Abstract
The effect of cyclophosphamide on the growth of Vero, BSC-1, and HeLa cells in monolayer cultures was studied. By using hemocytometer counts and tritiated thymidine uptake as indicators of growth, it was found that cyclophosphamide significantly interfered with the metabolism of Vero and BSC-1 cells when sustained in Leibovitz medium. Vero cells and HeLa cells grown in Eagle medium were not affected by exposure to cyclophosphamide. Vaccinia virus replication in Vero cell monolayer cultures incubated with cyclophosphamide was markedly augmented, and this enhanced growth was reflected by virus quantitation techniques and metabolic studies using tritiated thymidine uptake. No difference in the distribution of infectious particles was found when cyclophosphamide-treated and control infected cultures were compared. Pathways other than through hepatic enzymes appear available to activate cyclophosphamide in vitro. These effects are dependent on both the cell type and the medium in which the cells are grown. Cyclophosphamide can facilitate vaccinia virus replication in vitro through metabolic interactions at the cellular level. The precise mechanisms underlying this effect require further study.
Full text
PDF
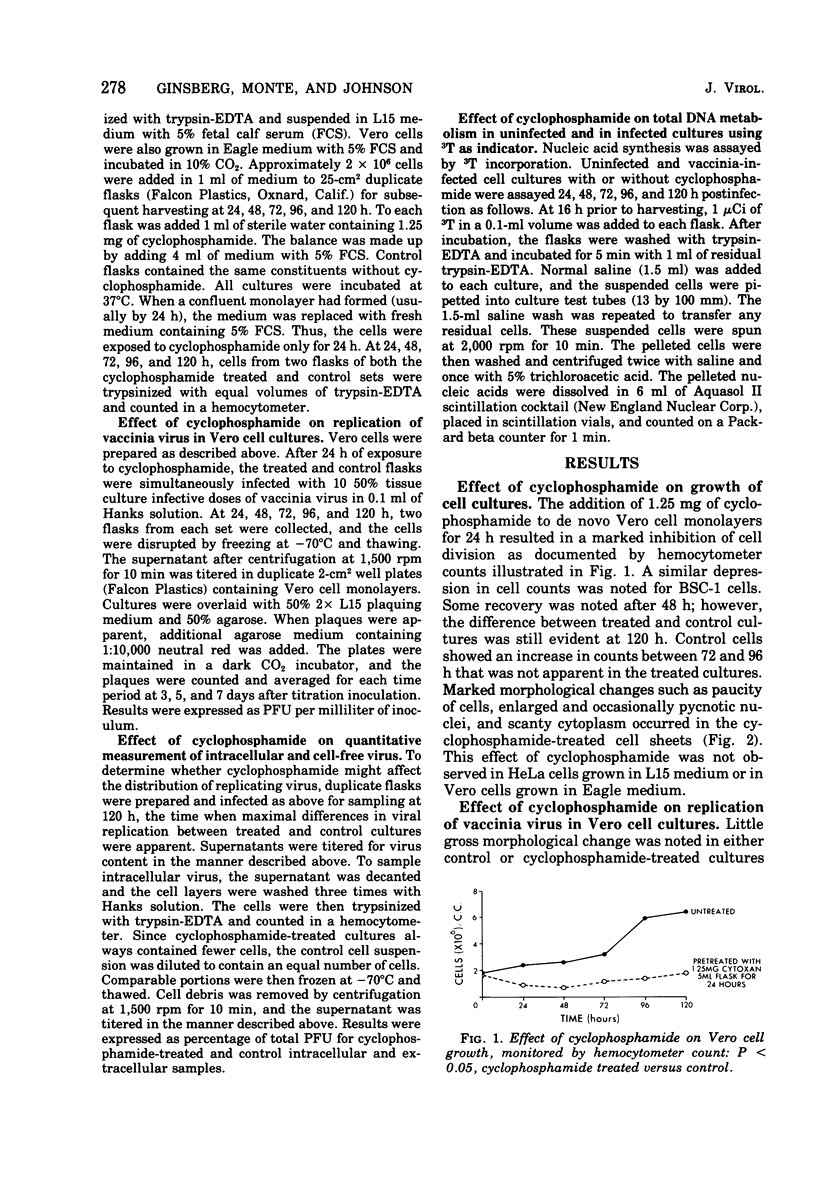

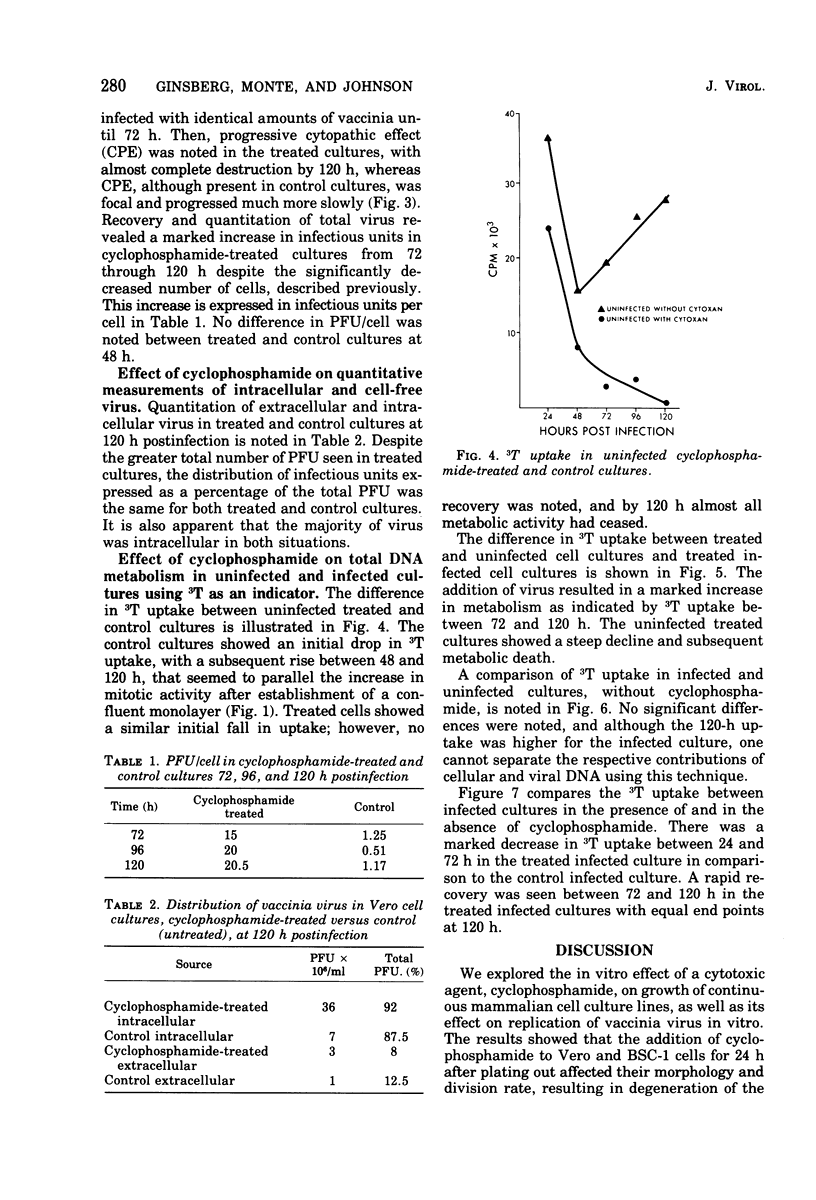
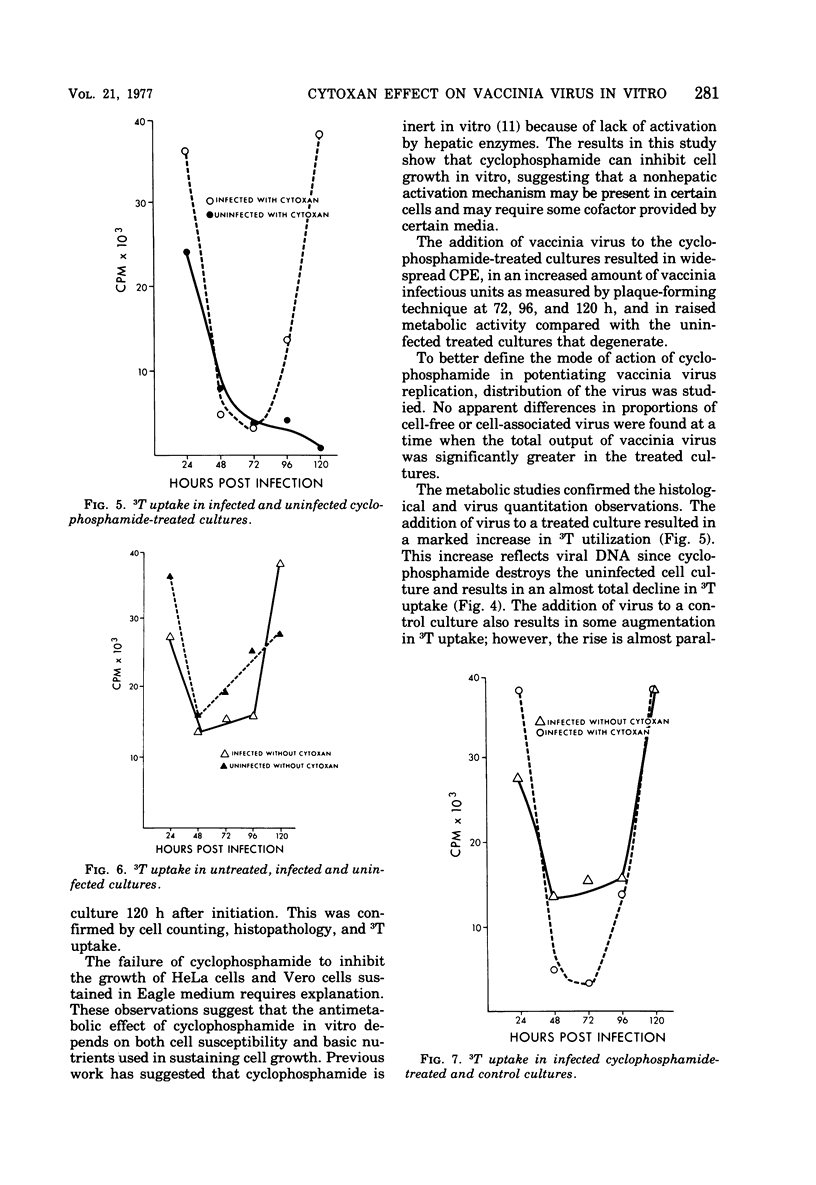


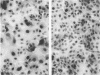
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FOLEY G. E., FRIEDMAN O. M., DROLET B. P. Studies on the mechanism of action of cytoxan. Evidence of activation in vivo and in vitro. Cancer Res. 1961 Jan;21:57–63. [PubMed] [Google Scholar]
- Green J. A., Baron S. 5-iododeoxyuridine potentiation of the replication in vitro of several unrelated RNA and DNA viruses. Science. 1975 Dec 12;190(4219):1099–1101. doi: 10.1126/science.1188388. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Koziorowska J., Chlopkiewicz B. Activation of virus production in vaccinia virus transformed cells by methotrexate. Arch Gesamte Virusforsch. 1973;41(4):334–343. doi: 10.1007/BF01250205. [DOI] [PubMed] [Google Scholar]
- O'Loughlin J. M. Infections in the immunosuppressed patient. Med Clin North Am. 1975 Mar;59(2):495–501. doi: 10.1016/s0025-7125(16)32051-x. [DOI] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Moss B. Inhibition of host protein synthesis by vaccinia virus: fate of cell mRNA and synthesis of small poly (A)-rich polyribonucleotides in the presence of actinomycin D. J Virol. 1975 Jul;16(1):34–42. doi: 10.1128/jvi.16.1.34-42.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek N. E. Bioassay and relative cytotoxic potency of cyclophosphamide metabolites generated in vitro and in vivo. Cancer Res. 1973 Jun;33(6):1150–1158. [PubMed] [Google Scholar]
- Sladek N. E. Therapeutic efficacy of cyclophosphamide as a function of its metabolism. Cancer Res. 1972 Mar;32(3):535–542. [PubMed] [Google Scholar]
- Worthington M. Mechanism of recovery from systemic infection with vaccinia virus. 3. Effects of antithymocyte serum. J Infect Dis. 1973 May;127(5):518–524. doi: 10.1093/infdis/127.5.518. [DOI] [PubMed] [Google Scholar]
- Worthington M., Rabson A. S., Baron S. Mechanism of recovery from systemic vaccinia virus infection. I. The effects of cyclophosphamide. J Exp Med. 1972 Aug 1;136(2):277–290. doi: 10.1084/jem.136.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]