Abstract
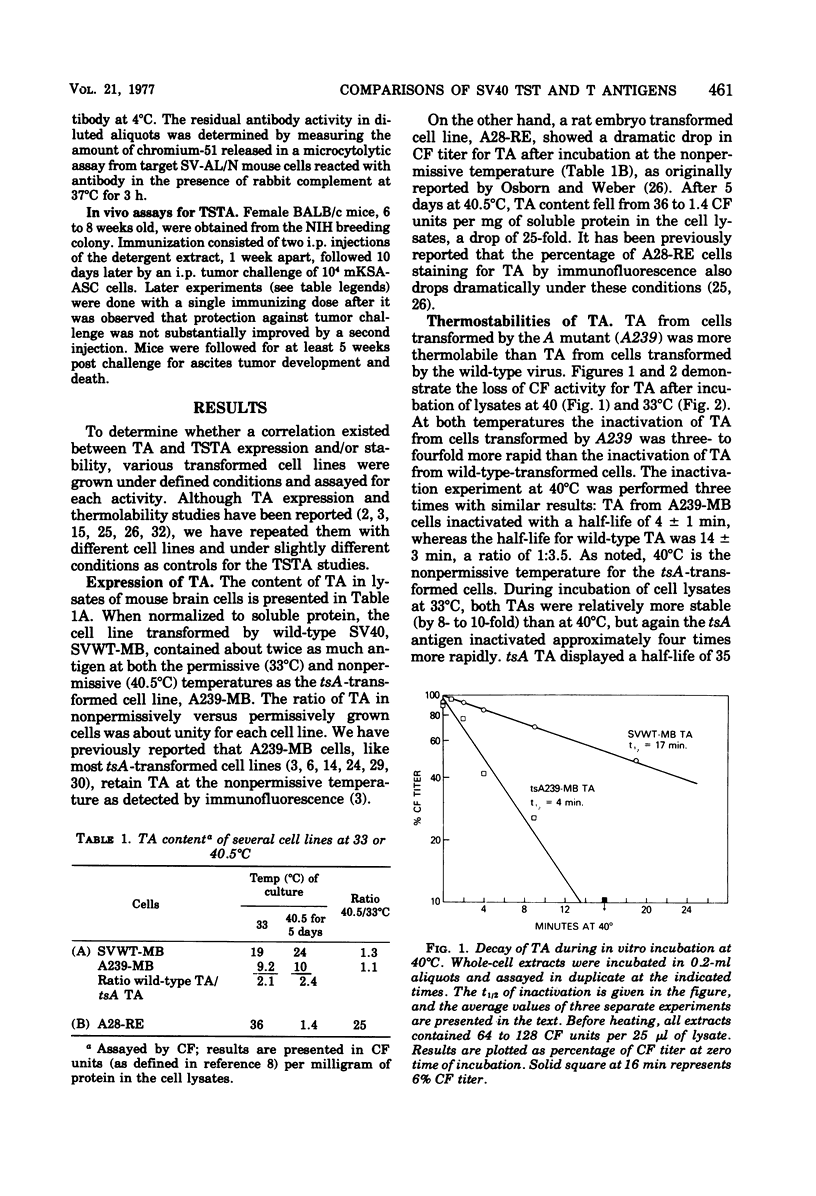
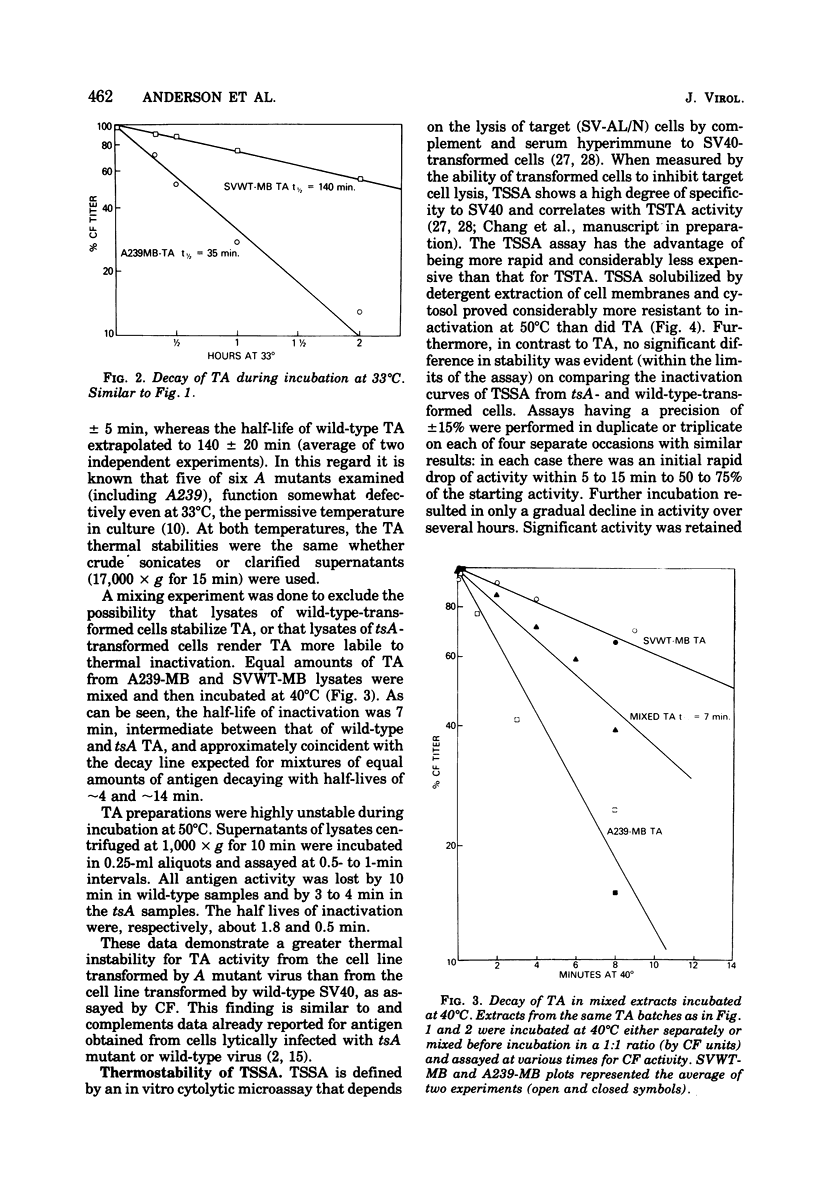
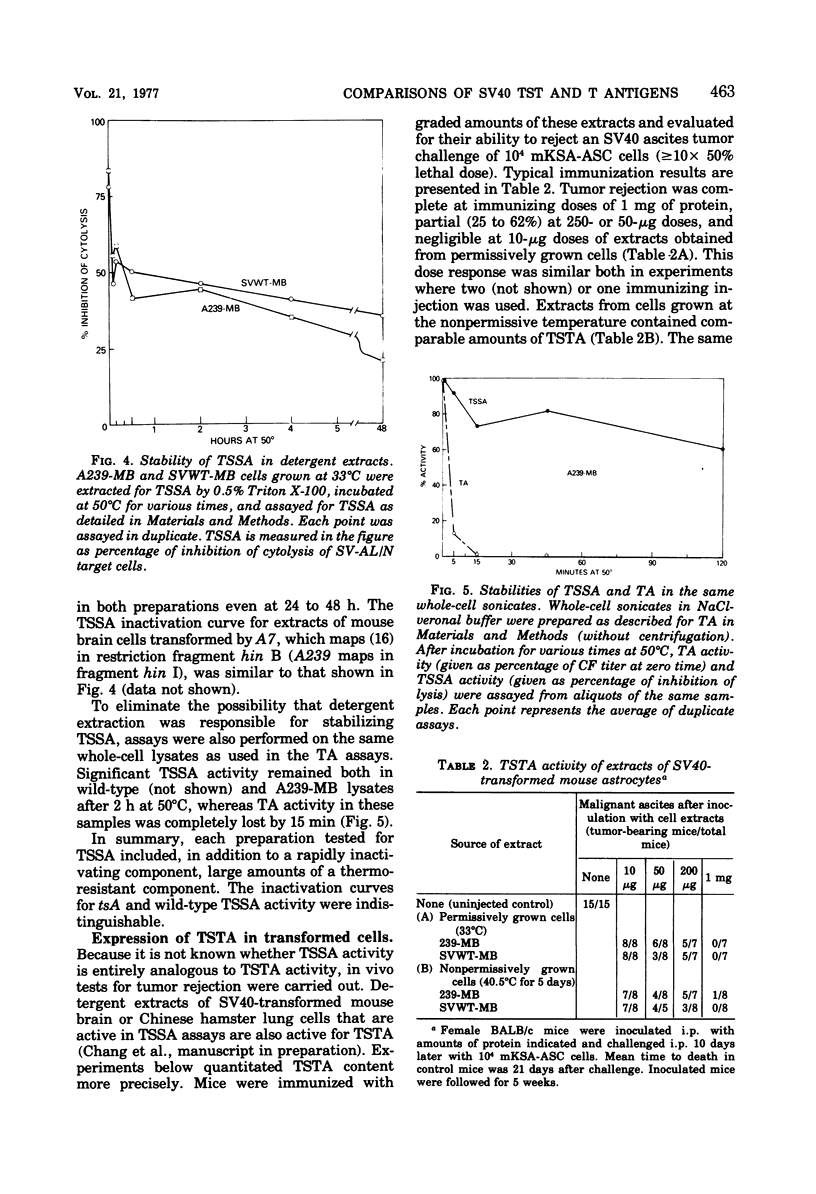
We have explored aspects of a suggested relationship between the expression of simian virus 40 tumor-specific transplantation antigen (TSTA) and tumor antigen (TA). A unique rat embryo cell line transformed by a temperature-sensitive A mutant that loses TA during exposure to the nonpermissive temperature (A28-RE) was found to lose TSTA. On the other hand, a typical control tsA-transformed cell line (A239-MB) expressed both TA and TSTA at the non-permissive temperature. TA in lysates obtained from A239-MB cells was found to be three to four times more thermolabile by covwt-mb) when incubated at either 33 or 40 degrees C. These data complement previous reports using TA from lytic infection and are consistent with the suggestion that TA is virus encoded. In contrast to TA, which even in wild-type-transformed cells was completely destroyed in less than 10 min at 50 degrees C, TSTA, assayed in vivo by tumor rejection, and tumor-specific surface antigen(s) TSSA) defined by an in vitro cytolytic assay, were thermostabile. Even after 24 h of incubation of extracts of 50 degrees C, high levels of TSTA and TSSA activity were present. Since these surface antigens when obtained from cells transformed by tsA mutants were also thermostabile, they could not be distinguished from the wild-type antigens. These results (i) indicate a coordinate expression of TA and TSTA in transformed cells; (ii) confirm that TA is virus encoded; and (iii) confirm that tha antigenic and immunogenic determinants that characterize TA and TSTA activities are distinct. However, the possibility that TSTA, like TA, is of viral rather than cellular origin is not excluded.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Allet B., Greenblatt J., Weil R. Two forms of simian-virus-40-specific T-antigen in abortive and lytic infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1097–1101. doi: 10.1073/pnas.73.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. L., Martin R. G. SV40 transformation of mouse brain cells: critical role of gene A in maintenance of the transformed phenotype. J Cell Physiol. 1976 May;88(1):65–76. doi: 10.1002/jcp.1040880109. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Products of complementation between temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):127–136. doi: 10.1128/jvi.15.1.127-136.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deppert W., Walter G. Simian virus to (SV40) tumor-specific proteins in nucleus and plasma membrane of HeLa cells infected by adenovirus 2-SV40 hybrid virus Ad2+ND2. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2505–2509. doi: 10.1073/pnas.73.7.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin M. S., Appella E., Law L. W. Immunogenic properties of a soluble tumor-specific transplantation antigen induced by Simian virus 40. J Natl Cancer Inst. 1974 Jan;52(1):259–264. doi: 10.1093/jnci/52.1.259. [DOI] [PubMed] [Google Scholar]
- Holloway P. W. A simple procedure for removal of Triton X-100 from protein samples. Anal Biochem. 1973 May;53(1):304–308. doi: 10.1016/0003-2697(73)90436-3. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino T., Yamaguchi N. Characterization of T antigen in cells infected with a temperature-sensitive mutant of simian virus 40. J Virol. 1975 Jun;15(6):1302–1307. doi: 10.1128/jvi.15.6.1302-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lebowitz P., Kelly T. J., Jr, Nathans D., Lee T. N., Lewis A. M., Jr A colinear map relating the simian virus 40 (SV40) DNA segments of six adenovirus-SV40 hybrids to the DNA fragments produced by restriction endonuclease cleavage of SV40 DNA. Proc Natl Acad Sci U S A. 1974 Feb;71(2):441–445. doi: 10.1073/pnas.71.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. S., Oxman M. N., Henry P. H., Levin M. J., Diamandopoulos G. T., Enders J. F. Virus-specific deoxyribonucleic acid in simian virus 40-exposed hamster cells: correlation with S and T antigens. J Virol. 1970 Aug;6(2):199–207. doi: 10.1128/jvi.6.2.199-207.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Studies on nondefective adenovirus-simian virus 40 hybrid viruses. I. A newly characterized simian virus 40 antigen induced by the Ad2+ND 1 virus. J Virol. 1971 Feb;7(2):189–197. doi: 10.1128/jvi.7.2.189-197.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y., Avila J., Saral R. The semiautonomous replicon: a molecular model for the oncogenicity of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):17–24. doi: 10.1101/sqb.1974.039.01.005. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollester D. L. Isolation of meth A cell surface membranes possessing tumor-specific transplantation antigen activity. Cancer Res. 1970 Dec;30(12):2832–2837. [PubMed] [Google Scholar]
- Osborn M., Weber K. SV40: T antigen, the A function and transformation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):267–276. doi: 10.1101/sqb.1974.039.01.035. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancake S. J., Mora P. T. Limitations and utility of a cytolytic assay for measuring simian virus 40-induced cell surface antigens. Cancer Res. 1976 Jan;36(1):88–94. [PubMed] [Google Scholar]
- Smith R. W., Mora P. T. Cytolytic microassays and studies of SV40 tumor-specific transplantation antigen. Virology. 1972 Oct;50(1):233–246. doi: 10.1016/0042-6822(72)90363-7. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Baygell P., Livingston D. M. Thermolabile T (tumor) antigen from cells transformed by a temperature-sensitive mutant of simian virus 40. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4351–4355. doi: 10.1073/pnas.72.11.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. W., Law L. W. Quantitative in vitro measurement of simian virus 40 tumor-specific antigens. Proc Natl Acad Sci U S A. 1971 May;68(5):973–976. doi: 10.1073/pnas.68.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]


