Abstract
Aliphatic hydrocarbons such as fatty alcohols and petroleum-derived alkanes have numerous applications in the chemical industry. In recent years, the renewable synthesis of aliphatic hydrocarbons has been made possible by engineering microbes to overaccumulate fatty acids. However, to generate end products with the desired physicochemical properties (e.g., fatty aldehydes, alkanes, and alcohols), further conversion of the fatty acid is necessary. A carboxylic acid reductase (CAR) from Mycobacterium marinum was found to convert a wide range of aliphatic fatty acids (C6–C18) into corresponding aldehydes. Together with the broad-substrate specificity of an aldehyde reductase or an aldehyde decarbonylase, the catalytic conversion of fatty acids to fatty alcohols (C8–C16) or fatty alkanes (C7–C15) was reconstituted in vitro. This concept was applied in vivo, in combination with a chain-length-specific thioesterase, to engineer Escherichia coli BL21(DE3) strains that were capable of synthesizing fatty alcohols and alkanes. A fatty alcohol titer exceeding 350 mg·L−1 was obtained in minimal media supplemented with glucose. Moreover, by combining the CAR-dependent pathway with an exogenous fatty acid-generating lipase, natural oils (coconut oil, palm oil, and algal oil bodies) were enzymatically converted into fatty alcohols across a broad chain-length range (C8–C18). Together with complementing enzymes, the broad substrate specificity and kinetic characteristics of CAR opens the road for direct and tailored enzyme-catalyzed conversion of lipids into user-ready chemical commodities.
Keywords: biofuel, metabolic engineering, synthetic biology, green chemistry
The production of a vast number of chemicals that are used in today’s society is strongly dependent on the finite supply of fossil fuels. Environmental concerns and rising fossil fuel prices have stimulated a search for alternative approaches that are both sustainable and renewable. The use of engineered microorganisms for the catalytic conversion of photosynthetic metabolites into desired end products is one such approach (1). With recent advances made in biological techniques and tools, microorganisms can be engineered to produce an array of chemicals based on inexpensive, renewable starting materials such as sugars (2).
Aliphatic hydrocarbons such as fatty alcohols and petroleum-derived alkanes have numerous applications as fuels, fragrances, emollients, plasticizers, thickeners, and detergents (Fig. 1A). In nature, the aliphatic backbone of these chemicals can be synthesized de novo via the well-known metabolic process of “fatty acid synthesis” (3). In this process, acetyl-CoA is initially condensed with malonyl-acyl carrier protein (ACP) to form oxoacyl-ACP. A repeated cycle of reactions comprising Claisen condensation, ketone reduction, dehydration, and a double-bond reduction elongates oxoacyl-ACP, two carbon units at a time, to form fatty acyl-ACP, an essential intermediate for membrane synthesis. In recent years, by using chain-specific fatty acyl-ACP thioesterases which cleave fatty acyl-ACP(s) to yield the respective fatty acid(s), the “fatty acid synthesis” pathway has been exploited for the production of aliphatic hydrocarbon chains (4, 5).
Fig. 1.
Conversion of biomass to fatty alcohols and alkanes. (A) Photoautotrophic organisms, via the harvesting of light energy, are able to generate primary carbon sources such as glucose or lipids that can be directed toward the synthesis of fatty alcohols and/or alkanes. AAR, acyl-ACP reductase; ACL, acyl-CoA ligase; ACP, acyl carrier protein; ACR, fatty acyl-CoA reductase [ACR1 (21) and ACR2 (25)]; ADC, cyanobacterial aldehyde decarbonylase; AHR, aldehyde reductase; CAR, carboxylic acid reductase; TES, thioesterase. (B) The catalytic cycle of CAR, adapted from ref. 9. (i) The fatty acid substrate enters the active site and binds within the vicinity of the ATP domain. (ii) An adenosyl moiety is added to the fatty acid, releasing diphosphate. (iii) The thiol group residing on the phosphopantetheine arm (angled solid line) reacts with the electrophilic carbonyl group, resulting in formation of a thioester bond. AMP is released in the process. (iv) The phosphopantetheine arm repositions the thioester intermediate within the NADPH domain. (v) The thioester intermediate is reduced, via hydride transfer from NADPH, to form the aldehyde product. The product along with NADP+ is released and the catalytic cycle is repeated.
However, to generate end products with the desired physicochemical properties, further conversion of the fatty acid is necessary, typically commencing by its “activation” to fatty acyl-CoA. Based on this, Steen et al. (6) devised a pathway for fatty alcohol production by utilizing a fatty acyl-CoA reductase in combination with a native aldehyde reductase (AHR) (Fig. 1A). A second recently discovered pathway toward fatty alcohol production commences with native fatty acids in a preactivated state, by direct conversion of acyl-ACP to aldehyde catalyzed by acyl-ACP reductase, although this system remains poorly understood (7).
Here, we report unique pathways for both fatty alcohol and alkane formation that proceed via another fatty acid activation route that depends on a class of enzymes called carboxylic acid reductase (CAR). These enzymes were previously known to catalyze the reduction of aromatic and short-chain carboxylic acids to their respective aldehydes in the presence of ATP and NADPH (8). A central catalytic feature of CAR enzymes is the prosthetic group 4′-phosphopantetheine, which is covalently linked via a phosphodiester bond to a serine residue. The formation and insertion of this prosthetic group is mediated by a phosphopantetheinyl transferase (9). Three key steps are involved in its reaction mechanism: (i) adenylylation of the bound fatty acid substrate to form an AMP-fatty acyl complex, (ii) formation of a thioester linkage between the fatty acyl moiety and the phosphopantetheine prosthetic group, and (iii) reduction of the thioester intermediate to the aldehyde (Fig. 1B).
In the following work, we (i) describe the biochemical properties, including the substrate profile and kinetic characteristics of a Mycobacterium marinum CAR, (ii) demonstrate how this enzyme can be applied to the microbial production of alkanes and alcohols, (iii) highlight the relevance of this enzyme in complementing fatty acid-generating biological systems, and (iv) illustrate how the broad compatibility between CAR and other downstream enzymes could open up the road for direct and tailored conversion of lipids into user-ready chemical commodities.
Results
Expression and in Vitro Activity of CAR.
A carboxylic acid reductase from Nocardia species was earlier discovered by He et al. (10) and shown to exhibit a broad substrate specificity for carboxylic acids (8). We speculated on whether the observed promiscuity of the CAR family could extend also to longer and linear fatty acids. A putative CAR sequence from Mycobacterium marinum (UniProt accession number B2HN69, CAR) was selected that contained the consensus sequences characteristic of a previously characterized CAR enzyme (10): (i) ATP domain, (ii) phosphopantetheine attachments site (LGGXSXXA), and (iii) Rossman fold for NADPH binding. A phosphopantetheinyl transferase, Sfp, from Bacillus subtilis was coexpressed alongside CAR because this was earlier shown to be essential for maximum activity (9). The CAR enzyme was purified by nickel-based affinity chromatography and its activity was verified in vitro, via the oxidation of NADPH, in the presence of benzoic acid (SI Appendix, Figs. S1 and S2). It was found to have a selective preference for NADPH over NADH with apparent Km values of 362 ± 13 μM, 48 ± 8 μM, and 115 ± 13 μM for benzoic acid, NADPH, and ATP, respectively, and a Vmax of 2.32 ± 0.1 μmol·mg−1 CAR (SI Appendix, Fig. S3). Optimal activity was observed at pH 7.5 with in vitro half-lives of 73, 70, and 48 h at 26, 30, and 37 °C, respectively, indicating it to be a relatively stable enzyme (SI Appendix, Figs. S4 and S5).
Kinetic Characterization of CAR.
The CAR enzyme was further characterized with respect to the aliphatic series of saturated and unsaturated fatty acids. Aldehyde formation was detected by GC-MS for substrates ranging from C4 to C18 (SI Appendix, Fig. S6). Apparent and observed catalytic rates (kcat) were thereafter determined in a high-throughput manner by monitoring of NADPH oxidation. The kcat values ranged from 0.3 ± 0.1 to 289 ± 7 min−1 and decreased progressively with increasing chain length (Fig. 2A). In comparison with its saturated counterpart, the unsaturated C18:1, C18:2, and C18:3 fatty acids elicited faster reaction rates, although the rates were hindered with a higher degree of unsaturation. The apparent Km values, determined for the C4–C12 range of substrates, decreased with increasing substrate chain length, resulting in an improvement in catalytic efficiencies (kcat/Km) toward longer-chain substrates (Fig. 2 B and C and SI Appendix, Fig. S7). Overall, the biochemical characteristics of CAR reveal an enzyme that accepts a broad range of aliphatic fatty acids.
Fig. 2.
The kinetic characterization of CARhis. (A) The observed and apparent kcat values were obtained for fatty acid substrates ranging from C4 to C18, including the C18 unsaturated counterparts. In addition, the (B) Km and (C) catalytic efficiencies (kcat/Km) were determined for the C4–C12 fatty acid substrates.
CAR-Dependent in Vivo Production of Fatty Alcohols and Alkanes.
We considered the possibility of exploiting the CAR enzyme as a biotechnological catalyst for the chemical manipulation of aliphatic fatty acids. The CAR enzyme, in combination with other enzymes, could be used to convert fatty acids to relatively stable aliphatic hydrocarbon end products such as fatty alcohols and alkanes (11). For large-scale synthesis of these chemical commodities, continual replenishment of the cosubstrates of CAR, NADPH, and ATP would be required to enable an economically sustainable process, thus necessitating an in vivo approach. Based on a previous engineering approach (6), along with new insight on the enzymatic capability of CAR, we devised a unique metabolic pathway for the synthesis of fatty alcohols. This pathway comprised four gene products: Escherichia coli TesA for the liberation and metabolic overaccumulation of free fatty acids from the native acyl-ACP/CoA metabolites, B. subtilis Sfp for activation of the CAR enzyme, M. marinum CAR for activation and subsequent reduction of the free fatty acid to the fatty aldehyde, and the previously characterized AHR from Synechocystis species PCC 6803, slr1192 (12), for further reduction of the fatty aldehyde to the fatty alcohol. Induction of the engineered pathway in E. coli under aerobic conditions in complex media resulted in the accumulation of both saturated and unsaturated fatty alcohols (C12–C18) with a titer of ∼200 mg·L−1 of culture (Fig. 3A). Furthermore, replacement of AHR with aldehyde decarbonylase (ADC) led to the formation of undecane, tridecane, pentadecene, and heptadecene with a titer of ∼2 mg·L−1 of culture (Fig. 3A).
Fig. 3.
Application of the CAR enzyme for fatty alcohol and alkane synthesis. (A) In vivo production of fatty alcohols and alkanes. The introduced metabolic pathway is graphically illustrated at the top of A. The recombinant strains TPC- slr1192his (solid black line) and TPC-ADChis (solid blue line) were cultivated for 24 h at 30 °C, 180 rpm. A BL21 (DE3) control strain harboring “empty” plasmids was also included for comparison (solid gray line). After metabolite extraction (Methods), the following chromatographic peaks were identified: 1, C11 alkane; 2, C13 alkane ; 3, C12 aldehyde; 4, C12 alcohol; 5, C12:1 alcohol; 6, C15:1 alkene; 7, C14:1 aldehyde; 8, C14:1 alcohol; 9, C14 alcohol; 10, C17:1 alkene; 11, C14 fatty acid; 12, C16:1 alcohol; 13, C16 alcohol; 14, C16:1 fatty acid; 15, C16 fatty acid; 16, C18:1 alcohol; and 17, C18:1 fatty acid. (B) In vivo production of fatty alcohols in glucose-supplemented minimal media. Various E. coli strains (SI Appendix, Table S3) were cultivated in glucose-supplemented minimal media for up to 48 h. Intracellular fatty alcohol accumulation were determined at specific time intervals. (C) Effect of TesA, CAR, and Ahrhis on the in vivo production of fatty alcohols. All strains were cultivated in minimal media for 24 h at 180 rpm, 30 °C and the total fatty alcohol content was quantified from whole cell cultures (Methods).
Coexpression with E. coli Ahr Results in Enhanced Accumulation of in Vivo Fatty Alcohols.
Relative to other recent studies using minimal media, the in vivo production of fatty alcohols was found to be low. Fatty alcohol titers reached only as high as 49 ± 1 mg·L−1 at a stoichiometric efficiency of 1% (Fig. 3B), and peak production occurred during the midexponential to early stationary phase. The low fatty alcohol titer and stoichiometric efficiency was found to be due to the poor conversion of aldehyde to alcohol since soluble lysate fractions showed relatively weak in vitro AHR activity (SI Appendix, Fig. S8). To further improve the efficiency and yield of the engineered pathway, we considered E. coli as a possible source of AHR candidates, given that fatty alcohol synthesis ensued even for the TPC strain, which lacked the recombinant slr1192(his) (Fig. 3B). A BLAST analysis revealed that E. coli YjgB (hereafter Ahr) had the highest amino acid sequence similarity to slr1192 (64% similarity) and, furthermore, contained the conserved cysteine residues required for zinc binding (13) (SI Appendix, Fig. S9). Preliminary characterization confirmed Ahr to be an aldehyde reductase capable of accepting a broad range of commercially available aldehydes with a selective preference for NADPH. Replacement of slr1192(his) with Ahrhis led to a threefold increase in fatty alcohol titers (148 ± 6 mg·L−1 of culture) along with an improvement in stoichiometric efficiency to 4.3% (Fig. 3B). Total production was further enhanced by cultivation in complex media, or in minimal media with increased glucose and NH4Cl content, resulting in titers of 357 ± 3 and 363 ± 15 mg·L−1 (Fig. 3C), respectively. The inclusion of TesA and CARhis, along with oxygen, was found to be necessary for fatty alcohol production. The improvement in yields for the TPC-Ahrhis strain was attributed to increased AHR activity, arising from elevated levels of Ahrhis (SI Appendix, Fig. S8). We noted also that the “his” variants of slr1192 and Ahr resulted in higher protein expression levels relative to their wild-type counterparts, suggesting that inclusion of an N-terminal his-tag encoding sequence may impart a degree of stability at the (post)transcriptional and/or (post)translational level.
Metabolic Platform for the Broad-Range Synthesis of Fatty Alcohols and Alkanes.
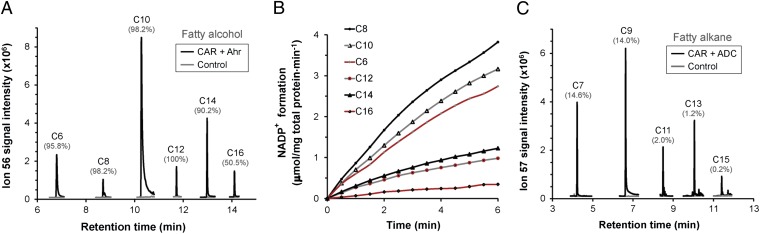
As with the CARhis enzyme, Ahrhis was found to accept a broad range of substrates with specificity for NADPH (SI Appendix, Figs. S10–S12). This display of promiscuity by both enzymes is particularly advantageous because it potentially allows the formation of end products of different chain lengths. To demonstrate this, a series of in vitro reactions was carried out in the presence of both CARhis and Ahrhis with fatty acids (C6 to C16) as the initial substrates. Reactions were supplemented with the necessary cosubstrates and incubated for up to 4 h without shaking at 30 °C. All of the tested fatty acid substrates were found to be reduced to their respective alcohols, as confirmed by GC-MS, with final product yields as high as 100% (Fig. 4A). The C8 fatty acid substrate elicited the highest rate of conversion at 1.12 μmol⋅min−1⋅mg of total protein−1, as deduced from NADPH oxidation (Fig. 4B and SI Appendix, Fig. S13 A–F). Moreover, by replacing Ahrhis with the recently characterized recombinant ADChis (7) from Prochlorococcus marinus, aldehydes could be directed toward the formation of C7–C15 alkanes with product yields exceeding no more than 15% (Figs. 4C and SI Appendix, Fig. S13 G–K). The poor rate of conversion to alkanes was not surprising given the slow turnover (<10 h−1) of the decarbonylase enzyme (14).
Fig. 4.
Broad-range conversion of fatty acids to fatty alcohols and alkanes. (A) In vitro formation of fatty alcohols. Reactions were carried out in 50 mM Tris⋅HCl buffer containing CARhis (10–200 μg·mL−1), Ahrhis (10 μg·mL−1), 1 mM ATP, 10 mM MgCl2, 1 mM NADPH, and 0.25 mM C6–C16 fatty acids. (B) In vitro rate of conversion of fatty acids to alcohols. Reactions were monitored at 340 nm for 6 min at 30-s intervals. (C) In vitro formation of alkanes. Same as A except Ahrhis was replaced with ADChis. In A and C, the percentage yield of product per substrate over a 4-h period is indicated in brackets above each peak.
Coupling of Triacylglycerol Hydrolysis to CAR-Dependent Reduction of Fatty Acids.
Triacylglycerol (TAG), which is highly rich in fatty acyl moieties, could be used as a potential source of substrates for the CAR enzyme. The biochemical conversion of TAG to aldehydes could be accomplished in two steps: (i) lipase-mediated hydrolysis of TAG to yield glycerol and free fatty acids followed by (ii) reduction of the fatty acids to the respective aldehydes by CAR. In vitro reactions containing purified lipase and active CAR, along with the necessary cosubstrates, were incubated with C8 TAG as the starting substrate. Based on size-exclusion HPLC, depletion of the TAG substrate was observed alongside accumulation of the aldehyde product (SI Appendix, Fig. S14A). Omission of either the CAR or lipase enzyme failed to result in aldehyde production, whereas the use of C12 TAG resulted in the generation of the longer C12 aldehyde (SI Appendix, Fig. S14 B–D).
Complementation of CAR-Dependent Routes with Fatty Acid-Generating Systems.
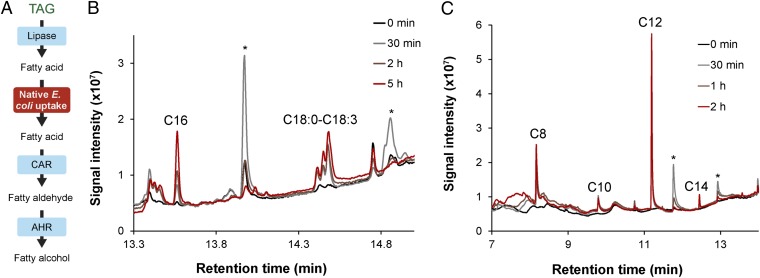
The concept of using TAGs for conversion to fatty alcohols was further developed by engineering a biological system that could convert natural oils, which are highly abundant in TAGs, to fatty alcohols. We noted that in supplementing CAR-overexpressed E. coli cells with a range of fatty acids (C4–C12), in addition to glucose, aldehyde “fragrances” could be detected by smell. This suggested that free fatty acids could be taken up by E. coli cells and made available to the CAR enzyme, exclusive of the FadD coupling mechanism hypothesized by Klein et al. (15). From this, we reasoned that the free fatty acids, upon liberation from the oil substrates by an exogenous lipase, could naturally be assimilated by recombinant E. coli cells, harboring the CAR and Ahrhis enzymes, and metabolically converted to the respective alcohol in the presence of glucose. To test this, preinduced, stationary-phase cells of PC-Ahrhis were supplemented with exogenous Candida rugosa lipase, glucose (required for the cellular synthesis of the cofactors, ATP and NADPH), and three distinct sources of TAGs: algae (Chlamydomonas reinhardtii), coconut oil, and palm oil. In the case of algae, a naturally occurring cell wall-less cc406 strain (16) was used to facilitate the release of oil bodies. As predicted, we observed the formation of C8–C18 fatty alcohols, including their unsaturated counterparts, over a short time period (5 h), (Fig. 5 and SI Appendix, Fig. S15). Maximal production rates of 25.8 ± 0.4, 1.2 ± 0.1, and 2.3 ± 0.4 μg total fatty alcohol⋅h−1⋅mg−1 dry cell weight were obtained for the coconut oil, palm oil, and algal oil bodies substrates, respectively, with a conversion efficiency of ∼10–20%.
Fig. 5.
Conversion of natural oils to fatty alcohols. A simplified reaction scheme is shown in A. Harvested cells from a preinduced culture of PC-Ahr were resuspended in 50 mM potassium phosphate buffered medium, supplemented with glucose and lipase (0.1–1 mg·mL−1), and incubated at 30 °C with shaking at 150 rpm for up to 5 h in the presence of (B) algae (C. reinhardtii strain cc406) or (C) coconut oil. *Fatty acid intermediate.
Discussion
Based on the findings in this work, the CAR enzyme has relevance in the synthesis of fatty acid-derived biofuels and/or chemical commodities due to its catalytic capacity to convert a range of fatty acids to their respective fatty aldehydes, the latter of which serves as a branching point for the synthesis of alkanes and alcohols.
The high levels of fatty alcohol production (>350 mg·L−1) in this study were obtained under relatively simple conditions without high-density cultivation or alteration of the host genome. However, the yields exceed that reported in previous work (6, 7, 17) relating to fatty alcohol production in E. coli, in which maximum productivities of ∼60 mg·L−1 (C12–C16), ∼330 mg·L−1 (C6–C10), and ∼140 mg·L−1 (C14–C18) were reported, in some cases only after extensive reengineering of the host organism. Still, the stoichiometric efficiency of strains expressing the CAR pathway (<5%) is far below the potential maximum, suggesting that further optimization is still possible. The predicted metabolic flux distribution of strains with maximum fatty alcohol yield (SI Appendix, Stoichiometric Evaluation) shows considerable redistribution of central carbon metabolism (high pentose phosphate pathway flux and no fermentation products) relative to strains in which only biomass is the objective function. Optimization of host metabolism and stimulation of fatty acid biosynthesis (4) could therefore lead to higher fatty alcohol yields, assuming that the toxicity of fatty alcohols will not be limiting. Alkane production remains somewhat problematic in E. coli due to the low reported kcat of the decarbonylase enzyme (7 h−1) (14) and the interception by native aldehyde reductases (18), which generally have far greater kcat values (up to 105 min−1) (12). High titers can therefore only be realistically expected in an E. coli background that is devoid of most, if not all, AHR activity after the components and/or environment required for optimal decarbonylase activity have been identified.
A CAR-dependent route could be used to complement systems capable of generating fatty acids from sources such as fatty acyl-ACP (19) or fatty acyl-CoA, as recently shown in the reverse β-oxidation approach by Dellomonaco et al. (17). This concept was demonstrated with a TesA-based pathway and by direct biochemical conversion of oils from plants and whole-cell algae to fatty alcohols. The latter approach is of particular interest because it allows fatty acids to be derived from alternative biological sources such as plants and algae, which are known to accumulate high levels of oils (20). However, the low product yields from such sources (∼10–20%) clearly indicate that a number of potential metabolic limitations (e.g., fatty acid uptake and ATP and NADPH supply) may need to be overcome to further improve the conversion efficiency of these first-generation systems.
One interesting observation with the lipase/TAG pathway relates to the intracellular conversion of fatty acids to the respective fatty alcohols and has implications in our current understanding of the uptake of fatty acids by prokaryotes. A hypothesis put forward by Klein et al. (15) is that fatty acid uptake is tightly coupled to its acylation to fatty acyl-CoA, via the activity of FadD, in a process known as “vectorial acylation.” Because CAR is not able to use fatty acyl-CoA as a substrate, this suggests that the intracellular uptake of free fatty acid can proceed independently of the coupling activity of FadD. A diffusive, flip-flop mechanism is unlikely to account for the near-complete uptake of fatty acid that is observed. Rather, an active transport mechanism must be involved. Together, this suggests that (i) vectorial acylation is not as tightly coupled as previously thought and/or (ii) that an unknown mechanism is in operation that is independent of vectorial acylation. Another possibility is that the CAR and AHR reactions together contribute to vectorial uptake, assuming that the rapid removal of fatty acids from the inner membrane is the essential contribution of FadD to fatty acid uptake. From a thermodynamic standpoint, it is unlikely that CAR alone can substitute for FadD, although CAR and AHR collectively could (SI Appendix, Table S1). Either way, elucidating the mechanism would be advantageous for the future engineering of prokaryotic hosts that depend on an external supply of fatty acids.
The chain length and the presence or lack of chemical functional groups will strongly influence the physicochemical properties and sensory attributes of the end products. Controlling these parameters would potentially allow the synthesis of a range of hydrocarbon end products with numerous applications. Up until now, no system, either in vitro or in vivo, has demonstrated the conversion of a fatty acid chain-length profile as broad as C6 to C18. In the work described by Steen et al. (6), the chain length range was limited to C12 to C16, a constraint that arises from the narrow substrate profile of the ACR1 enzyme (21). The recently discovered acyl-ACP reductases from cyanobacteria similarly display a very narrow chain-length specificity (C14 to C18) (7), whereas Dellomonaco et al. (17) reported chain lengths predominantly within the C4 to C10 range. In the present study, the broad and overlapping substrate profiles of the CAR, Ahr, and ADC enzymes allow a much wider range of products (C6–C18), including both fatty alcohols and alkanes, to be formed from the respective fatty acids. Providing that chain-length specificity can be controlled at the level of the thioesterase (22), the CAR-based platform provides a sound foundation for the “metabolic tuning” of the chain length of alkanes and fatty alcohols. Of course, the use of broad-range enzymes such as CAR and ADC could compromise the survival and integrity of the host system by unwanted conversion of key metabolites from other pathways. The engineering of host systems for efficient and high-yield synthesis of a narrow range of end products will more than likely depend on enzymes with a constricted, rather than broad, range of substrate specificity. Nevertheless, further refinements of the system could be made by selecting for enzyme isoforms with the desired kinetic characteristics.
The main rationale for engineering the CAR–dependent pathways was to broaden the substrate chain-length range of fatty alcohol and alkane synthesis. For the purpose of metabolic engineering, however, these pathways offer several other potential benefits, in comparison with other reported fatty alcohol pathways (Fig. 1), that are summarized in SI Appendix, Tables S1 and S2. First, both CAR and FadD use ATP hydrolysis, resulting in pathways with an overall standard free-energy change that is more favorable (−35.9 kJ·mol−1) than the cyanobacterial pathway (−3.9 kJ·mol−1). Second, the in vitro catalytic rates of the enzymes required for the CAR-dependent pathways greatly exceed those of the AAR- and FadD-dependent pathways. Third, the enhanced AHR activity afforded by Ahr overexpression greatly stimulates fatty alcohol synthesis (Fig. 3). In addition, the CAR enzyme with its phosphopantetheine cofactor could well present an advantage over the CoA-dependent FadD pathway. Unlike FadD, which requires a steady supply of free CoA for fatty acid activation, the covalently attached phosphopantetheine forms the structural and chemical prerequisite for fatty acid activation, thus avoiding a direct need for CoA. Furthermore, to facilitate the two-step conversion of fatty acid to aldehyde, the fatty acid substrate remains tethered to CAR (via a thioester linkage). Such a mechanism is likely to confer a spatial and steric advantage over the FadD pathway, which is dependent on the sequential release of distinct intermediates from the enzyme active site. It is difficult to speculate upon the relative significance of the above parameters for pathway flux, because there are also other unknown factors, such as substrate localization and total in vivo activity of key enzymes (CAR, ACR1, and AAR) that are likely to influence pathway functionality. In addition, the yield differences between this and other reported studies cannot be attributed to changes in pathway stoichiometry because the potential maximum yield of fatty alcohols is only 1.7–1.9% greater for the cyanobacteria pathway (SI Appendix, Stoichiometric Evaluation). Nevertheless, where known, the above parameters are all favorable for the CAR–AHR pathway, which may explain its higher productivity compared with previously engineered pathways (6, 7, 17).
Aside from CAR, another important pathway component identified in the present study is Ahr. In terms of enzyme functionality, this compares very favorably with the E. coli aldehyde reductase, YqhD, and would most likely be well suited for engineering purposes. Although the activity of Ahr has recently been hinted at (18), we unequivocally demonstrate that this enzyme catalyzes the reduction of aliphatic aldehydes to alcohols with a substrate profile ranging, at least, from C4 to C16 and a selective preference for NADPH. Based on the experimental verification of its catalytic activity in this study, as well as others (18, 23), a replacement of the current gene name, yjgB, with ahr (aldehyde reductase) is proposed to denote the catalytic function of this gene product.
In summary, given its capacity to accept a broad range of aliphatic fatty acid substrates, the CAR enzyme family will, undoubtedly, be a useful catalyst for the synthesis of fatty acid-derived chemical commodities such as fatty alcohols and alkanes.
Methods
Full protocols are given in SI Appendix, Methods.
Strains and Plasmids.
Genes encoding M. marinum CAR and Sfp from B. subtilis were codon-optimized for E. coli (Genscript), whereas tesA, slr1192, and ahr were amplified from E. coli BL21(DE3) and Synechocystis species PCC 6803 genomes by PCR. Plasmid vectors were constructed by standard methods and are described in SI Appendix, Tables S3–S5. Multiple genes were assembled in artificial operons (24). Plasmids were used to transform E. coli BL21 (DE3) to generate the strains listed in SI Appendix, Table S6 with the gene products listed in SI Appendix, Table S7.
Protein Expression and Purification.
Cells were grown in Overnight Express Instant TB Medium (Novagen) and lysed with lysozyme (2 mg·mL−1) and 2% (vol/vol) hexane. Recombinant proteins were purified with His-Select Nickel Affinity Gel (Sigma Aldrich) according to the manufacturer’s instructions.
Enzyme Characterization.
For CAR assays, reactions contained CAR (0–10 μg·mL−1), 1 mM NADPH, 1 mM ATP, 10 mM MgCl2, and 0.5 mM fatty acids. For AHR assays, reactions contained Ahrhis (1 or 10 μg·mL−1), 1 mM NADPH, and 0.5 mM C4–C12 aldehydes or C4–C12 alcohols. For qualitative confirmation of aldehydes and alcohols, reaction mixtures (500 μL) were mixed with chloroform or acetone and the organic phase/supernatant was analyzed by GC-MS.
In Vivo Production of Fatty Alcohols.
Strains were cultivated either in Overnight Express Instant TB Medium (Novagen) or in defined minimal medium. For total fatty alcohol quantification, 100 μL of cell culture was vigorously mixed with 200 μL of acetone and microfuged (17,000 × g, 5 min) and the resulting supernatant was analyzed by GC-MS. All stoichiometric evaluations were made in minimal media. Where an error is given in the text it represents the SE (SEM, n = 2–4). The calculation of the stoichiometric efficiency is described in SI Appendix, Methods.
In Vitro and in Vivo Production of Fatty Alcohols and Alkanes.
In vitro alkane synthesis was carried out as described previously (14) with the addition of CARhis (100 μg·mL−1), 1 mM NADPH, 1 mM ATP, and 0.5 mM fatty acid (C4–C16). For in vitro fatty alcohol formation, ADChis was replaced with Ahrhis (10 μg·mL−1). For in vitro conversion of TAG to aldehydes, fatty acid substrate was substituted with lipase (100 μg·mL−1) and 1 mM suspensions of the commercially purified TAGs. For lipase-mediated in vivo formation of fatty alcohols, cells were resuspended in potassium phosphate buffer with TAG sources (1), C. reinhardtii cc406 (2), palm oil (Afroase), and (3) coconut oil (Biona Organic).
Supplementary Material
Acknowledgments
We thank B. A. Nebel for setting up size-exclusion HPLC analysis, O. Kruse and M. Jokel for supplying the cc406 algal strain, N. Marsh for supplying the pET28-ADChis plasmid, and A. Pasztor and F. Guerrero for the cloning of adc and tesA, respectively. This work was supported by the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/European Research Council Grant Agreement 260661 (to P.R.J.), and a Wolfson Research Merit Award from the Royal Society (to N.J.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216516110/-/DCSupplemental.
References
- 1.Keasling JD. Manufacturing molecules through metabolic engineering. Science. 2010;330(6009):1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- 2.Keasling JD. Synthetic biology and the development of tools for metabolic engineering. Metab Eng. 2012;14(3):189–195. doi: 10.1016/j.ymben.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Chan DI, Vogel HJ. Current understanding of fatty acid biosynthesis and the acyl carrier protein. Biochem J. 2010;430(1):1–19. doi: 10.1042/BJ20100462. [DOI] [PubMed] [Google Scholar]
- 4.Liu T, Vora H, Khosla C. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab Eng. 2010;12(4):378–386. doi: 10.1016/j.ymben.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Brune D, Vermaas W, Curtiss R., III Production and secretion of fatty acids in genetically engineered cyanobacteria. Proc Natl Acad Sci USA. 2010;108(17):6899–6904. doi: 10.1073/pnas.1001946107. [DOI] [PubMed] [Google Scholar]
- 6.Steen EJ, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature. 2010;463(7280):559–562. doi: 10.1038/nature08721. [DOI] [PubMed] [Google Scholar]
- 7.Schirmer A, Rude MA, Li X, Popova E, del Cardayre SB. Microbial biosynthesis of alkanes. Science. 2010;329(5991):559–562. doi: 10.1126/science.1187936. [DOI] [PubMed] [Google Scholar]
- 8.Venkitasubramanian P, Daniels L, Rosazza JPN. In: Biocatalytic Reduction of Carboxylic Acids: Mechanism and Applications Biocatalysis in Pharmaceutical and Biotechnology Industries. Patel R, editor. Boca Raton, FL: CRC; 2006. pp. 425–440. [Google Scholar]
- 9.Venkitasubramanian P, Daniels L, Rosazza JP. Reduction of carboxylic acids by Nocardia aldehyde oxidoreductase requires a phosphopantetheinylated enzyme. J Biol Chem. 2007;282(1):478–485. doi: 10.1074/jbc.M607980200. [DOI] [PubMed] [Google Scholar]
- 10.He A, Li T, Daniels L, Fotheringham I, Rosazza JP. Nocardia sp. carboxylic acid reductase: Cloning, expression, and characterization of a new aldehyde oxidoreductase family. Appl Environ Microbiol. 2004;70(3):1874–1881. doi: 10.1128/AEM.70.3.1874-1881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Rodriguez S, Keasling JD. Metabolic engineering of microbial pathways for advanced biofuels production. Curr Opin Biotechnol. 2011;22(6):775–783. doi: 10.1016/j.copbio.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Vidal R, López-Maury L, Guerrero MG, Florencio FJ. Characterization of an alcohol dehydrogenase from the cyanobacterium Synechocystis sp. strain PCC 6803 that responds to environmental stress conditions via the Hik34-Rre1 two-component system. J Bacteriol. 2009;191(13):4383–4391. doi: 10.1128/JB.00183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid MF, Fewson CA. Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol. 1994;20(1):13–56. doi: 10.3109/10408419409113545. [DOI] [PubMed] [Google Scholar]
- 14.Eser BE, Das D, Han J, Jones PR, Marsh EN. Oxygen-independent alkane formation by non-heme iron-dependent cyanobacterial aldehyde decarbonylase: investigation of kinetics and requirement for an external electron donor. Biochemistry. 2011;50(49):10743–10750. doi: 10.1021/bi2012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein K, Steinberg R, Fiethen B, Overath P. Fatty acid degradation in Escherichia coli: An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- 16.Davies DR, Plaskitt A. Genetical and structural analyses of cell-wall formation in Chlamydomonas reinhardi. Genet Res. 1971;17(1):33–43. [Google Scholar]
- 17.Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature. 2011;476(7360):355–359. doi: 10.1038/nature10333. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez GM, Atsumi S. Isobutyraldehyde production from Escherichia coli by removing aldehyde reductase activity. Microb Cell Fact. 2012;11(1):90. doi: 10.1186/1475-2859-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho H, Cronan JE., Jr Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J Biol Chem. 1995;270(9):4216–4219. doi: 10.1074/jbc.270.9.4216. [DOI] [PubMed] [Google Scholar]
- 20.Durrett TP, Benning C, Ohlrogge J. Plant triacylglycerols as feedstocks for the production of biofuels. Plant J. 2008;54(4):593–607. doi: 10.1111/j.1365-313X.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- 21.Reiser S, Somerville C. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J Bacteriol. 1997;179(9):2969–2975. doi: 10.1128/jb.179.9.2969-2975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jing F, et al. Phylogenetic and experimental characterization of an acyl-ACP thioesterase family reveals significant diversity in enzymatic specificity and activity. BMC Biochem. 2011;12:44. doi: 10.1186/1471-2091-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koma D, Yamanaka H, Moriyoshi K, Ohmoto T, Sakai K. Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway. Appl Environ Microbiol. 2012;78(17):6203–6216. doi: 10.1128/AEM.01148-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akhtar MK, Jones PR. Engineering of a synthetic hydF-hydE-hydG-hydA operon for biohydrogen production. Anal Biochem. 2008;373(1):170–172. doi: 10.1016/j.ab.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Willis RM, Wahlen BD, Seefeldt LC, Barney BM. Characterization of a fatty acyl-CoA reductase from Marinobacter aquaeolei VT8: A bacterial enzyme catalyzing the reduction of fatty acyl-CoA to fatty alcohol. Biochemistry. 2011;50(48):10550–10558. doi: 10.1021/bi2008646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.