα mRNA levels differed among all three social groups (subordinate<dominant<ascending) (GLZM between-subjects factor, F(2,33)=10.06, P<0.001) in the Dl (P=0.005), Vs (P<0.001), and ATn (P<0.001). In the Vv (P=0.004), Ce (P=0.004), and pituitary (P<0.001), subordinate males had lower levels of ERα compared to both ascending and dominant males. In the POA, dominant males had lower ERα mRNA levels compared to both ascending and subordinate animals (P=0.035). There was no difference in ERα mRNA levels among social states in Dm (P=0.159) or VTn (P=0.239) (Fig. 6).
Fig. 6.
Relative mRNA levels of oestrogen receptors and aromatase in the brain and pituitary of male A. burtoni. mRNA levels are normalised to the geometric mean of the reference genes 18s and g3pdh. n=9–12 fish per social group. Different letters indicate significant differences among social groups at P<0.05. ns, not significant. See list for abbreviations.
ERβa mRNA levels in the Vs were higher in ascending and dominant males compared to subordinate animals (GLZM between-subjects factor, F(2,33)=6.37, P=0.005; Vs, P=0.045) (Fig. 6). In the pituitary, ascending males had higher ERβa mRNA levels than both subordinate and dominant males, and dominant males also had lower levels compared to subordinates (P<0.001). There was no difference in ERβa mRNA levels in Dm (P=0.672), Dl (P=0.463), Vv (P=0.593), POA (P=0.311), VTn (P=0.373), ATn (P=0.302) or Ce (P=0.572) among social groups (Fig. 6).
In the Vv and ATn, ERβb mRNA levels were highest in subordinate males (GLZM between-subjects factor, F(2,33)=3.8, P=0.037) (Vv: P=0.009; ATn: P=0.003) (Fig. 6). There was no difference in ERβb levels in Dm (P=0.469), Dl (P=0.574), Vs (P=0.316), POA (P=0.556), VTn (P=0.430), Ce (P=0.287), or the pituitary (P=0.187) among social groups.
In the Vv and VTn, dominant males had higher mRNA levels of aromatase compared to both subordinate and ascending males (GLZM between-subjects factor, F(2,33)=4.41, P=0.020) (Vv: P=0.006; VTn: P=0.013) (Fig. 6). In the Ce, ascending males had higher aromatase mRNA levels compared to both subordinate and dominant males, but dominant males also had higher levels compared to subordinates (P=0.027). In the pituitary, subordinate males had lower aromatase mRNA levels compared to both ascending and dominant males (P<0.001). There was no difference in aromatase mRNA in Dm (P=0.179), Dl (P=0.393), Vs (P=0.535), POA (P=0.671), or ATn (P=0.906) among social groups.
Stepwise linear discriminant function analysis (Wilks’ Lambda with an F-value of 3.84 for predictor entry and 2.71 for removal) which used mRNA levels of steroid receptors and aromatase in all brain regions to predict social status delineated five variables (ATn-ERα, Vv-ERα, VTn-Arom, Vv-ARα, Dm-ARβ) that were able to correctly classify 100% of the individuals that belong to each social grouping (98% with leave-one-out cross-validation approach). This LDA revealed two significant functions (function 1, X2=92.7, P<0.001; function 2, X2=34.9, P<0.001), with function 1 explaining 72.4% of the variance and the remaining 27.6% explained by function 2 (Fig. 7). Function 1 was most heavily loaded by ERα mRNA levels in the ATn and Vv, which separated ascending males (group centroid=2.76) from both stable subordinate (−2.71) and dominant (−0.050) states. Function 2 was most heavily loaded by aromatase mRNA levels in VTn and ARα mRNA levels in Vv, which discriminated dominant males (group centroid=1.95) from ascending (−0.95) and subordinate (−1.00) males.
Fig. 7.
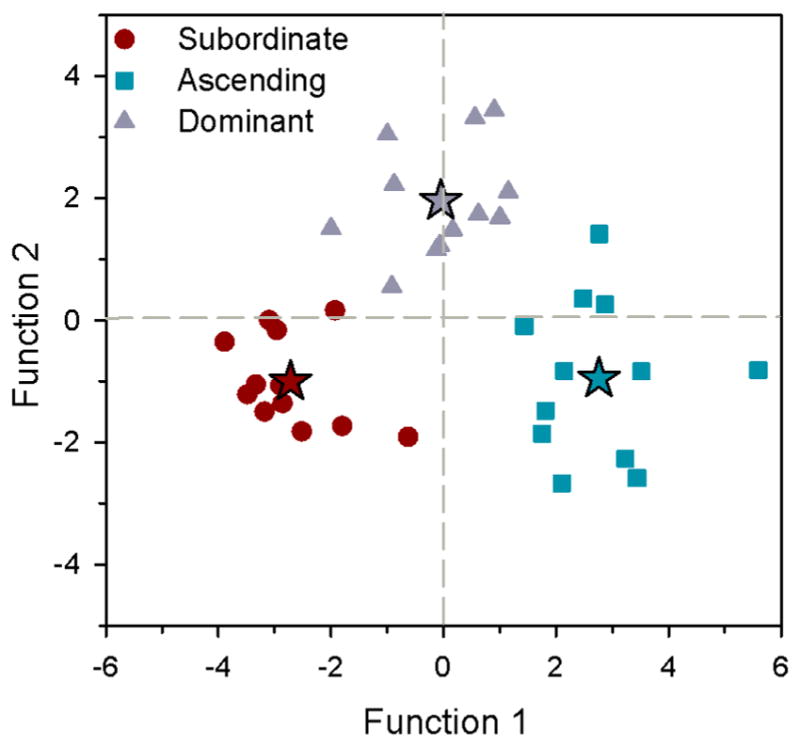
Linear discriminant function analysis of mRNA levels of sex-steroid receptors and aromatase in microdissected brain regions of male A. burtoni. Function 1 separates ascending males (squares) from subordinate (circles) and dominant (triangles) states and was most heavily loaded by ERα mRNA levels in ATn and Vv, while function 2 discriminates dominant males from subordinate and ascending males based on aromatase mRNA levels in VTn and ARα levels in Vv. Discriminant scores are plotted and stars represent the centroid of each social group. n=12 fish per social group.
Correlation analyses in all males together, adjusted for multiple comparisons, showed only two relationships between circulating steroid levels and mRNA levels of aromatase or steroid receptors: 1) T was positively correlated with aromatase in the VTn (r=0.49, P=0.002), and 2) E2 was positively correlated with ERα in the pituitary (r=0.60, P<0.001). Further, the only relationships observed between steroid receptor mRNA levels and IEG mRNA levels were positive correlations between ERα and cfos in the ATn (r=0.67, P<0.001) and VTn (r=0.67, P<0.001).
Discussion
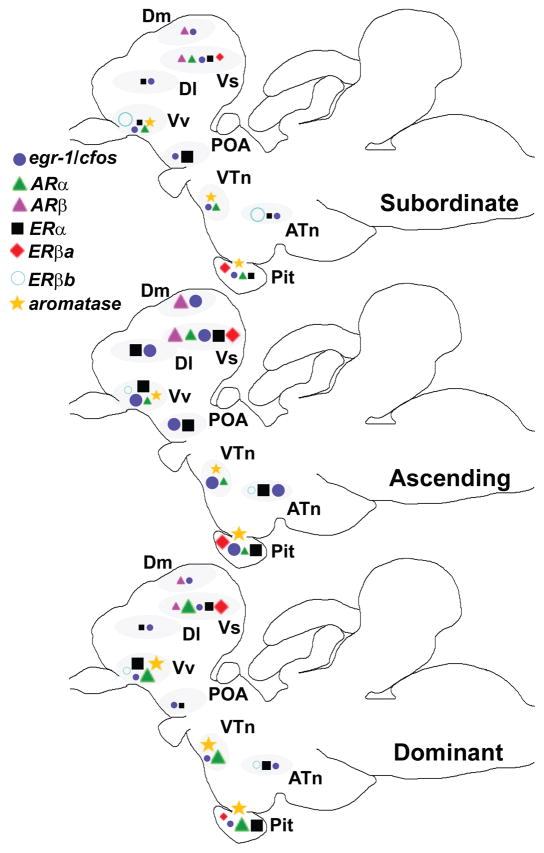
How does the brain respond to a social opportunity, and how might activation of specific brain regions be modulated by steroid hormones to then influence behavioural outputs? We used the natural social transition in male A. burtoni to address these questions and found that within 30 min, socially-suppressed males that were given an opportunity to rise in rank and become dominant, had higher levels of circulating sex- and stress-related steroids (T, 11-KT, E2, cortisol), higher IEG mRNA levels (egr-1, cfos) in brain regions associated with social behaviour, and distinct changes in steroid receptor mRNA levels within nuclei of the social behaviour network (Fig. 8). To our knowledge, this is the first study to show that perception of a social opportunity induces collective activation of all nuclei within the SBN that is also coincident with rapid changes in sex steroid receptor mRNA levels. This remarkably rapid transcriptional and endocrine response may be part of a complex cascade that integrates social inputs with internal physiological state to facilitate the status transition and to prepare the animal for a dominant position, which ultimately leads to improved reproductive fitness for the previously suppressed individual.
Fig. 8.
Schematic summary of transcriptional changes associated with changing social status in the brain of male A. burtoni. Relative size of the symbols in each sagittal brain section indicates the relative mRNA levels of each gene transcript among the three social groups (subordinate, ascending, dominant). Within each identified brain nucleus (grey ovals), only those genes that showed differences among social groups are shown. Note that the ascending phenotype has the greatest distribution of higher mRNA levels throughout the brain (i.e., more large symbols), and the subordinate phenotype has the lowest (i.e., more small symbols). The Vs (putative extended amygdala homolog), Vv (putative septum homolog), and pituitary also showed the greatest number of changes in mRNA levels with social state compared to other brain regions. Locations of each nucleus within the sagittal fish brain are depicted to minimize overlap and are therefore only approximate. Changes in the cerebellum are not shown. Rostral is to the left.
In A. burtoni, we found that circulating levels of the sex steroids, T, 11-KT, and E2, were all higher in males at 30 min after they were given a social opportunity compared to levels in subordinate males. This rapid increase may be triggered by the social interactions that occur at opportunity, as proposed by the ‘challenge hypothesis’ (42). A non-mutually exclusive possibility is that sex steroid increase is a consequence of the rapidly up-regulated reproductive axis and activation of the steroid-producing testes (24, 25, 43). Regardless of the mechanism, however, the quick increase could serve important behavioural functions such as increasing male motivation to court or perform aggressive displays (44, 45). In fact, both testosterone and oestradiol levels were positively correlated with aggressive index in ascending male A. burtoni (43). Further, O’Connell and Hofmann (46) showed that oestrogen treatment increases aggressive behaviours in A. burtoni males regardless of their social status, while androgen treatment (dihydrotestosterone, DHT) increases courtship behaviours only in dominant males. Thus, the rapid increases in both oestradiol and androgens at social ascent may contribute to their increased displays of aggressive and reproductive behaviours, respectively. The quick elevation in hormone levels in ascending males also results in circulating concentrations similar to those found in dominant males, and occurs despite their small testes. Previous studies showed however, that subordinate male testes still contain Leydig, Sertoli, and germ cells of all stages (25), maintain spermatogenic potential (27), and have measurable mRNA levels of steroidogenic acute regulatory protein (StAR; rate-limiting enzyme for T synthesis) (43), suggesting that they have some physiological capacity to rapidly produce and release sex steroids to the circulation. Thus, the rapid increase in circulating androgens and oestradiol may function to modulate behaviours as well as reproductive physiology via actions at multiple sites along the hypothalamic-pituitary-gonadal axis.
Immediate early gene mRNA levels of both egr-1 and cfos were higher throughout the brain in A. burtoni males that were given an opportunity to acquire a vacant territory and become dominant compared to control stable males that had been either subordinate or dominant for several weeks. This response is likely due to the recognition of the social opportunity because it is not elicited in males who are already dominant and performing similar territorial and reproductive behaviours. Interestingly, this response occurred in all of the nuclei of the SBN, suggesting that many neuronal populations respond to this social opportunity, possibly to initiate phenotypic changes necessary for the social transition and preparation for dominant status. In mammals, the pattern of IEG activation in SBN nuclei often differs between aggressive and sexual contexts, with nuclei such as the preoptic area showing activation during sexual contexts but not aggression, and the anterior hypothalamus showing activation during aggression but not reproduction (10). In contrast, social ascent in A. burtoni includes a simultaneous opportunity for both aggressive and reproductive behaviours, as well as representing a social stressor, which may explain the higher levels of both IEGs observed across all SBN nuclei in ascending males. To disentangle the brain IEG response resulting from aggression versus reproduction versus stress, new experimental paradigms designed to specifically test these contexts are needed. It is also possible that the social opportunity simply triggers a general and widespread response to initiate changes in neural and cognitive processing required for ascent and dominance status, including activation of regions involved in stress coping. It is important to note however, that the protein products of IEGs are transcription factors that can either promote or inhibit the expression of downstream target genes, so future studies are needed to determine the cellular identity of co-localised IEG expression and what functional consequences each IEG response might have on the behaviour, physiology, and expression levels of downstream genes in ascending males. It will also be relevant to test whether this IEG activation occurs in specific subregions or neurone/glial types within these nuclei during social ascent. IEG levels in the pituitary were also higher in ascending males, suggesting that the rapid increase in luteinising hormone (LH) and follicle stimulating hormone (FSH) shown previously (26), may involve the transcription factors egr-1 and cfos, as shown for mammals (47, 48).
In addition to opportunity, the ascent transition in male A. burtoni is likely also a stressful event, as evidenced by the rapid ~5-fold elevation in circulating cortisol levels within 30 min, suggesting activation of the hypothalamic-hypophyseal-adrenal (HPA) axis (or HPI, hypothalamic-hypophyseal interrenal, in fishes). Further, many of the SBN brain regions showing higher IEG expression in ascending fish are similar to those activated in response to various stressors in mammals (49, 50), supporting the hypothesis that the SBN represents an important ‘core’ circuitry for processing social information, including stressors, of different valence across vertebrates. There is often overlap between brain areas involved in processing social stimuli and those involved in the stress response, which could indicate that stress is a natural part of many social interactions, or that components of the circuitry that mediate social perception are shared by the stress system.
In A. burtoni, we found that within 30 min of a social opportunity, ascending males had higher levels of circulating E2 and higher mRNA levels of ERα in several regions of the social forebrain that are putative homologs to the mammalian hippocampus/medial pallium (Dl), septal formation (Vv), extended amygdala (Vs), and part of the ventromedial hypothalamus (ATn), as well as the cerebellum and pituitary, compared to control subordinate males. In addition to controlling female behaviours, oestrogenic signalling pathways are known to be important in the regulation of context-dependent male-typical behaviours including aggression, mating, and communicative displays across vertebrate taxa (51–54). For example, within minutes, increases in circulating or brain levels of E2 can rapidly influence male sexual behaviours in fish (45) and mammals (55). Further, in male mice, deletion of the ERα gene decreases male aggression, causes deficits in social recognition tasks, and inhibits sexual behaviours (52, 56, 57). Aggressive behaviours were also positively correlated with ERα-expressing cells in a specific subset of social nuclei in the mammalian brain (lateral septum, BNST, anterior hypothalamus) (58), suggesting that localised changes in oestrogen sensitivity may contribute to modulation of behaviours (55, 59). In fact, there is increasing evidence that in addition to ER expression in neuronal somata, ERs (ERα, ERβ) are localised to dendritic spines, axons, terminals, and glia, suggesting that oestrogens binding to ERs can also act locally to modulate cell signalling pathways with immediate rapid effects on cell function, without regulation of nuclear transcription (60, 61). Among vertebrates, teleost fishes have the greatest capacity for oestrogen production in the brain due to their high levels of aromatase, particularly in the forebrain (62). In A. burtoni, the combined increase in circulating E2 and ERα mRNA levels in the brain of ascending males suggests that increased oestrogen sensitivity in specific social nuclei may be important for the expression of behaviours (territorial and/or reproductive) and physiological changes that occur during social transition to dominance [see also (43, 46)]. Similarly, dominant type I male midshipman fish Porichthys notatus show higher levels of aromatase mRNA expression (measured by in situ hybridization) in the brain during the reproductive nesting period compared to before and after this time, which parallels the seasonal changes in circulating sex steroid levels in this species (63), suggesting that oestrogen signalling is also important during seasonal transitions associated with breeding in males. Our LDA analysis also identified ERα mRNA levels and aromatase as the variables that contributed most to distinguishing amongst social groups, highlighting that oestrogen signalling pathways may play a significant role in male social transition and preparing the animal for a dominant lifestyle. In fact, in A. burtoni, oestradiol treatment increases aggressive behaviours in both dominant and subordinate males (46), suggesting that elevated E2 at ascent may help facilitate expression of aggressive behaviours needed to establish territory ownership.
Another interesting finding of our study was that ERβb mRNA levels in the ATn and Vv were higher in subordinate males, but lower in ascending and dominant males. While the functions of ERβ-subtypes in the brain are not well understood, especially in fishes, this receptor has been implicated in mediating anxiety-like behaviours, controlling reactivity to social stimuli, and modulating aggression in mammals (64). For example, ERβ knock-out mice are more aggressive than wild-type littermates, suggesting that activation of ERβ has an inhibitory effect on the expression of aggressive behaviours in mice. The ATn of teleost fishes is a putative homolog in part to the ventromedial hypothalamus and Vv is a putative homolog in part to the septal formation, which are both regions implicated in the control of aggression in mammals (10, 65). Thus, we speculate that the higher mRNA levels of ERβb in these nuclei in subordinate males might function to inhibit aggressive behaviours as an adaptive subordination response, while the rapid removal of this inhibition upon social opportunity may contribute to the increase in aggressive/territorial behaviours needed to acquire the territory and assert dominance towards neighboring males. This idea is further supported by the fact that oestradiol-activation of ERα and ERβ tend to have opposite effects on male aggression in rodents (64), suggesting that the lower ERα and higher ERβb mRNA levels in particular nuclei (e.g., ATn and Vv) of subordinate A. burtoni males might act synergistically to lower aggression and promote subordination as an adaptive survival behaviour.
Androgen receptor mRNA levels also differed among social states in several brain regions including the Dm (ARβ), Vv (ARα), Vs (ARα, ARβ), VTn (ARα), and pituitary (ARα). In male mice, winning a fight was associated with more AR-immunoreactive cells in the bed nucleus of the stria terminalis, a key brain region that controls social aggression and reproduction (66). In A. burtoni, we also saw increased AR mRNA levels in the Vs, the proposed teleost homolog of the extended amygdala (which in mammals includes the BNST and medial amygdala), in ascending (ARα and ARβ) and dominant (ARα) males compared to subordinates. Previous studies suggested that the perception of winning a fight may in itself be a major factor driving physiological changes (e.g., increases in AR expression and steroid production) (66, 67), which may help explain the rapid increase in mRNA of both AR subtypes when A. burtoni males perceive the opportunity to rise in rank and potentially win territorial contests. Selective deletion of AR in the male mouse nervous system does not eliminate mating or sexual behaviours, but rather, causes deficits in specific components of male-typical behaviours, suggesting that activation of AR by androgens regulates the extent of male sexual and territorial displays (68). Further, a previous study in A. burtoni showed that AR agonists increased courtship behavior in dominant, but not subordinate, males (46). This raises the possibility that the region-specific differences in AR mRNA levels seen here may help modulate status-specific behavioural displays by increasing androgen sensitivity in regions such as Vv, Vs, and VTn in dominant males, which may in turn regulate sexual behaviors. The different patterns of expression between ARα and ARβ even within the same brain regions in A. burtoni also further supports the hypothesis of divergent functions for these two receptor subtypes, which may include roles in social aggression and reproduction.
A previous study in A. burtoni showed that social dominance influenced androgen and oestrogen receptor mRNA levels in the brain such that dominant males had higher levels of multiple receptor subtypes primarily in the anterior brain (entire telencephalon and portion of anterior POA) compared to subordinate males (39). In the present study, we used microdissection to increase the neuroanatomical resolution in the forebrain and discovered that for the limited number of nuclei that we sampled, there were relatively few cases where dominant males had higher mRNA levels of any sex steroid receptor compared to subordinates. However, function 2 of our LDA analysis did identify ARα mRNA levels in Vv and aromatase levels in VTn as the best predictors for separating dominant males from subordinate and ascending males, suggesting that dominant individuals have increased steroid sensitivity in at least some SBN regions, which may help to maintain their behavioural repertoire and status position. In light of the recent studies that show rapid steroid-induced changes in neurone response properties and localised changes in neurosteroid production in the brain in response to social cues (69–72), changes in steroid sensitivity of even a few neurones within a circuit could fine-tune neural activity and potentially have important behavioural and physiological consequences. Thus, future studies should focus with even finer-scale neuroanatomical resolution to examine the dynamic changes in steroid receptor expression associated with social plasticity.
Summary and Conclusions
We used the social African cichlid fish A. burtoni to demonstrate that social opportunity is associated with rapidly (30 min) increased levels of circulating sex and stress steroids, higher IEG mRNA levels, and changes in mRNA levels of androgen and oestrogen receptors within nuclei of the conserved social behaviour network. The SBN is thought to influence behaviours by weighing neural activity/inputs from different nodes and then inducing a patterned release of neurochemicals that is ultimately translated into motor outputs (11). Thus, social perception, or changes in the salience of social inputs, may alter the relative neural activity or functional connectivity in the SBN by adjusting how each node detects and responds to sex steroids via changes in receptor abundance or availability. These localised changes in transcriptional profile could have profound effects on the behaviours and physiological changes that occur during social transition to prepare the male for a dominant lifestyle, which may differ substantially from the mechanisms responsible for maintenance of either stable subordinate or stable dominant states. Interestingly, the two brain regions that showed the most changes in sex steroid receptor expression among social groups were the Vs and Vv (see Fig. 8), which are putative homologs in part to the extended amygdala and septal formation of mammals, respectively. These regions are involved in regulating both social aggression and reproductive behaviours across vertebrates, and are recognised as important functional links between the ‘social behaviour network’ and the ‘mesolimbic reward system’ because they are thus far identified as the only two regions shared by both networks (9). Thus, the rapid activation and changes in sex steroid receptor mRNA expression in Vs and Vv suggests these regions serve as crucial neuroendocrine substrates for integration of social salience with internal hormonal state, followed by translation of this information into appropriate behavioural actions. What remains enigmatic, however, is how changes in these transcription factors (IEGs and sex steroid receptors) might influence the transcription of specific downstream genes to alter neuronal plasticity and function leading to physiological and behavioural change. While future studies are needed to fully understand the endocrine, neuroendocrine, and transcriptional plasticity that result from social opportunity, our results in A. burtoni highlight how rapidly the perception of social cues can influence so many different levels of biological organization in this model vertebrate system.
Acknowledgments
We thank Scott Juntti, Caroline Hu, and the reviewers for helpful comments on the manuscript. This research was funded in part by the Stanford University Undergraduate Research Fund (AZ), NIH F32NS061431 (KPM), NIH NS 034950 (RDF), and NSF IOS-0923588 (RDF).
Abbreviations
- A
nterior thalamic nucleus
- ALLn
Anterior lateral line nerve
- ATn
Anterior tuberal nucleus
- Ce
Cerebellum
- CCeG
Granular layer of the corpus cerebelli
- CCeM
Molecular layer of the corpus cerebelli
- CP
Central posterior thalamic nucleus
- Dc
Central part of the dorsal telencephalon
- Dc-2
Subdivision 2 of Dc
- Dd
Dorsal nucleus of the dorsal telencephalon
- Dl
Lateral part of the dorsal telencephalon
- Dld
Dorsal division of the lateral part of the dorsal telencephalon
- Dlg
Granular division of the lateral part of the dorsal telencephalon
- Dlv
Ventral division of the lateral part of the dorsal telencephalon
- Dm
Medial part of the dorsal telencephalon
- Dm-1,2,3
Subdivisions 1,2 and 3 of Dm
- Dm-2r
Rostral part of Dm-2
- Dp
Posterior part of the dorsal telencephalon
- DP
Dorsal posterior thalamic nucleus
- EG
Granular eminence
- Gn
Glomerular nucleus
- Ha
Habenula
- M
Medulla
- MgON
Magnocellular octaval nucleus
- MON
Medial octavolateralis nucleus
- MLF
Medial longitudinal fasciculus
- NDIL
Diffuse nucleus of the inferior lobe
- nPVO
Nucleus of the paraventricular organ
- OB
Olfactory bulb
- ON
Optic nerve
- PAG
Periaqueductal grey
- PCo
Posterior commissure
- PG
Periventricular granular cell mass of caudal cerebellar lobe
- PGa
Anterior preglomerular nucleus
- PGl
Lateral preglomerular nucleus
- Pit
Pituitary gland
- POA
Preoptic area
- PPr
Rostral periventricular pretectal nucleus
- PS
Pineal stalk
- PSn
Superficial pretectal nucleus
- RM
Medial reticular nucleus
- T
Tectum
- TL
Torus longitudinalis
- TLa
Nucleus of the torus lateralis
- TPp
Periventricular nucleus of the posterior tuberculum
- TS
Torus semicircularis
- Vc
Central nucleus of the ventral telencephalon
- Vdc
Caudal part of Vd
- Vde
Descending tract of the trigeminal nerve
- VIIs
Facial sensory nucleus
- Vl
Lateral nucleus of the ventral telencephalon
- VM
Ventromedial thalamic nucleus
- Vp
Postcommissural nucleus of the ventral telencephalon
- Vs
Supracommissural nucleus of the ventral telencephalon
- Vsm
Medial part of Vs
- Vsl
Lateral part of Vs
- VTn
Ventral tuberal nucleus
- Vv
Ventral nucleus of the ventral telencephalon
References
- 1.Maruska KP, Fernald RD. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology. 2011:26412–23. doi: 10.1152/physiol.00032.2011. [DOI] [PubMed] [Google Scholar]
- 2.Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322(5903):896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufmann JH. On the definitions and functions of dominance and territoriality. Biol Rev. 1983:581–20. [Google Scholar]
- 4.Fernald RD. Social regulation of reproduction: what changes and why? Hormones, Brain and Behavior. 2009:1683–91. [Google Scholar]
- 5.O’Connell LA, Hofmann HA. Genes, hormones, and circuits: An integrative approach to study the evolution of social behavior. Front Neuroendocrinol. 2011 doi: 10.1016/j.yfrne.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Fernald RD, Hirata NR. Field study of Haplochromis burtoni: quantitative behavioral observations. Animal Behavior. 1977;25(4):964–75. [Google Scholar]
- 7.Renn SC, Aubin-Horth N, Hofmann HA. Fish and chips: functional genomics of social plasticity in an African cichlid fish. J Exp Biol. 2008;211(Pt 18):3041–56. doi: 10.1242/jeb.018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sneddon LU, Schmidt R, Fang Y, Cossins AR. Molecular correlates of social dominance: a novel role for ependymin in aggression. PLoS One. 2011;6(4):e18181. doi: 10.1371/journal.pone.0018181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol. 2011 doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- 10.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999:877242–57. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 11.Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crews D. The development of phenotypic plasticity: where biology and psychology meet. Dev Psychobiol. 2003;43(1):1–10. doi: 10.1002/dev.10115. [DOI] [PubMed] [Google Scholar]
- 13.Goodson JL, Bass AH. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: Convergence with terrestrial vertebrates reveals conserved traits. Journal of Comparative Neurology. 2002;448(3):298–322. doi: 10.1002/cne.10258. [DOI] [PubMed] [Google Scholar]
- 14.Desjardins JK, Klausner JQ, Fernald RD. Female genomic response to mate information. Proc Natl Acad Sci U S A. 2010;107(49):21176–80. doi: 10.1073/pnas.1010442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoke KL, Ryan MJ, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc Natl Acad Sci U S A. 2005;102(30):10712–7. doi: 10.1073/pnas.0502361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44(4):397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- 17.Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155(2):307–18. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3(11):e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol. 2008;20(6):665–72. doi: 10.1111/j.1365-2826.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 20.Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14(8):4825–30. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74(3):185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann HA. The neuroendocrine action potential. Winner of the 2008 Frank Beach Award in Behavioral Neuroendocrinology. Horm Behav. 2010;58(4):555–62. doi: 10.1016/j.yhbeh.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann HA, Benson ME, Fernald RD. Social status regulates growth rate: consequences for life-history strategies. Proc Natl Acad Sci U S A. 1999;96(24):14171–6. doi: 10.1073/pnas.96.24.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruska KP, Fernald RD. Behavioral and physiological plasticity: Rapid changes during social ascent in an African cichlid fish. Horm Behav. 2010:58230–40. doi: 10.1016/j.yhbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruska KP, Fernald RD. Plasticity of the reproductive axis caused by social status change in an African cichlid fish: II. testicular gene expression and spermatogenesis. Endocrinology. 2011:152291–302. doi: 10.1210/en.2010-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruska KP, Levavi-Sivan B, Biran J, Fernald RD. Plasticity of the reproductive axis caused by social status change in an African cichlid fish: I. pituitary gonadotropins. Endocrinology. 2011:152281–90. doi: 10.1210/en.2010-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kustan JM, Maruska KP, Fernald RD. Subordinate male cichlids retain reproductive competence during social suppression. Proc R Soc B. 2011 doi: 10.1098/rspb.2011.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White SA, Nguyen T, Fernald RD. Social regulation of gonadotropin-releasing hormone. J Exp Biol. 2002;205(Pt 17):2567–81. doi: 10.1242/jeb.205.17.2567. [DOI] [PubMed] [Google Scholar]
- 29.Fernald RD, Shelton LC. The organization of the diencephalon and the pretectum in the cichlid fish, Haplochromis burtoni. J Comp Neurol. 1985;238(2):202–17. doi: 10.1002/cne.902380207. [DOI] [PubMed] [Google Scholar]
- 30.Burmeister SS, Munshi RG, Fernald RD. Cytoarchitecture of a cichlid fish telencephalon. Brain Behav Evol. 2009;74(2):110–20. doi: 10.1159/000235613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munchrath LA, Hofmann HA. Distribution of sex steroid hormone receptors in the brain of an African cichlid fish, Astatotilapia burtoni. J Comp Neurol. 2010;518(16):3302–26. doi: 10.1002/cne.22401. [DOI] [PubMed] [Google Scholar]
- 32.Munoz-Cueto JA, Sarasquete C, Zohar AH, Kah O. An atlas of the brain of the gilthead seabream (Sparus aurata) College Park: Maryland Sea Grant; 2001. [Google Scholar]
- 33.Wulliman MF, Rupp B, Reichert H. Neuroanatomy of the zebrafish brain: a topological atlas. Basel, Switzerland: Birkhauser Verlag; 1996. [Google Scholar]
- 34.Maler L, Sas E, Johnston S, Ellis W. An atlas of the brain of the electric fish Apteronotus leptorhynchus. J Chem Neuroanat. 1991;4(1):1–38. doi: 10.1016/0891-0618(91)90030-g. [DOI] [PubMed] [Google Scholar]
- 35.Wullimann MF, Mueller T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J Comp Neurol. 2004;475(2):143–62. doi: 10.1002/cne.20183. [DOI] [PubMed] [Google Scholar]
- 36.Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119(3):251–60. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- 37.Maruska KP, Fernald RD. Steroid receptor expression in the fish inner ear varies with sex, social status, and reproductive state. BMC Neurosci. 2010;11:58. doi: 10.1186/1471-2202-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12(8):1047–64. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burmeister SS, Kailasanath V, Fernald RD. Social dominance regulates androgen and estrogen receptor gene expression. Horm Behav. 2007;51(1):164–70. doi: 10.1016/j.yhbeh.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maruska KP, Fernald RD. Reproductive status regulates expression of sex steroid and GnRH receptors in the olfactory bulb. Behav Brain Res. 2010:213208–17. doi: 10.1016/j.bbr.2010.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burmeister SS, Fernald RD. Evolutionary conservation of the egr-1 immediate-early gene response in a teleost. J Comp Neurol. 2005;481(2):220–32. doi: 10.1002/cne.20380. [DOI] [PubMed] [Google Scholar]
- 42.Wingfield JC, Hegner R, Dufty A, Jr, Ball GF. The “challenge hypothesis”: theoretical implications for patterns of testosterone secretion, mating system, and breeding strategies. American Naturalist. 1990:136829–46. [Google Scholar]
- 43.Huffman LS, Mitchell MM, O’Connell LA, Hofmann HA. Rising StARs: behavioral, hormonal, and molecular responses to social challenge and opportunity. Horm Behav. 2012;61 (4):631–41. doi: 10.1016/j.yhbeh.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 44.Forlano PM, Bass AH. Neural and hormonal mechanisms of reproductive-related arousal in fishes. Horm Behav. 2010;59(5):616–29. doi: 10.1016/j.yhbeh.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lord LD, Bond J, Thompson RR. Rapid steroid influences on visually guided sexual behavior in male goldfish. Horm Behav. 2009;56(5):519–26. doi: 10.1016/j.yhbeh.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Connell LA, Hofmann HA. Social status predicts how sex steroid receptors regulate complex behavior across levels of biological organization. Endocrinology. 2012:153. doi: 10.1210/en.2011-1663. [DOI] [PubMed] [Google Scholar]
- 47.Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol. 2010;31(3):322–40. doi: 10.1016/j.yfrne.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer SI, Willars GB, Nishida E, Thiel G. Elk-1, CREB, and MKP-1 regulate Egr-1 expression in gonadotropin-releasing hormone stimulated gonadotrophs. J Cell Biochem. 2008;105(5):1267–78. doi: 10.1002/jcb.21927. [DOI] [PubMed] [Google Scholar]
- 49.Martinez M, Calvo-Torrent A, Herbert J. Mapping brain response to social stress in rodents with c-fos expression: a review. Stress. 2002;5(1):3–13. doi: 10.1080/102538902900012369. [DOI] [PubMed] [Google Scholar]
- 50.Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17(22):8842–55. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soma KK, Tramontin AD, Wingfield JC. Oestrogen regulates male aggression in the non-breeding season. Proc Biol Sci. 2000;267(1448):1089–96. doi: 10.1098/rspb.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proc Natl Acad Sci U S A. 2000;97(26):14737–41. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166(1):110–23. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Remage-Healey L, Bass AH. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006;1126(1):27–35. doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 55.Taziaux M, Keller M, Bakker J, Balthazart J. Sexual behavior activity tracks rapid changes in brain estrogen concentrations. J Neurosci. 2007;27(24):6563–72. doi: 10.1523/JNEUROSCI.1797-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imwalle DB, Scordalakes EM, Rissman EF. Estrogen receptor alpha influences socially motivated behaviors. Horm Behav. 2002;42(4):484–91. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A. 1997;94(4):1476–81. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor alpha in select brain nuclei are associated with individual differences in aggression. Horm Behav. 2006;50 (2):338–45. doi: 10.1016/j.yhbeh.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126(1):2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429(3):355–71. [PubMed] [Google Scholar]
- 61.Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31(45):16056–63. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forlano PM, Schlinger BA, Bass AH. Brain aromatase: new lessons from non-mammalian model systems. Front Neuroendocrinol. 2006;27(3):247–74. doi: 10.1016/j.yfrne.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 63.Forlano PM, Bass AH. Seasonal plasticity of brain aromatase mRNA expression in glia: divergence across sex and vocal phenotypes. J Neurobiol. 2005;65(1):37–49. doi: 10.1002/neu.20179. [DOI] [PubMed] [Google Scholar]
- 64.Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for estrogen receptor beta in adult brain function. J Neuroendocrinol. 2011 doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470(7333):221–6. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci U S A. 2010;107(27):12393–8. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirschenhauser K, Wittek M, Johnston P, Mostl E. Social context rather than behavioral output or winning modulates post-conflict testosterone responses in Japanese quail (Coturnix japonica) Physiol Behav. 2008;95(3):457–63. doi: 10.1016/j.physbeh.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 68.Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66(2):260–72. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–34. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31 (9):3271–89. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 2010;57(4–5):381–9. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011;32(3):287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]