Abstract
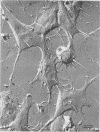
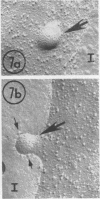
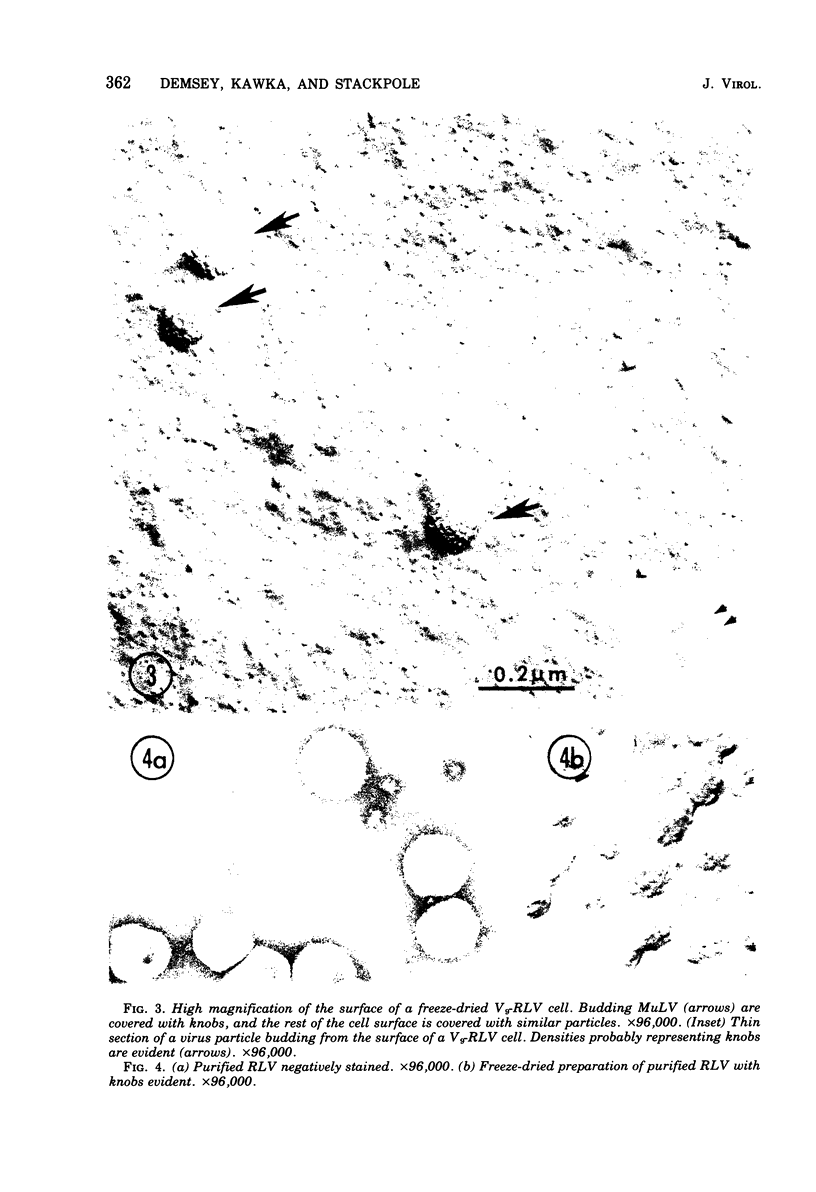
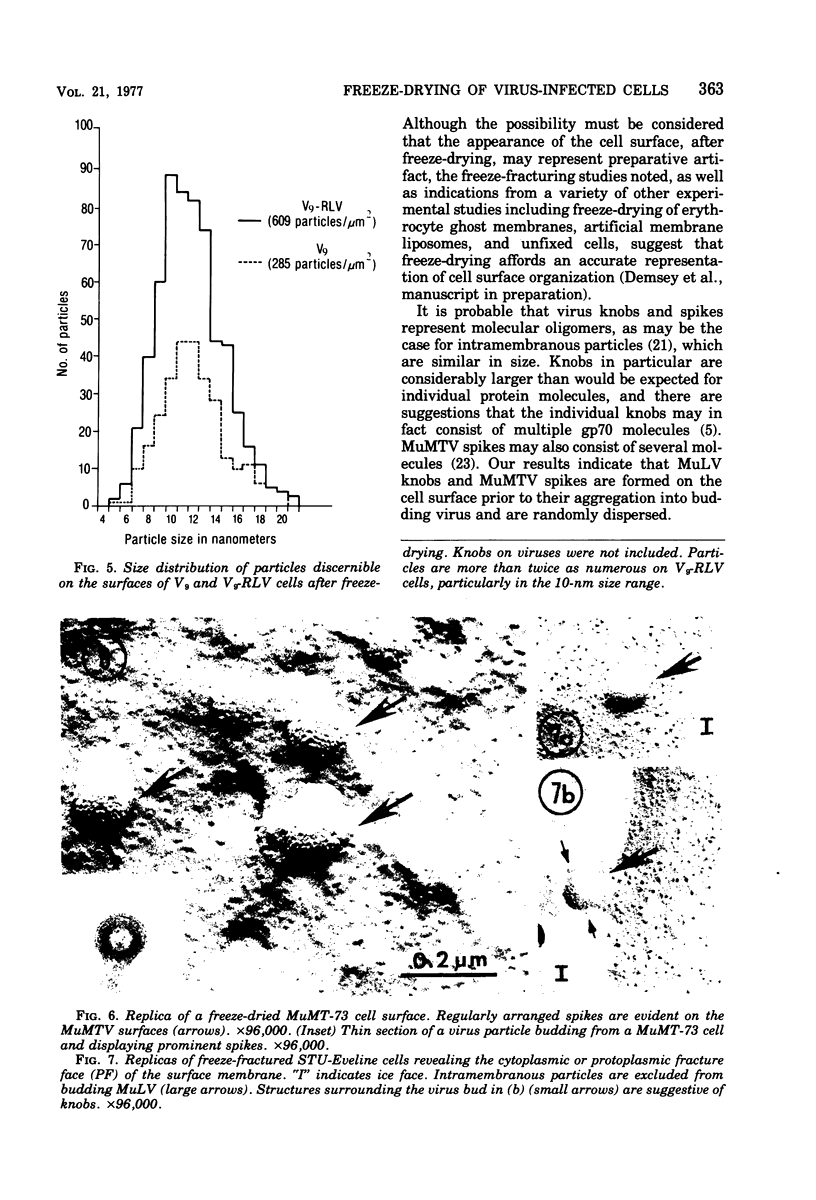
Using a method for freeze-drying intact cells, uninfected and murine leukemia virus (MuLV)-infected JLSV9 cell surfaces, as well as murine mammary tumor virus (MuMTV)-infected cell surfaces, were examined by electron microscopy. The 10-nm knobs of MuLV and the 5-nm spikes of MuMTV were clearly revealed on the surfaces of budding viruses and were also found dispersed over the cell surface. The MuLV knobs are randomly arranged on the virus surface, whereas the MuMTV spikes are much more ordered. Because freeze-fractured budding viral envelopes are devoid of intramembranous particles, the observed surface particles do not appear to be merely accentuated intramembranous particles. This technique should permit further analysis of the morphogenesis of viral envelopes without the need for externally applied labels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- August J. T., Bolognesi D. P., Fleissner E., Gilden R. V., Nowinski R. C. A proposed nomenclature for the virion proteins of oncogenic RNA viruses. Virology. 1974 Aug;60(2):595–600. doi: 10.1016/0042-6822(74)90356-0. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Strauss E. G., Strauss J. H. Replication of Sindbis virus. 3. An electron microscopic study of virus maturation using the surface replica technique. Virology. 1973 Dec;56(2):429–438. doi: 10.1016/0042-6822(73)90047-0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. Structural components of rna tumor viruses. Adv Virus Res. 1974;19:315–359. doi: 10.1016/s0065-3527(08)60663-6. [DOI] [PubMed] [Google Scholar]
- Davis W. C. H-2 antigen on cell membranes: an explanation for the alteration of distribution by indirect labeling techniques. Science. 1972 Mar 3;175(4025):1006–1008. doi: 10.1126/science.175.4025.1006. [DOI] [PubMed] [Google Scholar]
- Del Vellano B. C., Nave B., Croker B. P., Lerner R. A., Dixon F. J. The oncornavirus glycoprotein gp69/71: a constituent of the surface of normal and malignant thymocytes. J Exp Med. 1975 Jan 1;141(1):172–187. doi: 10.1084/jem.141.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Reese T. S. Structural changes in the membrane of vero cells infected with a paramyxovirus. J Cell Biol. 1975 Dec;67(3):551–565. doi: 10.1083/jcb.67.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H. W., Cooper T. W. Electron microscope studies of the microvilli of HeLa cells. J Cell Biol. 1967 Aug;34(2):569–576. doi: 10.1083/jcb.34.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. D., Follett E. A. The structure of the major cell processes of isolated BHK21 fibroblasts. Exp Cell Res. 1969 Oct;57(2):263–276. doi: 10.1016/0014-4827(69)90150-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Kanzaki T. Surface ultrastructure of tissue cultured keratinocytes. J Ultrastruct Res. 1974 Nov;49(2):252–269. doi: 10.1016/s0022-5320(74)80036-5. [DOI] [PubMed] [Google Scholar]
- Hunsmann G., Claviez M., Moennig V., Schwarz H., Schäfer W. Properties of mouse leukemia viruses. X. Occurrence of viral structural antigens on the cell surface as revealed by a cytotoxicity test. Virology. 1976 Jan;69(1):157–168. doi: 10.1016/0042-6822(76)90203-8. [DOI] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Pister L., Seifert E., Schäfer W. Properties of mouse leukemia viruses. VIII. The major viral glycoprotein of Friend leukemia virus. Seroimmunological, interfering and hemagglutinating capacities. Virology. 1974 Dec;62(2):307–318. doi: 10.1016/0042-6822(74)90394-8. [DOI] [PubMed] [Google Scholar]
- Hunsmann G., Moennig V., Schäfer W. Properties of mouse leukemia viruses. IX. Active and passive immunization of mice against Friend leukemia with isolated viral GP71 glycoprotein and its corresponding antiserum. Virology. 1975 Jul;66(1):327–329. doi: 10.1016/0042-6822(75)90203-2. [DOI] [PubMed] [Google Scholar]
- Kennel S. J., Del Villano B. C., Levy R. L., Lerner R. A. Properties of an oncornavirus glycoprotein: evidence for its presence on the surface of virions and infected cells. Virology. 1973 Oct;55(2):464–475. doi: 10.1016/0042-6822(73)90188-8. [DOI] [PubMed] [Google Scholar]
- Kennel S. J., Feldman J. D. Distribution of viral glycoprotein gp 69/71 on cell surfaces of producer and nonproducer cells. Cancer Res. 1976 Jan;36(1):200–208. [PubMed] [Google Scholar]
- Luftig R. B., McMillan P. N., Bolognesi D. P. An ultrastructural study of C-type virion assembly in mouse cells. Cancer Res. 1974 Dec;34(12):3303–3310. [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Phillips E. R., Perdue J. F. Ultrastructural distribution of cell surface antigens in avian tumor virus-infected chick embryo fibroblasts. J Cell Biol. 1974 Jun;61(3):743–756. doi: 10.1083/jcb.61.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto da Silva P., Branton D. Membrane splitting in freeze-ethching. Covalently bound ferritin as a membrane marker. J Cell Biol. 1970 Jun;45(3):598–605. doi: 10.1083/jcb.45.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Dion A. S. Polypeptides of the mouse mammary tumor virus. I. Characterization of two group-specific antigens. Virology. 1975 Apr;64(2):471–491. doi: 10.1016/0042-6822(75)90125-7. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. Surface structure of mouse mammary tumor virus. Virology. 1974 Sep;61(1):38–55. doi: 10.1016/0042-6822(74)90240-2. [DOI] [PubMed] [Google Scholar]
- Sarkar N. H., Taraschi N. E., Pomenti A. A., Dion A. S. Polypeptides of the mouse mammary tumor virus. II. Identification of two major glycoproteins with the viral structure. Virology. 1976 Feb;69(2):677–690. doi: 10.1016/0042-6822(76)90496-7. [DOI] [PubMed] [Google Scholar]
- Schwarz H., Hunsmann G., Moenning V., Schäfer W. Properties of mouse leukemia viruses. XI. Immunoelectron microscopic studies on viral structural antigens on the cell surface. Virology. 1976 Jan;69(1):169–178. doi: 10.1016/0042-6822(76)90204-x. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Demsey A., Frank H., Hunsmann G., Lange J., Moennig V., Pister L., Bolognesi D. P., Green R. W., Luftig R. B. Morphological, chemical, and antigenic organization of mammalian C-type viruses. Bibl Haematol. 1975;(40):497–515. doi: 10.1159/000397568. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Lange J., Fischinger P. J., Frank H., Bolognesi D. P., Pister L. Properties of mouse leukemia viruses. II. Isolation of viral components. Virology. 1972 Jan;47(1):210–228. doi: 10.1016/0042-6822(72)90253-x. [DOI] [PubMed] [Google Scholar]
- Seifert E., Claviez M., Frank H., Hunsmann G., Schwarz H., Schäfer W. XII. Produktion grösserer Mengen von Friend-Virus durch eine permanente Zell-Suspensions-Kultur (Eveline-Suspensions-Zellen) Z Naturforsch C. 1975 Sep-Oct;30(5):698–700. [PubMed] [Google Scholar]
- Sheffield J. B. Envelope of mouse mammary tumor virus studied by freeze-etching and freeze-fracture techniques. J Virol. 1973 Sep;12(3):616–624. doi: 10.1128/jvi.12.3.616-624.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield J. B. Membrane alterations which accompany MuMTV maturation. I. Studies by freeze-cleave techniques. Virology. 1974 Jan;57(1):287–290. doi: 10.1016/0042-6822(74)90130-5. [DOI] [PubMed] [Google Scholar]
- Silva P. P., Nicolson G. L. Freeze-etch localization of concanavalin A receptors to the membrane intercalated particles of human erythrocyte ghost membranes. Biochim Biophys Acta. 1974 Sep 23;363(3):311–319. doi: 10.1016/0005-2736(74)90071-6. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Revel J. P. Mapping of concanavalin A binding sites on the surface of several cell types. Dev Biol. 1972 Mar;27(3):434–441. doi: 10.1016/0012-1606(72)90183-2. [DOI] [PubMed] [Google Scholar]
- Stackpole C. W., De Milio L. T., Hämmerling U., Jacobson J. B., Lardis M. P. Hybrid antibody-induced topographical redistribution of surface immunoglobulins, alloantigens, and concanavalin A receptors on mouse lymphoid cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):932–936. doi: 10.1073/pnas.71.3.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Tillack T. W., Marchesi V. T. Demonstration of the outer surface of freeze-etched red blood cell membranes. J Cell Biol. 1970 Jun;45(3):649–653. doi: 10.1083/jcb.45.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Weissman I. L., Kaplan H. S. Structural characteristics of some murine RNA tumor viruses studied by lactoperoxidase iodination. Proc Natl Acad Sci U S A. 1973 Jan;70(1):36–40. doi: 10.1073/pnas.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter R., Frank H., Moennig V., Hunsmann G., Lange J., Schäfer W. Properties of mouse leukemia viruses. IV. Hemagglutination assay and characterization of hemagglutinating surface components. Virology. 1973 Aug;54(2):330–345. doi: 10.1016/0042-6822(73)90147-5. [DOI] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Hardy W. D., Jr, Fleissner E. Common cell surface antigen associated with mammalian C-type RNA viruses. Cell membrane-bound gs antigen. J Exp Med. 1974 Apr 1;139(4):925–942. doi: 10.1084/jem.139.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Normal distribution, patching and capping of lymphocyte surface immunoglobulin studied by electron microscopy. Nat New Biol. 1973 Feb 28;241(113):257–259. doi: 10.1038/newbio241257a0. [DOI] [PubMed] [Google Scholar]