Abstract
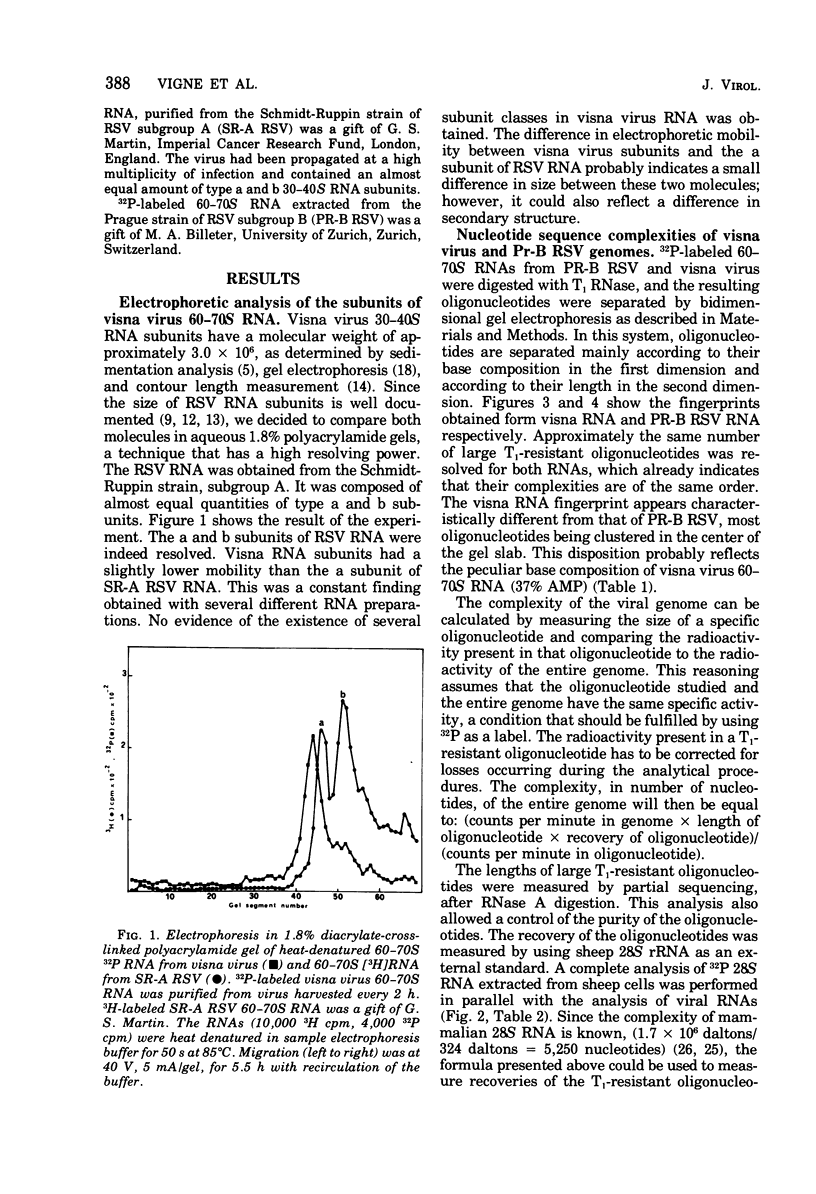
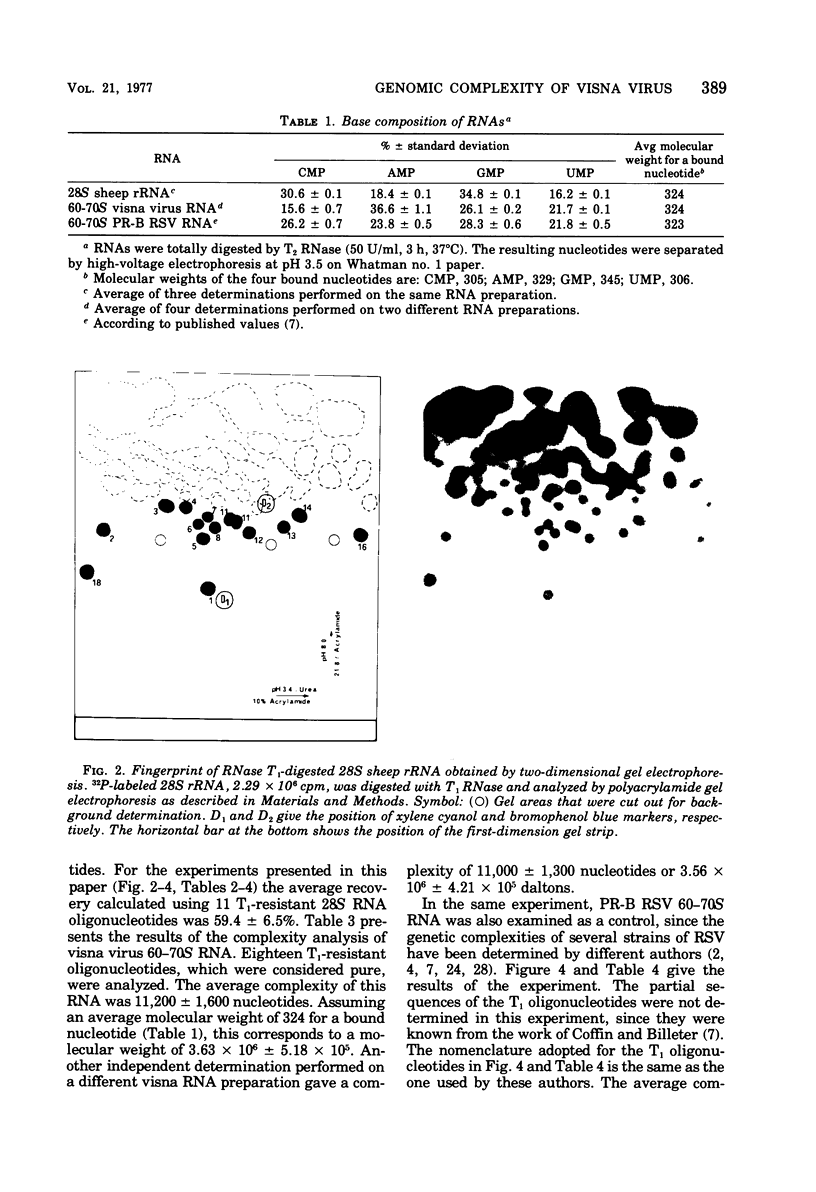
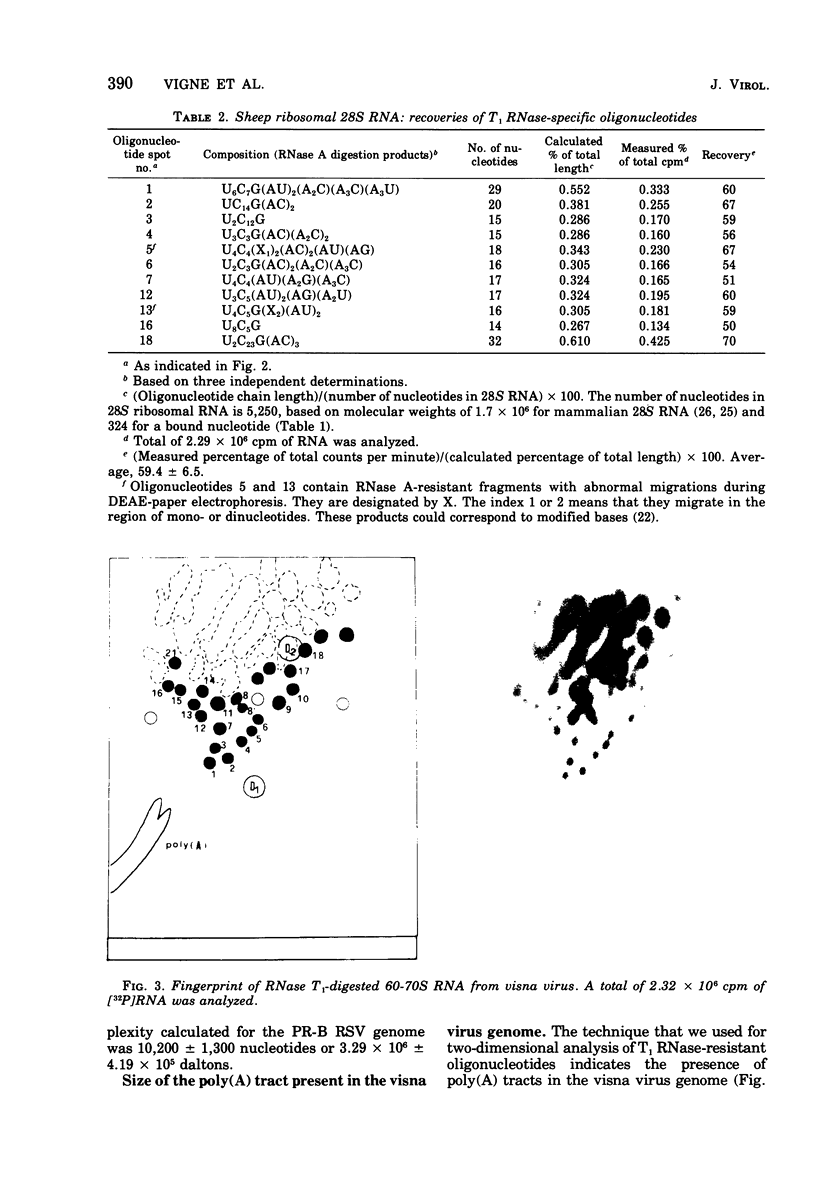
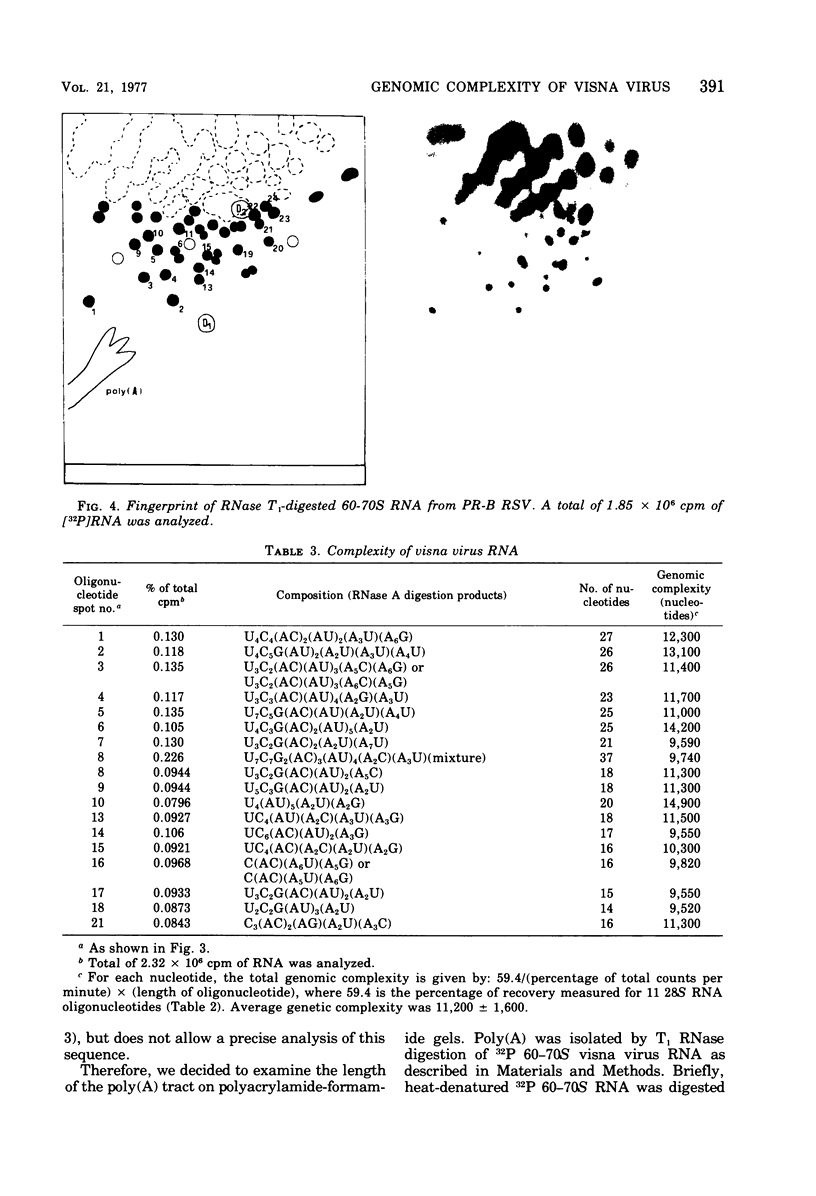
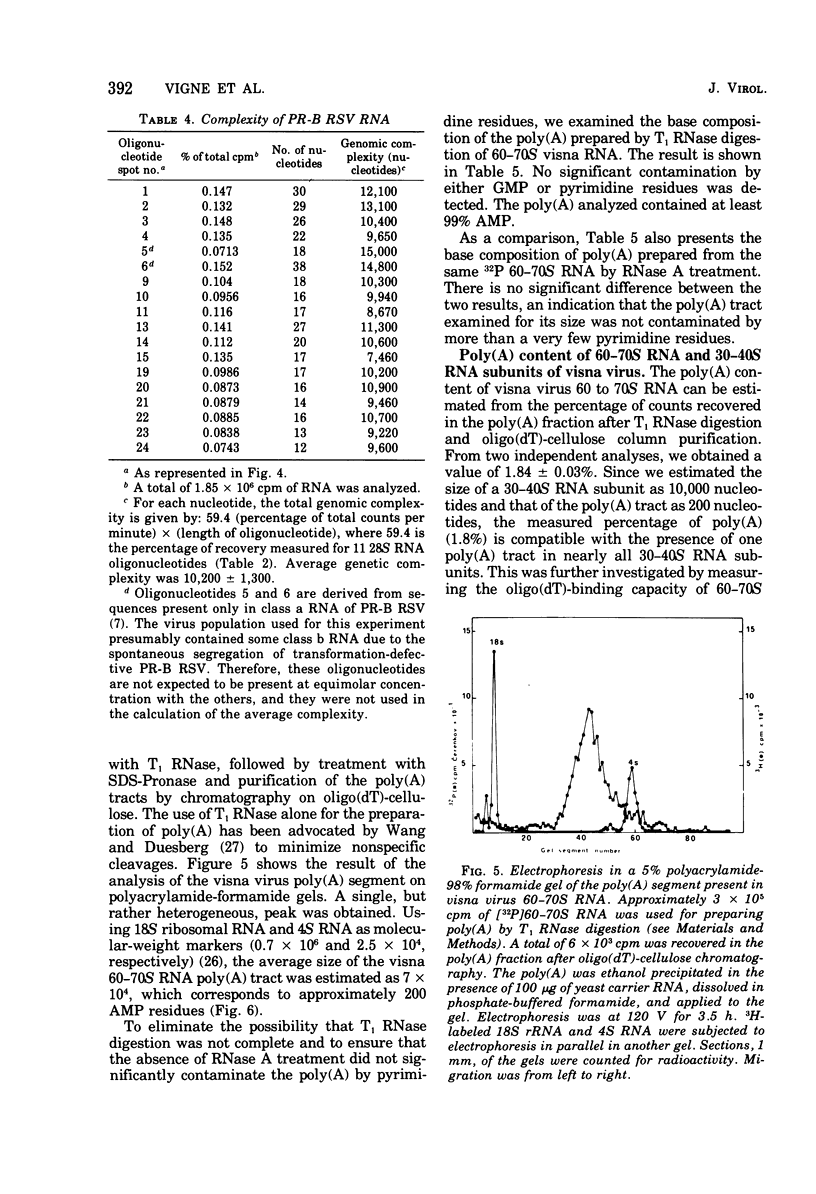
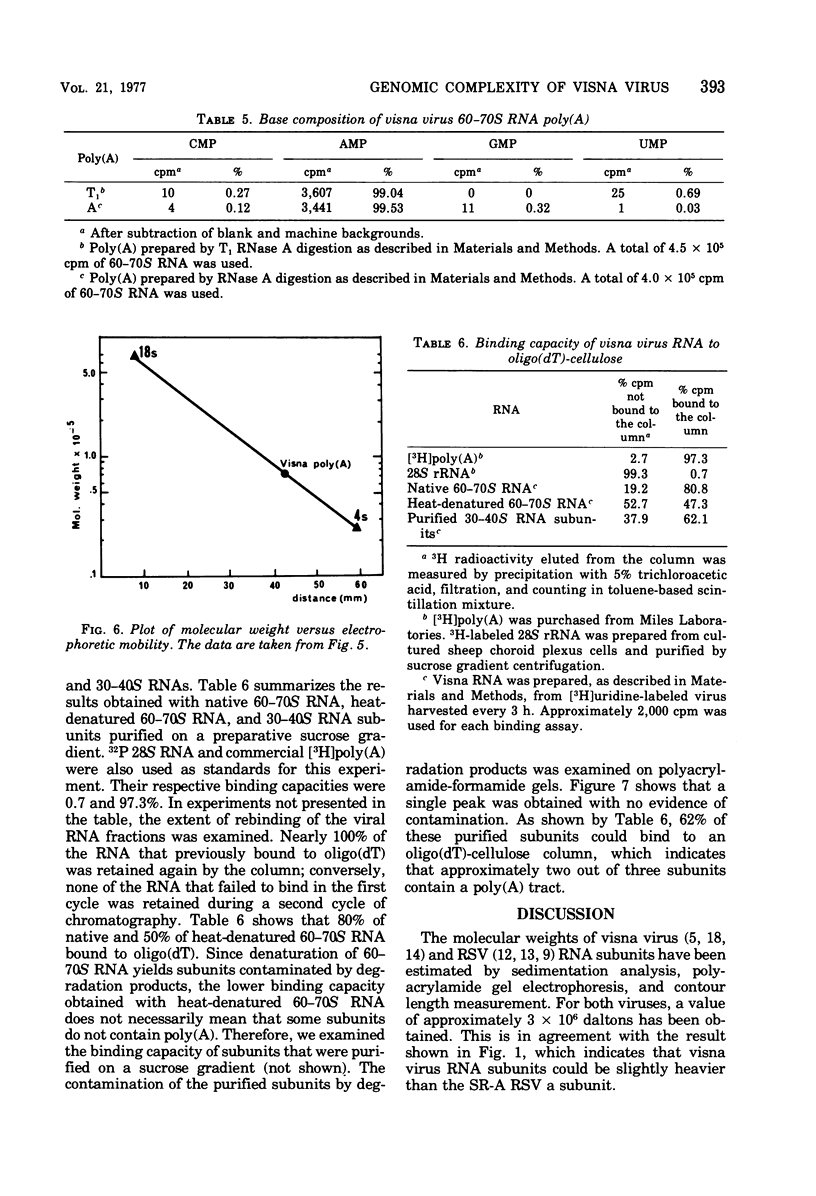
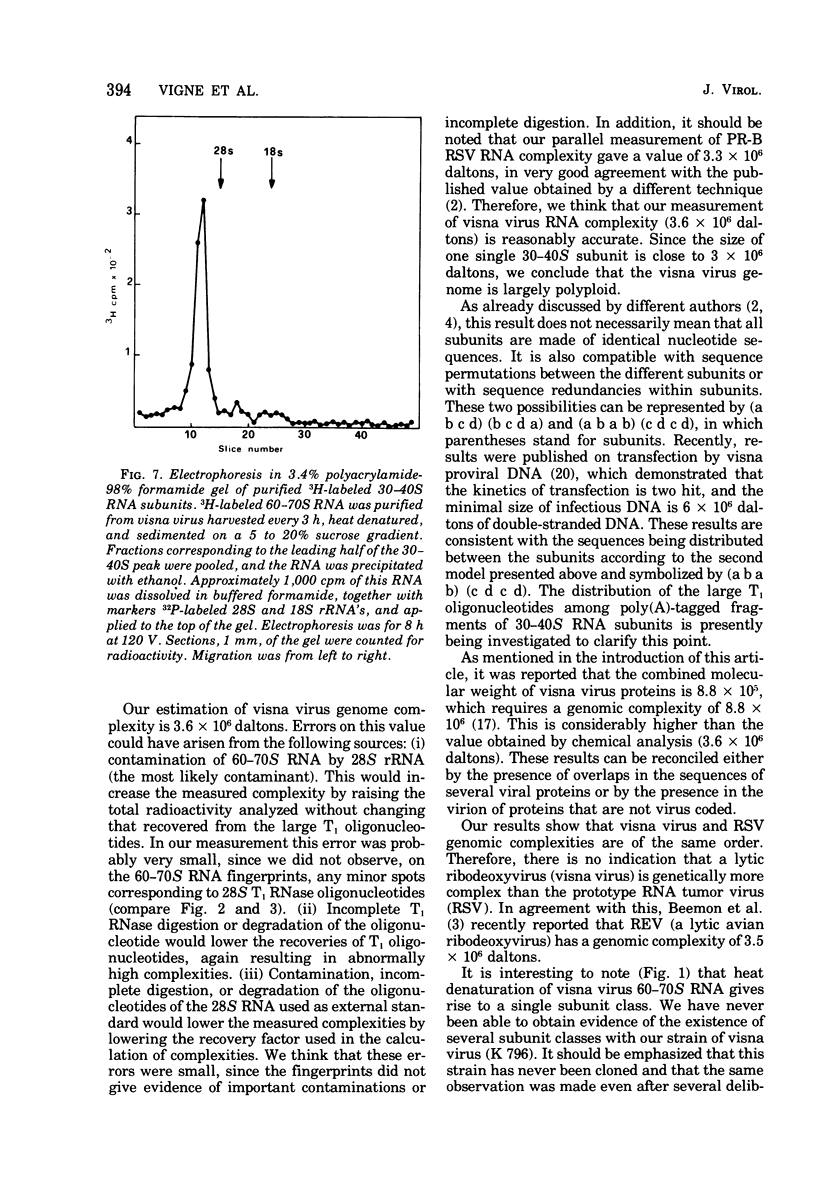
The genomic complexity of visna virus was measured by quantitative analysis of 18 RNase T1-resistant oligonucleotides from 60-70S RNA. T1-resistant oligonucleotides were separated by two-dimensional polyacrylamide gel electrophoresis. Visna virus had a genomic complexity of 3.6 X 10(6) daltons, very close to the size of a single 30-40S RNA subunit. It was therefore concluded that the visna virus genome is largely polyploid. Visna virus 60-70S RNA polyadenylic acid segment was purified by T1 RNase digestion followed by oligodeoxythymidylic acid-cellulose column chromatography. It contained over 99% AMP and had a size of about 200 nucleotides. The binding capacities on oligodeoxythymidylic acid-cellulose of native 60-70S RNA and purified 30-40S RNA subunits were examined. It was concluded that two out of three intact subunits contain a polyadenylic acid segment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K. L., Faras A. J., Hasse A. T., Duesberg P. H., Maisel J. E. Genomic complexities of murine leukemia and sarcoma, reticuloendotheliosis, and visna viruses. J Virol. 1976 Feb;17(2):525–537. doi: 10.1128/jvi.17.2.525-537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Tamalet J., Filippi P., Delbecchi L. The high molecular weight RNA of Visna virus. Biochimie. 1973;55(8):885–891. doi: 10.1016/s0300-9084(73)80165-8. [DOI] [PubMed] [Google Scholar]
- Brahic M., Vigne R. Properties of visna virus particles harvested at short time intervals: RNA content, infectivity, and ultrastructure. J Virol. 1975 May;15(5):1222–1230. doi: 10.1128/jvi.15.5.1222-1230.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Temin H. M. Infectious rous sarcoma virus and reticuloendotheliosis virus DNAs. J Virol. 1974 Nov;14(5):1132–1141. doi: 10.1128/jvi.14.5.1132-1141.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Duesberg P. H., Mangel W. F. Electron microscope measurements of rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):835–843. doi: 10.1101/sqb.1974.039.01.097. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. The RNA of influenza virus. Proc Natl Acad Sci U S A. 1968 Mar;59(3):930–937. doi: 10.1073/pnas.59.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Gel electrophoresis of avian leukosis and sarcoma viral RNA in formamide: comparison with other viral and cellular RNA species. J Virol. 1973 Sep;12(3):594–599. doi: 10.1128/jvi.12.3.594-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann A., Coward J. E., Harter D. H., Lipset J. S., Morgan C. Electron microscopic studies of visna virus ribonucleic acid. J Gen Virol. 1974 Oct;25(1):93–104. doi: 10.1099/0022-1317-25-1-93. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Takemoto K., Robert M., Gallo R. C. Polyadenylic acid in Visna virus RNA. Science. 1973 Mar 30;179(4080):1328–1330. doi: 10.1126/science.179.4080.1328. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Baringer J. R. The structural polypeptides of RNA slow viruses. Virology. 1974 Jan;57(1):238–250. doi: 10.1016/0042-6822(74)90124-x. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Garapin A. C., Faras A. J., Taylor J. M., Bishop J. M. A comparison of the high molecular weight RNAs of visna virus and Rous sarcoma virus. Virology. 1974 Jan;57(1):259–270. doi: 10.1016/0042-6822(74)90126-3. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Levinson W. Inhibition of RNA slow viruses by thiosemicarbazones. Biochem Biophys Res Commun. 1973 Apr 16;51(4):875–880. doi: 10.1016/0006-291x(73)90008-9. [DOI] [PubMed] [Google Scholar]
- Haase A. T. The slow infection caused by visna virus. Curr Top Microbiol Immunol. 1975;72:101–156. doi: 10.1007/978-3-642-66289-8_4. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Traynor B. L., Ventura P. E., Alling D. W. Infectivity of visna virus DNA. Virology. 1976 Mar;70(1):65–79. doi: 10.1016/0042-6822(76)90236-1. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Lee K. L., Kennedy F. T. Fractionation of 34 S ribonucleic acid subunits from oncornaviruses on polyuridylate-sepharose columns. J Biol Chem. 1974 Jan 10;249(1):38–42. [PubMed] [Google Scholar]
- Khan M. S., Maden B. E. Nucleotide sequences within the ribosomal ribonucleic acids of HeLa cells, Xenopus laevis and chick embryo fibroblasts. J Mol Biol. 1976 Feb 25;101(2):235–254. doi: 10.1016/0022-2836(76)90375-2. [DOI] [PubMed] [Google Scholar]
- Lai M. M., Duesberg P. H. Adenylic acid-rich sequence in RNAs of Rous sarcoma virus and Rauscher mouse leukaemia virus. Nature. 1972 Feb 18;235(5338):383–386. doi: 10.1038/235383c0. [DOI] [PubMed] [Google Scholar]
- Quade K., Smith R. E., Nichols J. L. Evidence for common nucleotide sequences in the RNA subunits comprising Rous sarcoma virus 70 S RNA. Virology. 1974 Sep;61(1):287–291. doi: 10.1016/0042-6822(74)90263-3. [DOI] [PubMed] [Google Scholar]
- Schibler U., Wyler T., Hagenbüchle O. Changes in size and secondary structure of the ribosomal transcription unit during vertebrate evolution. J Mol Biol. 1975 May 25;94(3):503–517. doi: 10.1016/0022-2836(75)90217-x. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. Properties and location of poly(A) in Rous sarcoma virus RNA. J Virol. 1974 Dec;14(6):1515–1529. doi: 10.1128/jvi.14.6.1515-1529.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]





