Abstract
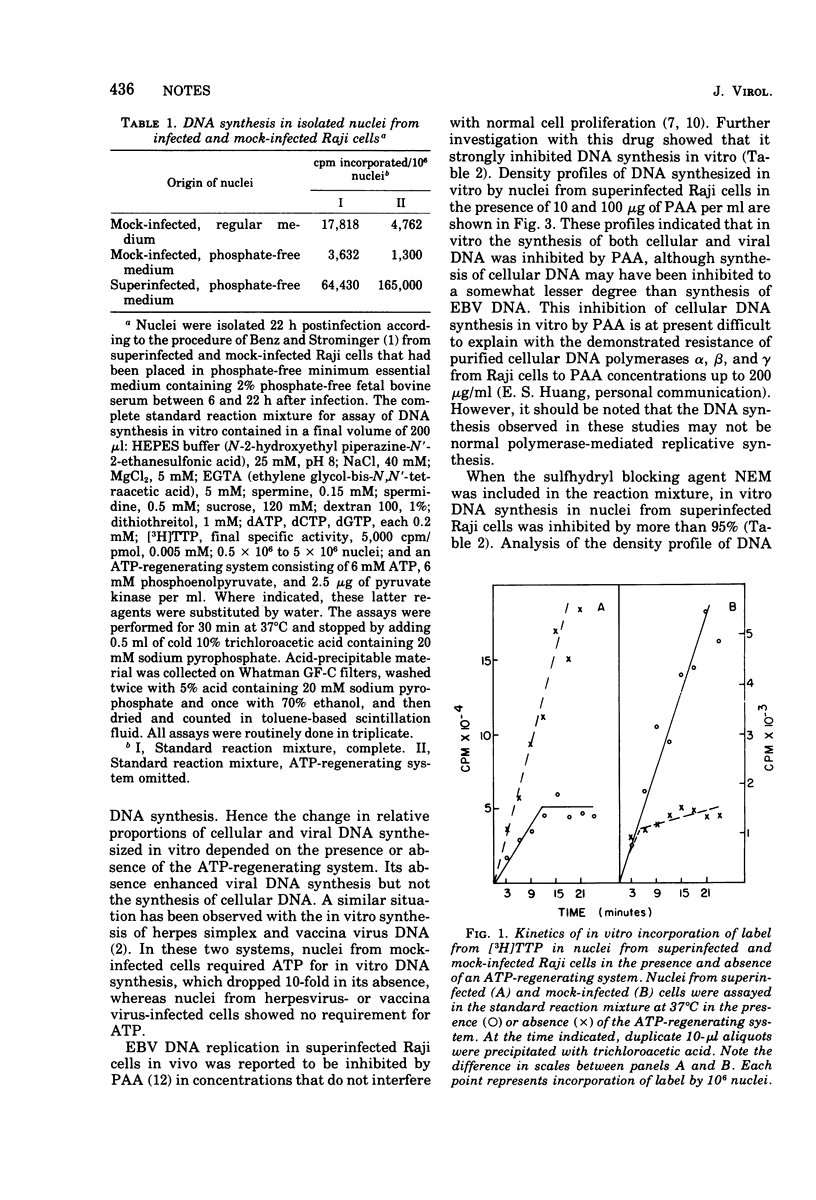
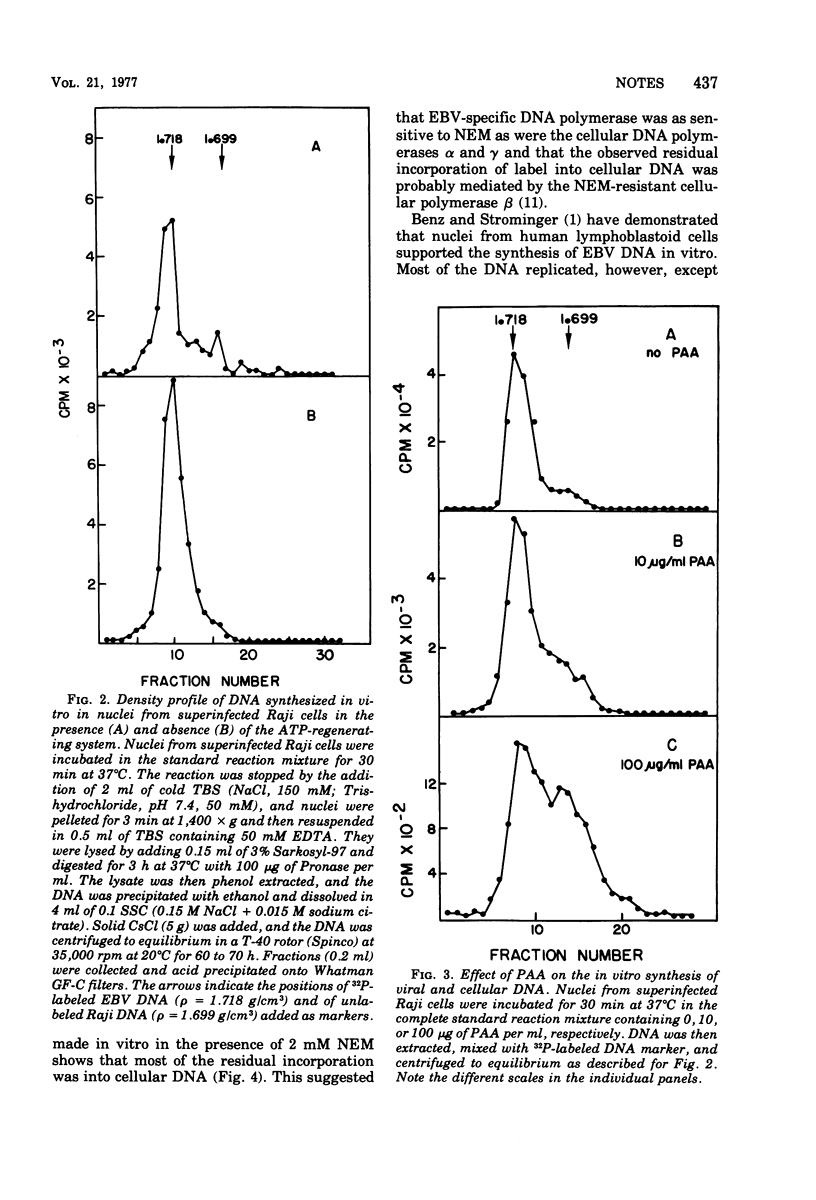
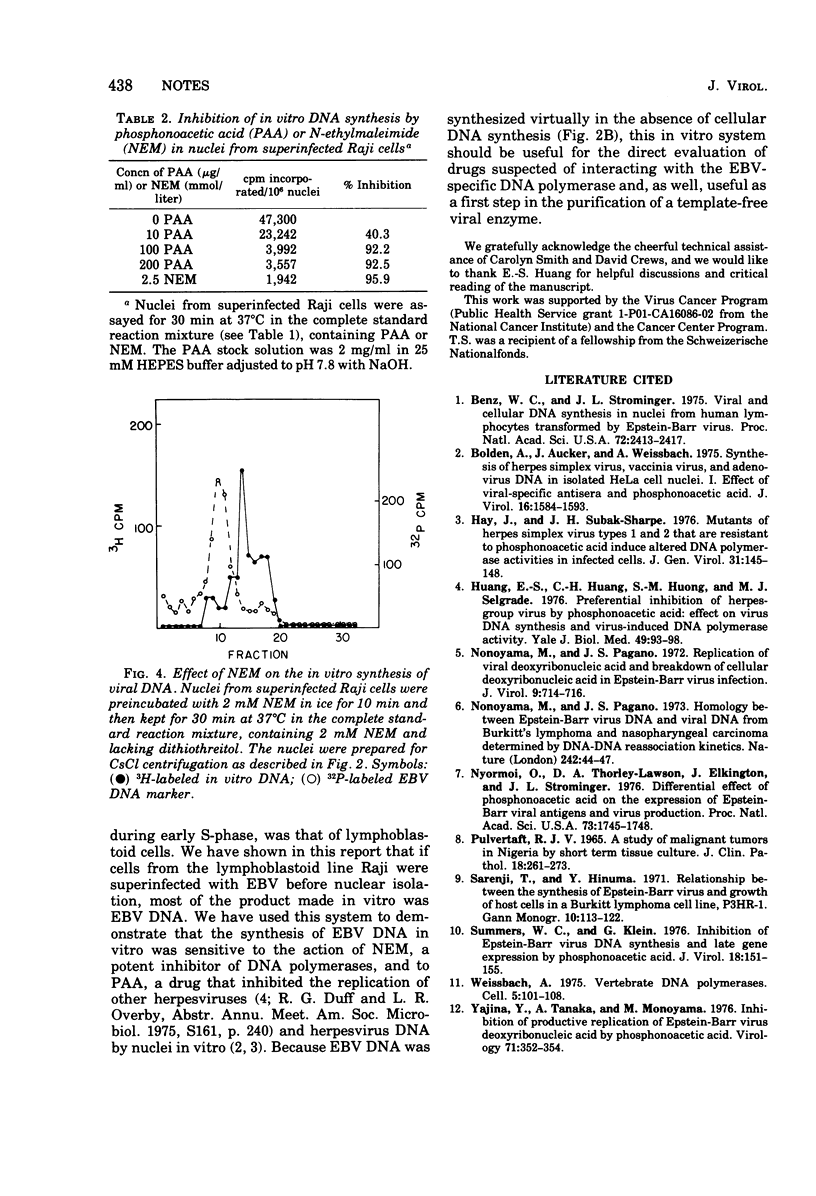
Nuclei from superinfected Raji cells synthesized Epstein-Barr Virus (EBV) DNA in vitro in the absence of cell DNA synthesis. The synthesis of EBV DNA in vitro was inhibited by phosphonoacetic acid and N-ethylmaleimide, and maximum synthesis was achieved in the absence of an ATP-regenerating system. Nuclei from mock-infected cells required an ATP-regenerating system for maximum DNA synthesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz W. C., Strominger J. L. Viral and cellular DNA synthesis in nuclei from human lymphocytes transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2413–2417. doi: 10.1073/pnas.72.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden A., Aucker J., Weissbach A. Synthesis of herpes simplex virus, vaccinia virus, and adenovirus DNA in isolated HeLa cell nuclei. I. Effect of viral-specific antisera and phosphonoacetic acid. J Virol. 1975 Dec;16(6):1584–1592. doi: 10.1128/jvi.16.6.1584-1592.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Huang C. H., Huong S. M., Selgrade M. Preferential inhibition of herpes-group viruses by phosphonoacetic acid: effect on virus DNA synthesis and virus-induced DNA polymerase activity. Yale J Biol Med. 1976 Mar;49(1):93–99. [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Replication of viral deoxyribonucleic acid and breakdown of cellular deoxyribonucleic acid in Epstein-Barr virus infection. J Virol. 1972 Apr;9(4):714–716. doi: 10.1128/jvi.9.4.714-716.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyormoi O., Thorley-Lawson D. A., Elkington J., Strominger J. L. Differential effect of phosphonoacetic acid on the expression of Epstein-Barr viral antigens and virus production. Proc Natl Acad Sci U S A. 1976 May;73(5):1745–1748. doi: 10.1073/pnas.73.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C., Klein G. Inhibition of Epstein-Barr virus DNA synthesis and late gene expression by phosphonoacetic acid. J Virol. 1976 Apr;18(1):151–155. doi: 10.1128/jvi.18.1.151-155.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A. Vertebrate DNA polymerases. Cell. 1975 Jun;5(2):101–108. doi: 10.1016/0092-8674(75)90017-3. [DOI] [PubMed] [Google Scholar]
- Yajima Y., Tanaka A., Nonoyama M. Inhibition of productive replication of Epstein-Barr virus DNA by phosphonoacetic acid. Virology. 1976 May;71(1):352–354. doi: 10.1016/0042-6822(76)90119-7. [DOI] [PubMed] [Google Scholar]


