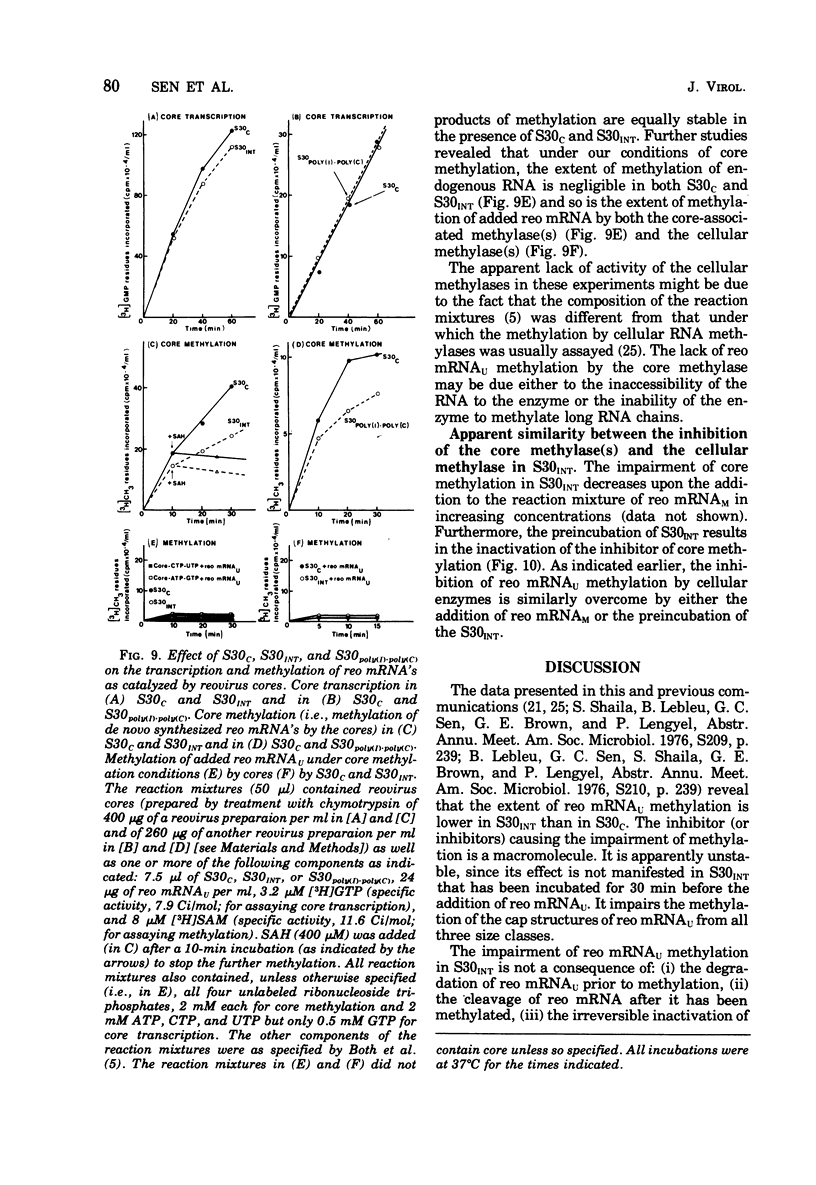
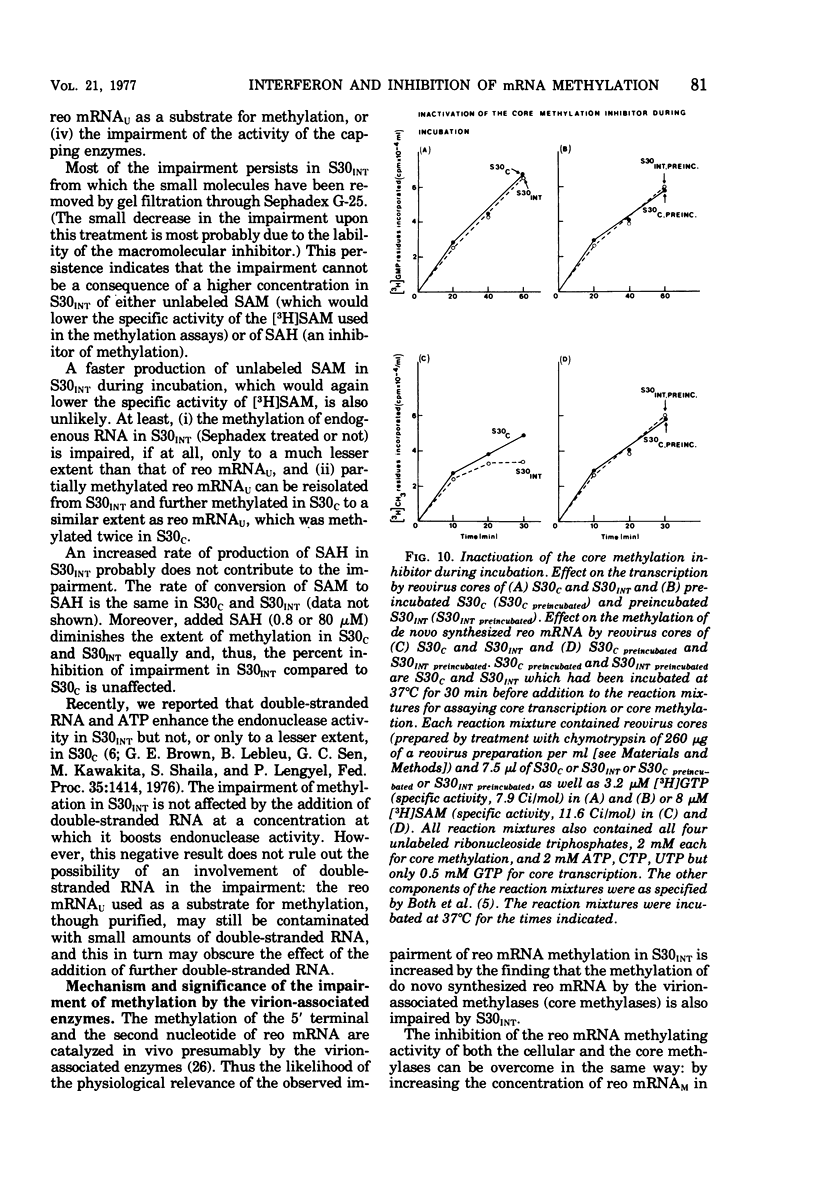
Abstract
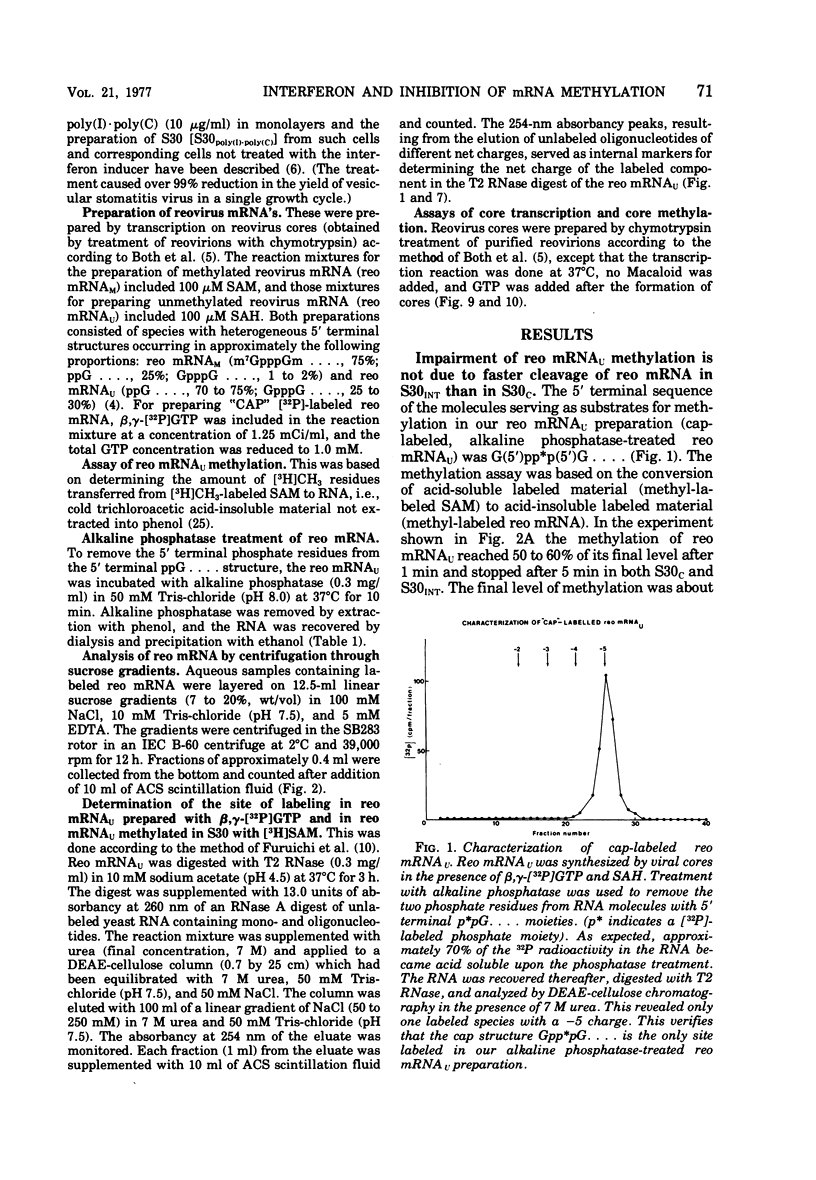
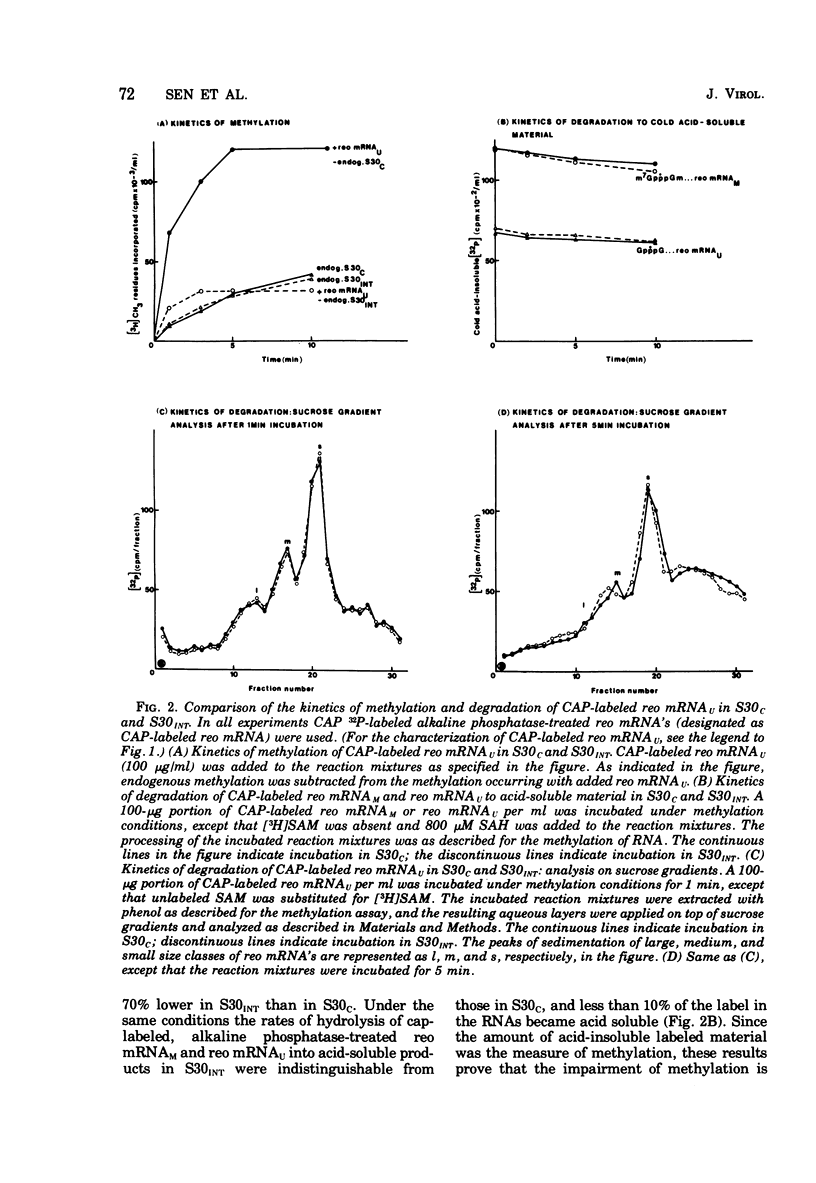
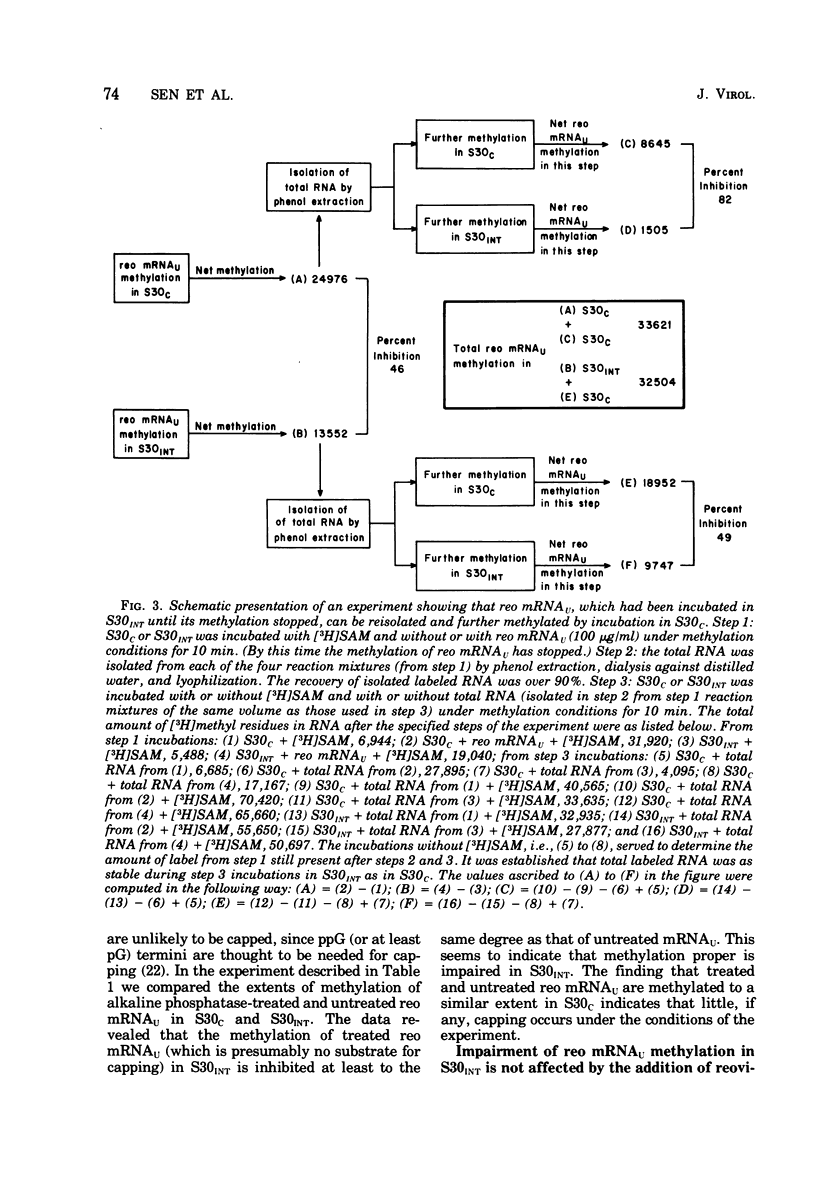
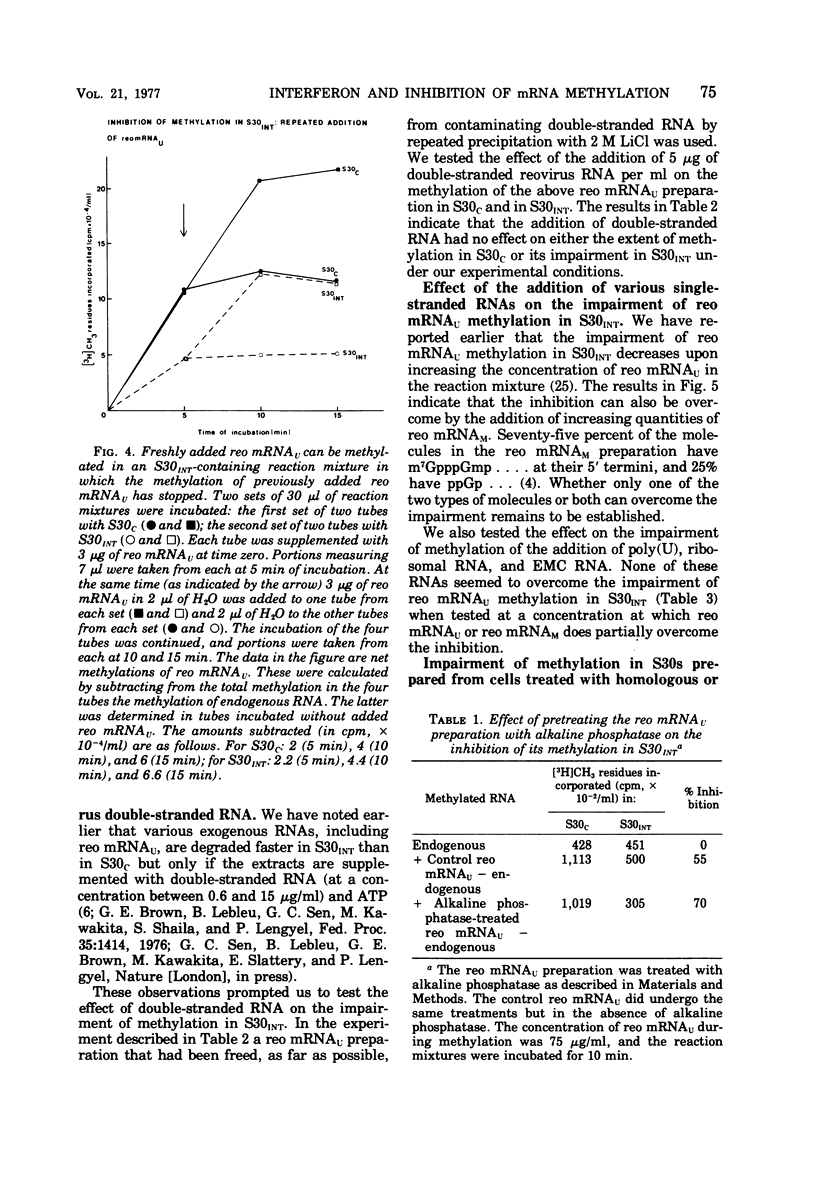
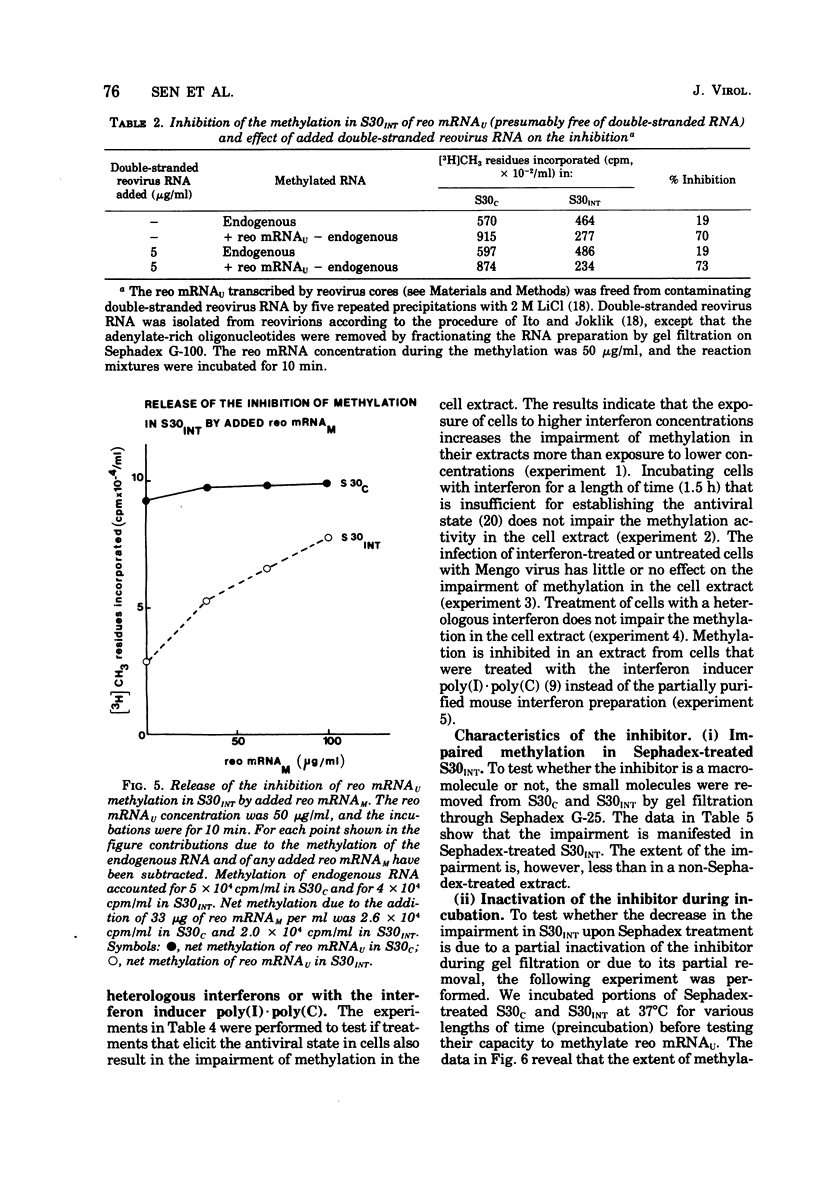
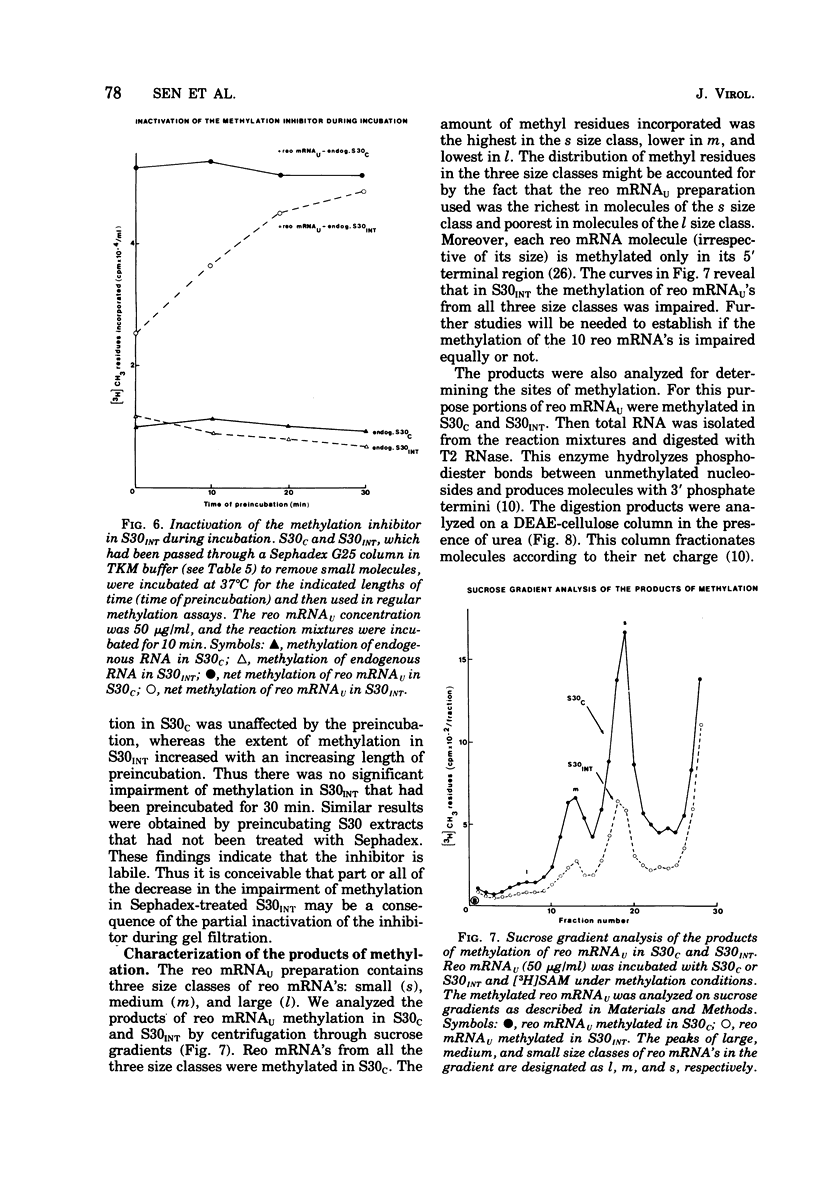
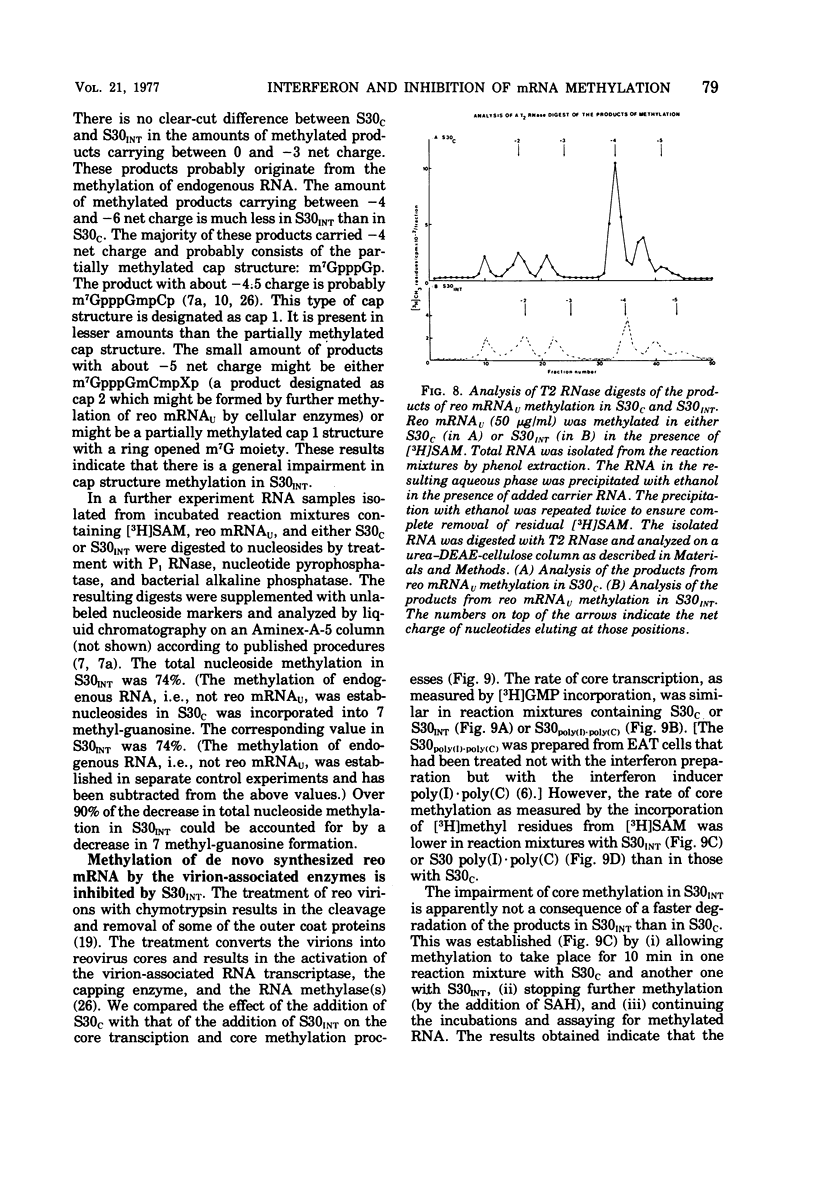
We reported earlier that the methylation of unmethylated reovirus mRNA (reo mRNAU) by the cellular methylating enzymes is impaired in extracts of uninfected, interferon-treated Ehrilich ascites tumor cells (S30INT). We find now that after the methylation of reo mRNAU has stopped in S30INT, the RNA can be reisolated and further methylated in an extract of control cells (S30C). Thus the impairment of methylation in S30INT cannot be due to cleavage or irreversible inactivation of reo mRNAU. Freshly added reo mRNAU can be methylated in S30INT in which the methylation of previously added reo mRNAU has stopped. This indicates that the impairment is due to the depletion of S-adenosylme thionine (the methyl donor), the accumulation of S-adenosylhomocysteine (an inhibitor of methylation), or the irreversible inactivation of reo mRNAU. Freshly added reo mRNAU can be methylated in S30INT in which the methylation of previously added reo mRNAU has stopped. This indicates that the impairment is not due to the depletion of S-adenosylmethionine (the methyl donor), the accumulation of S-adenoxylhomocysteine (an inhibitor of methylation), or the irreversible inactivation of the methylating enzymes. It may be due, however, to the unavailability of reo mRNAU for methylation. The extent of the impairment of reo mRNAU methylation in S30INT decreases with an increasing concentration of reo mRNAU but is not affected by added poly (U), ribosomal RNA, or encephalomyocarditis virus RNA (an mRNA that is probably not capped or methylated at its 5' end). The methylation of reo mRNAU is also impaired in an extract from cells that have not been treated with interferon but with the interferon inducer poly(I) - poly(C). The inhibitor is apparently a macromolecule that is inactivated during incubation. It decreases the methylation at the 7 position of the 5' terminal guanylate residue. In vitro, the rate of reo mRNA synthesis by reovirus cores in the presence of S30INT is the same as in the presence of S30C. However, the methylation of the de novo synthesized reo mRNA by the core-associated methylating enzyme(s) in vitro is inhibited by S30INT but not by S30C. The relevance of these phenomena to the inhibition of reovirus replication in interferon-treated cells remains to be established.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Furuichi Y., Muthukrishnan S., Shatkin A. J. Ribosome binding to reovirus mRNA in protein synthesis requires 5' terminal 7-methylguanosine. Cell. 1975 Oct;6(2):185–195. doi: 10.1016/0092-8674(75)90009-4. [DOI] [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Brown G. E., Lebleu B., Kawakita M., Shaila S., Sen G. C., Lengyel P. Increased endonuclease activity in an extract from mouse Ehrlich ascites tumor cells which had been treated with a partially purified interferon preparation: dependence of double-stranded RNA;. Biochem Biophys Res Commun. 1976 Mar 8;69(1):114–122. doi: 10.1016/s0006-291x(76)80280-x. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Friderici K. H., Rottman F. M. Characterization of Novikoff hepatoma mRNA methylation and heterogeneity in the methylated 5' terminus. Biochemistry. 1975 Oct 7;14(20):4367–4374. doi: 10.1021/bi00691a004. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Darnell J. E. Structural difference between the 5' termini of viral and cellular mRNA in poliovirus-infected cells: possible basis for the inhibition of host protein synthesis. J Virol. 1976 May;18(2):719–726. doi: 10.1128/jvi.18.2.719-726.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. E., Brown M. R. The influence of nutrient limitation in a chemostat on the sensitivity of Pseudomonas aeruginosa to polymyxin and to EDTA. J Antimicrob Chemother. 1975 Dec;1(4):379–386. doi: 10.1093/jac/1.4.379. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Shatkin A. J. 5'-Terminal m-7G(5')ppp(5')G-m-p in vivo: identification in reovirus genome RNA. Proc Natl Acad Sci U S A. 1975 Feb;72(2):742–745. doi: 10.1073/pnas.72.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galster R. L., Lengyel P. Formation and characteristics of reovirus subviral particles in interferon-treated mouse L cells. Nucleic Acids Res. 1976 Mar;3(3):581–598. doi: 10.1093/nar/3.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J. Effect of interferon on synthesis of ssRNA in reovirus type 3-infected L cell cultures. Biochem Biophys Res Commun. 1972 Jun 9;47(5):1228–1236. doi: 10.1016/0006-291x(72)90966-7. [DOI] [PubMed] [Google Scholar]
- Graziadei W. D., 3rd, Lengyel P. Translation of in vitro synthesized reovirus messenger RNAs into proteins of the size of reovirus capsid proteins in a mouse L cell extract. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1816–1823. doi: 10.1016/0006-291x(72)90056-3. [DOI] [PubMed] [Google Scholar]
- Groner Y., Hurwitz J. Synthesis of RNA containing a methylated blocked 5' terminus by HeLa nuclear homogenates. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2930–2934. doi: 10.1073/pnas.72.8.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. L., Graziadei W. D., 3rd, Weideli H., Sopori M. L., Lengyel P. Selective inhibition of viral protein accumulation in interferon-treated cells; nondiscriminate inhibition of the translation of added viral and cellular messenger RNAs in their extracts. Virology. 1974 Jan;57(1):49–63. doi: 10.1016/0042-6822(74)90107-x. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Joklik W. K. Temperature-sensitive mutants of reovirus. I. Patterns of gene expression by mutants of groups C, D, and E. Virology. 1972 Oct;50(1):189–201. doi: 10.1016/0042-6822(72)90359-5. [DOI] [PubMed] [Google Scholar]
- Jordan G. W. Quantitative aspects of interferon-induced plaque reduction: kinetics of interferon action. Virology. 1972 May;48(2):425–432. doi: 10.1016/0042-6822(72)90053-0. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottman F., Shatkin A. J., Perry R. P. Sequences containing methylated nucleotides at the 5' termini of messenger RNAs: possible implications for processing. Cell. 1974 Nov;3(3):197–199. doi: 10.1016/0092-8674(74)90131-7. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Lebleu B., Brown G. E., Rebello M. A., Furuichi Y., Morgan M., Shatkin A. J., Lengyel P. Inhibition of reovirus messenger RNA methylation in extracts of interferon-treated Ehrlich ascites tumor cells. Biochem Biophys Res Commun. 1975 Jul 8;65(1):427–434. doi: 10.1016/s0006-291x(75)80111-2. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Both G. W. Reovirus mRNA: transcription and translation. Cell. 1976 Mar;7(3):305–313. doi: 10.1016/0092-8674(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Van Ventrooij W. J., Henshaw E. C., Hirsch C. A. Nutritional effects on the polyribosome distribution and rate of protein synthesis in Ehrlich ascites tumor cells in culture. J Biol Chem. 1970 Nov 25;245(22):5947–5953. [PubMed] [Google Scholar]
- Vassef A., Beaud G., Paucker K., Lengyel P. Interferon assay based on the inhibition of double-stranded reovirus RNA accumulation in mouse L cells. J Gen Virol. 1973 Apr;19(1):81–87. doi: 10.1099/0022-1317-19-1-81. [DOI] [PubMed] [Google Scholar]
- Wiebe M. E., Joklik T. W. The mechanism of inhibition of reovirus replication by interferon. Virology. 1975 Jul;66(1):229–240. doi: 10.1016/0042-6822(75)90193-2. [DOI] [PubMed] [Google Scholar]


