Abstract
Population persistence in a new and stressful environment can be influenced by the plastic phenotypic responses of individuals to this environment, and by the genetic evolution of plasticity itself. This process has recently been investigated theoretically, but testing the quantitative predictions in the wild is challenging because (i) there are usually not enough population replicates to deal with the stochasticity of the evolutionary process, (ii) environmental conditions are not controlled, and (iii) measuring selection and the inheritance of traits affecting fitness is difficult in natural populations. As an alternative, predictions from theory can be tested in the laboratory with controlled experiments. To illustrate the feasibility of this approach, we briefly review the literature on the experimental evolution of plasticity, and on evolutionary rescue in the laboratory, paying particular attention to differences and similarities between microbes and multicellular eukaryotes. We then highlight a set of questions that could be addressed using this framework, which would enable testing the robustness of theoretical predictions, and provide new insights into areas that have received little theoretical attention to date.
Keywords: evolutionary demography, experimental evolution, extinction, changing environment, evolution of plasticity, generalism
1. Introduction
Abrupt environmental alterations can increase extinction risk and foster rapid phenotypic change, both of which are broadly observed in response to current climate change, species introductions and other anthropogenic modifications of the environment [1,2]. Evolution on the time-scale of population dynamics may affect the demography of a species, that is, the set of vital rates (survivals and fecundities) that determine the size and age/stage composition of a population [3,4]. In particular, evolutionary rescue (hereafter ER) describes the situation where adaptive evolution prevents population extinction in a stressful environment [5,6]. The details of this interaction between evolution and demography, however, depend on the underlying mechanism of phenotypic change, i.e. whether it is caused by a change in the genetic composition of the population in response to natural selection, or by a change in the phenotype of each individual in response to its environment of development or expression. Monitoring of wild populations with known pedigrees increasingly shows that rapid phenotypic change of traits affecting fitness often involves a combination of genetic change and phenotypic plasticity [7–10]. This suggests that phenotypic plasticity may play an important role in the interaction of demography and evolution. Furthermore, plasticity can vary genetically, and may thus itself evolve in response to natural selection [11], so the evolution of plasticity may also be important for ER.
On the basis of verbal arguments tracing back to Baldwin [12], recent theory has investigated how the interplay of phenotypic plasticity, genetic evolution and demography, affects population persistence in a new or changing environment [13–15]. Testing predictions from this theory can be challenging in the wild, because of the inherent lack of control on environmental conditions, and of the difficulties in accurately measuring fitness and how it relates to phenotypes and genotypes in natural populations, among other complications [16] (even though some cases of ER are documented in the wild, reviewed in [17]). Alternatively, current theoretical predictions and further questions they raise can be investigated in the laboratory using experimental evolution. While such experiments have rarely been performed so far in the context of plasticity interacting with ER, we argue below that (i) all the conceptual tools are available and (ii) many model organisms are adequate for such studies.
To argue our point, we start by briefly defining the concepts of plasticity and generalism in relation to fitness and population growth in variable environments. We then review theoretical predictions for the role of phenotypic plasticity and its evolution in ER following an abrupt environmental shift, and the experimental work that addresses components of this theory. We end by highlighting important questions that could be addressed by this approach, and outline simple prototype experiments.
2. Key concepts
In this section, we introduce the conceptual tools that will be discussed in the following sections. A thorough review of the many forms of plasticity and of their mechanistic underpinnings is beyond the scope of this paper, and is already available elsewhere [11,18–20]. Instead, we specifically focus on issues relevant to ER.
(a). Phenotypic plasticity and generalism
Many morphological, physiological or behavioural traits can change in response to an organism's environment. The curve that captures this relationship between trait and environment for a given genotype is the norm of reaction [19,21], a generic term that applies to fitness or to any other trait. However, in the context of evolutionary demography, it is useful to distinguish fitness and its life-history components (survival and fecundity) from other traits. This is because fitness has the specific attribute of defining adaptiveness for other traits (thus causing their evolution), and because it can determine population growth [4,22,23]. Importantly for ER, the focus is on absolute fitness (broadly defined as the expected number of offspring in the next generation), rather than on relative fitness (proportional contribution to the next generation, more commonly used in evolutionary genetics), because only the former affects demography. In practice, one can compute absolute fitness from the vital rates (age- or stage-specific survivals and fecundities) using standard life-history theory [3,4,24,25].
‘Phenotypic plasticity’ describes any change in the phenotype of a given genotype with its environment of development or expression, leading to non-flat reaction norms. The term plasticity is better suited to characterize effects of the environment on traits that are not direct components of fitness. While some authors have used ‘plasticity’ for fitness itself, this can lead to self-inconsistencies: a genotype whose fitness changes little across environments is described by some authors as very plastic (for putative underlying traits), and by others as showing low plasticity (for fitness itself). Changes in fitness across environments rather relate to the degree of ‘generalism’ (or ‘environmental tolerance’, ‘robustness’), and the corresponding norm of reaction is a tolerance curve [26,27]. Averaging these curves over the population yields a measure of niche breadth, as the range of environments over which mean fitness equals or exceeds unity (for geometric fitness in discrete generations), or zero (for Malthusian fitness in continuous generations) when numbers are low [28]. The two concepts can be related, as generalism may or may not result from plasticity of underlying traits (see figs 1 and 2 in [14]).
(b). Benefits and costs of plasticity and generalism
Plasticity is beneficial (or adaptive) and may help prevent extinction over a range of environments if it produces phenotypes with high fitnesses across these environments (i.e. a genotype with more beneficial plasticity is more of a generalist). But several factors may act to prevent plasticity from being beneficial. First, the environment that triggers the plastic response can differ from the one where the expressed trait affects fitness [15,29–34], for instance, if the former is experienced earlier in life than the latter, or if plasticity is in response to a partially unreliable cue used as a proxy for the fitness-determining environment (e.g. photoperiod for seasonality and food abundance). The resulting low predictability of the environment of selection determines whether (and how much) plasticity is beneficial: if environmental predictability is poor, being very responsive to the environment can be detrimental. The optimal level of plasticity (including possibly no plasticity) is thus a compromise between the environmental sensitivity of phenotypic selection (how much the selective pressure on the phenotype changes with the environment), and the correlation between the environments of development and of selection, which may depend on when dispersal occurs in the life cycle (for evolutionary models, see [29–31,35]; for a recent demographic model with no evolution, see [15,16]). Second, the reaction norm might produce detrimental phenotypes in environments that a species has never experienced before [36], or in extreme environments where homeostasis is disrupted, such that there is less genetic control on development. And third, costs of plasticity might reduce fitness regardless of the expressed phenotype [37,38]. These include the cost of maintaining a system enabling information to be acquired about the environment, or alternative phenotypes to be produced in different environments. Currently available empirical measurements suggest that these costs are rather weak [39] (despite some controversy about their measurement [40]). However, any such costs will always work against ER since, by definition, they reduce fitness and thus population growth rates [13,14].
(c). Measuring phenotypic plasticity and generalism
Reaction norms can be analysed and measured empirically by considering the same trait measured in different environments as different characters (one per environment), allowing for genetic correlations between these characters (the character state approach [41–43]). An alternative is to define a set of polynomial parameters describing the reaction norm shape in a reference environment (intercept, slope, quadratic term, etc.), and to treat these parameters themselves as genetically variable, and possibly correlated, traits (the polynomial approach [29], also referred to by some authors as the ‘reaction norm’ approach). These two models are often mathematically exchangeable [44], even though their assumptions may differ in specific contexts. Here, our argument will be mostly set in terms of the polynomial approach, since it provides a straightforward quantification of plasticity for nearly linear reaction norms (see below).
Regarding reaction norms for fitness, studies of tolerance curves initially focused on whether genotypes with broader tolerance also have lower fitness in their most favourable environment (generalist/specialist trade-off [26,45,46]), but more recent methods allow quantifying and investigating richer aspects of tolerance curve shape variation [47,48].
When reaction norms are genetically variable, they can evolve in response to natural selection on the expressed trait, which can be important in the context of ER as we will see below. The direction of reaction norm evolution is determined partly by the genetic constraints on their shape, quantified by the genetic covariances of trait values across environments, in the character-state approach, or by the genetic (co)variances of different polynomial coefficients, for instance, slope and curvature, in the polynomial approach.
3. Theory of evolutionary rescue with plasticity
We now briefly review recent theoretical predictions about the effect of phenotypic plasticity and its evolution on ER.
Consider a population experiencing an abrupt environmental change, such that the mean phenotype prior to the change becomes maladaptive, and causes the mean absolute fitness to be less than unity (or less than zero for Malthusian fitness [49]). If the phenotype does not change, births will not match deaths, and the population will decline inexorably to extinction. However, if the response to selection on heritable traits increases fitness sufficiently fast, then the population will not go extinct [5,50], which is described as ER.
Phenotypic plasticity was recently introduced by Chevin & Lande [13] into this classic ER scenario. Unlike earlier studies, they also included negative density dependence, and considered populations starting at carrying capacity. They assumed that the evolutionary demography of a population is mostly determined by optimizing selection on a quantitative trait with continuous polygenic variation, and that this trait is also phenotypically plastic, with a linear reaction norm whose slope quantifies plasticity. Linear reaction norms are a simple form of plasticity, and give a reasonably good description of patterns of variation in several empirical studies of quantitative traits related to climate adaptation, over the observed ranges of environments [7,8]. (For a heuristic argument about the role of plasticity on genetic evolution with more arbitrary reaction norms, but without explicit genetic variance in plasticity and genetic constraints, see [51]). The mean plasticity in the population was assumed to be partially adaptive before the environmental change, with plastic responses in the direction of changes in the optimum, but with smaller amplitude. This would occur, for instance, if the population was exposed to partially predictable environmental fluctuations (i.e. temporally variable but autocorrelated environments) prior to the abrupt environmental shift [11,31].
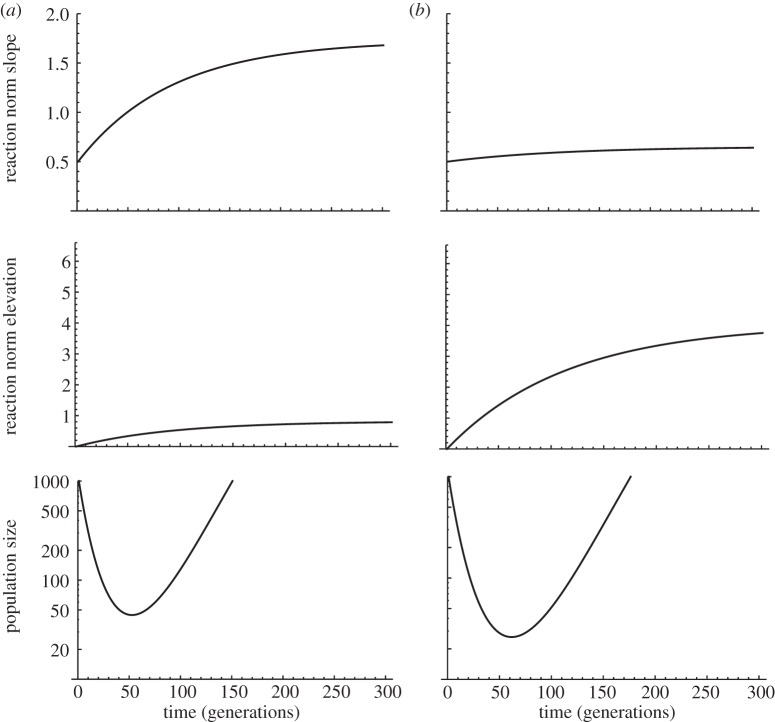
Phenotypic plasticity could also evolve in this model, as a result of quantitative genetic variance in reaction norm slopes. Variance in slopes entails that genetic variance of the plastic trait changes across environments (G × E interaction). Whether selection on the expressed trait translates into indirect selection on its plasticity depends on what proportion of the genetic variance of the trait is attributed to variance in reaction norm slopes [29]. Extending results from a previous study on plasticity evolution in a fluctuating environment [22] (which, however, had no demography and no cost of plasticity), Chevin & Lande [13] found that (i) the initial (partially adaptive) plasticity decreases the effective severity of the environmental shift, slowing the initial population decline and (ii) the evolution of plasticity accelerates adaptation (faster rate of fitness increase), in proportion to the contribution of variance in plasticity to overall genetic variance of the trait. Both factors favour persistence. The authors also provided quantitative predictions for the contribution of evolving plasticity to ER: if the magnitude of the environmental shift is large enough that indirect selection on plasticity (through its effect on the trait under optimizing selection) is initially stronger than the cost of plasticity, then ER is mostly caused by the evolution of phenotypic plasticity [13]. By contrast, under less severe environmental shifts, ER occurs without much change in the mean reaction norm slope.
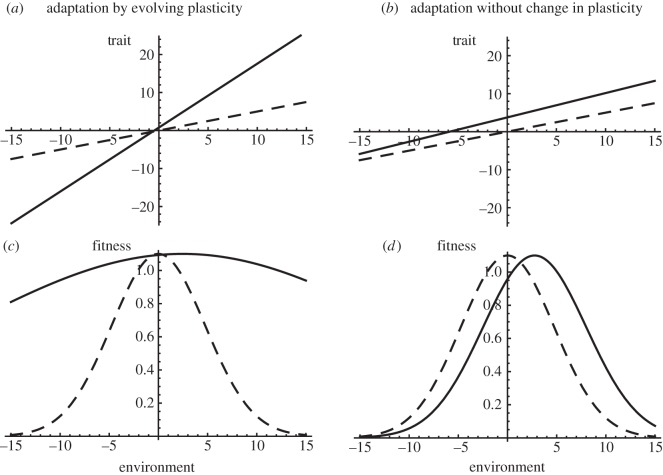
It is straightforward to translate these findings into predictions regarding the degree of generalism, within the assumptions of this model (Gaussian selection towards an optimum trait value, with linear changes in the optimum with the environment), and given perfect predictability of the environment of selection at the time of development. The breadth of environmental tolerance then is simply ω/(B−b), with ω the width of the fitness function on the trait, B the rate of change in the optimum with the environment and b the reaction norm slope (see figure 2). Other theoretical studies have investigated the influence of plasticity and/or genetic evolution on demography and extinction in a changing environment [14,15], but they do not specifically address adaptation to a sudden environmental change.
Figure 2.
Evolved reaction norms for the trait and fitness. The initial (dashed) and final reaction norms for the trait (a,b) and for absolute fitness (tolerance curves, c,d) are shown for the two scenarios shown in figure 1 (a,c versus b,d).
The quantitative predictions of this model rely on a number of assumptions, notably (i) linear reaction norms, (ii) substantial polymorphism at a number of loosely linked loci, allowing continuous (quantitative) genetic variation of traits, (iii) constant (co)variances of reaction norm parameters, and (iv) partially adaptive initial plasticity. These assumptions are a reasonable starting point, but are not likely to be realistic for all systems, and should all be violated to some extent for many organisms. Instead, they provide a first approximation of an idealized biological reality, providing a theoretical scaffold that can then be modified to generate predictions tailored to more complex situations. Whether this theory can accurately predict ER in real populations remains to be tested empirically. The study of ER and phenotypic plasticity with sufficient repeatability and control being particularly challenging in the wild, we here suggest investigating this question with laboratory experiments (some of them described in §5). While laboratory conditions represent model environments that do not capture all the complexity of natural systems, they allow deciphering with more precision those population processes that are likely to also play an important role in the wild [52,53]. Combining such experiments with data from natural populations should help identify which aspects highlighted by existing theory are most critical to ER, as well as raise questions in need of further theoretical developments.
4. Experimental evolutionary rescue and evolution of plasticity
To illustrate the feasibility of the proposed approach, we will now briefly review studies of experimental ER, and of experimental evolution of (or artificial selection on) plasticity. We will propose a few key questions that can be addressed with this methodology in the following section.
(a). Evolutionary rescue experiments
Over the past few years, a handful of studies have demonstrated ER in the laboratory. In a seminal paper, Bell & Gonzalez [54] showed that the likelihood a yeast population persists under salt stress increases with initial population size. Adaptation was limited by mutation in this study, and the results seemed to indicate that increasing the population size increased the probability that a single beneficial allele restoring population growth was sampled in the initial population, consistent with theoretical predictions by Orr & Unckless [55] (see also ref. [56] for comparisons of rescue probabilities by de novo mutations versus standing variation). Bell and Gonzalez later extended their experiment to ER in connected populations spread over a spatial gradient of salinity, and experiencing various rates of environmental change [57]. Both the speed of environmental change and the connectivity of populations appeared important, with complex interactions between them that are not yet related to specific theoretical predictions.
On technical grounds it should be noted that, owing to the stochastic nature of ER, especially when limited by mutation supply, the two yeast experiments described above necessitated very large numbers of replicates. The use of a robotic liquid handling system proved crucial to this task. Experimental studies of ER with microbes should also benefit from the ‘morbidostat’, a new selection device introduced by Toprak et al. [58], which dynamically tunes the level of stress (antibiotics in their case) so as to provoke population decline whenever the population size reaches an upper threshold. This makes it an ideal set-up to study successive ERs in the laboratory (see figure 1c in [58]).
Figure 1.
Evolving plasticity and evolutionary rescue. Evolutionary rescue caused by an increase in plasticity (a) or by adaptation with little change in plasticity (b). We assume linear reaction norms, with plasticity quantified by reaction norm slope, while the non-plastic component of the phenotype is described by the intercept, or elevation, in a reference environment. The environment changes from 0 to 3 on generation 0, and the optimum phenotype from 0 to 6, causing maladaptation and population decline (for more details see [13]). Whether evolutionary rescue is caused by the evolution of plasticity, or by genetic evolution of the phenotype with little change in plasticity, depends notably on the relative genetic variances of reaction slope and elevation. The initial and final reaction norms are plotted in figure 2. A quantitative genetic model was used, with genetic variance of reaction norm elevations Va = 0.1 (a) or Va = 0.4 in (b), and genetic variance of slopes Vb = 0.05 (a) or Vb = 0.005 (b), and no covariance between slope and elevation measured in a reference environment.
Performing ER experiments with higher eukaryotes is more challenging because of their larger body sizes and longer generation times. Nonetheless, a study recently exemplified how response to selection on standing genetic variation can allow a plant population to avoid extinction [59]. In this experiment, Mimulus guttatus populations were kept with or without pollinators. Within a few generations, pollinator-free populations evolved high degrees of selfing, allowing them to reach similar fecundities to those of populations with pollinators. Examples of potential ER in vertebrates are reviewed in [17] (albeit in the wild rather than in the laboratory). Overall, this literature reflects the possibility to study ER empirically in a variety of biological systems, from bacteria to large multicellular eukaryotes.
(b). Experimental evolution of phenotypic plasticity and generalism
Experimental evolution of phenotypic plasticity under artificial or pseudo-natural selection has been performed on a variety of organisms, with diverse mechanisms of adaptation at play (reviewed by Scheiner [60] and Garland & Kelly [61]). Some authors directly selected for plasticity by selecting a different trait value in different environments within a lineage [62], or for generalism by exposing populations to heterogeneous environments [63,64]. But more studies revealed increased phenotypic plasticity (or generalism) as a correlated response to selection in one environment [61,65–68], which is more directly related to theoretical predictions above for evolutionary demography following abrupt environmental change [13]. As argued by Scheiner [60], it is probable that more examples would be found if phenotypic plasticity and generalism across environments were systematically tested, after a phase of adaptation to a new environment. For instance, lines of E. coli selected at different pH by Hughes et al. [69] were recently reanalysed by placing them over a gradient of pH, revealing that lines selected in environments more different from their original one evolved broader tolerance, consistent with theoretical predictions by Lande [31] (R. Gallet and T. Lenormand 2012, in preparation).
5. Some outstanding questions in need of testing
Experimental evolution thus appears as a potentially fruitful method for studying the role of phenotypic plasticity and its evolution in ER. To illustrate more precisely the promise of this approach, we now indicate a few key questions that may be investigated this way. Each question addresses a specific prediction from theory, or relates to a question that deserves further theoretical development. It is followed by a simplified protocol designed to test it.
(a). When does evolutionary rescue in a new environment occur by the evolution of phenotypic plasticity?
Are populations rescued by a change in plasticity (as illustrated in figures 1a and 2a), or does the mean phenotype of adaptive traits evolve similarly across environments, with little or no change in plasticity (figures 1b and 2b)? Similarly, did the breadth of environmental tolerance increase during ER, or did just the optimal environment change (figure 2c,d)? And do the effects of the severity of the novel environment, and of the variance of plasticity in the initial population/mixture of clones, conform to predictions from theory [13]?
(i). Experiment
Perform ER starting with different combinations of genotypes with known reaction norms (investigated initially by placing them across a range of environments), under different levels of stress (quantified by the reduction in absolute fitness of the wild-type, relative to a benign environment). Compare the average reaction norms of rescued populations to those of ancestral populations, across the same environmental range, to find out whether higher plasticity evolved during the ER.
(b). Can the evolution of phenotypic plasticity cause ER after a change in environmental variation/predictability?
Phenotypic plasticity is more likely to be beneficial if the environments experienced in the past are informative about those to be encountered in the future. Yet a salient feature of current global change is an alteration of patterns of environmental variability and cross-correlation (e.g. temperature with photoperiod), leading to changes in environmental predictability. This can cause phenotypic plasticity to become detrimental, which has been suspected of causing population decline in well-documented cases [70,71]. A scenario of particular interest is thus the one where maladaptation and population decline are initially caused by an abrupt change in environmental predictability (rather than just an abrupt change in the environment), causing a mismatch between current plastic responses and patterns of selection. This would provide the potential for ER caused by the evolution of phenotypic plasticity (towards either stronger or weaker plasticity), as illustrated in figure 3. We are not aware of any quantitative predictions for this evolutionary demographic effect. Evolutionary theory has investigated the role of environmental predictability for the evolution of plasticity, but without demography [11,30,31,72]; Reed et al. [15] performed simulations on the interaction of plasticity and population growth in a fluctuating environment, but without evolution, and without changes in patterns of environmental variation. Despite the lack of quantitative predictions, basic qualitative predictions could be tested experimentally.
Figure 3.
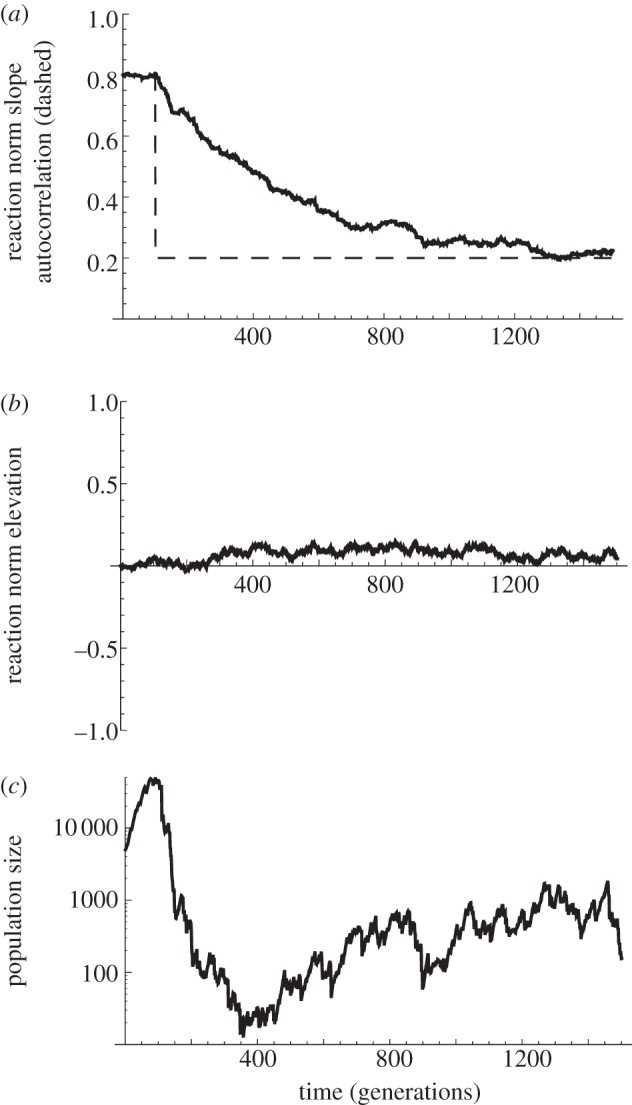
Plasticity, predictability and evolutionary rescue in a fluctuating environment. Evolutionary rescue following an abrupt change in environmental predictability is illustrated. (a) Relative plasticity, that is, the mean reaction norm slope scaled to the slope of changes in the optimum phenotype with the environment (environmental sensitivity of selection). The temporal autocorrelation of the environment during the time lag between development and selection on the plastic trait is also represented (dashed line). (b) The reaction norm elevation, i.e. its intercept in a reference environment. (c) Represents population size, under density-independent population growth. Autocorrelation of environmental fluctuations drops from 0.8 to 0.2 at generation 100 (a), with no change in the average environment. This less predictable environment causes the high level of plasticity that was previously optimal to become detrimental, and the population starts declining (b). In this example, evolutionary rescue is afforded by the evolution of a decreased level of plasticity that matches the current environmental predictability (a), with little change in reaction norm elevation (b). Quantitative genetic simulations were used as in figure 1, but with fluctuations in the optimum, with variance 1.5 and autocorrelation as given above. All genetic parameters are as in figure 1a.
(i). Experiment 1: change in cue reliability
For a species known to use a cue as a proxy for the environment of selection (e.g. photoperiod for seasonality and food abundance [70], or chemical kairomones for presence of a predator [73]), artificially disconnecting cues from selection pressure in the laboratory, therefore inducing the expression of the wrong plastic response, can cause a population to initially decline because of maladaptive phenotypic plasticity. A first prediction is thus that decreasing the match between cue and selective pressure should increase the level of stress, and accelerate the initial decline of plastic populations, but not for populations with little or no plasticity. Such a pattern was recently found in an elegant study combining experiments with Escherichia coli and Saccaromyces cerevisiae, where the authors showed that changing the order in which environments (such as exposure to specific sugars, oxidative stress or increased temperature) are experienced can turn phenotypic plasticity from beneficial to detrimental [74] (see also [75]). The most favourable sequence of environments for the wild-type was the one found in its natural habitat (e.g. host digestive tract for E. coli) [74]. A second prediction is that populations that did manage to escape extinction by ER have evolved a level of phenotypic plasticity that matches the imposed covariance between cue and selection. Exposing ancestral and evolutionarily rescued populations to various levels of the cue can reveal whether the reaction norms actually evolved along this trend during ER.
(ii). Experiment 2: change in patterns of fluctuations
In species for which plastic responses are caused by the same environmental factor that causes selection, what is important is the autocorrelation of this factor between the time of initiation of the plastic response, and the time when selection occurs [31]. Suddenly reducing or increasing temporal autocorrelation (the ‘colour’ of environmental noise [76]) can cause the initial level of plasticity to become detrimental, and provoke population decline (figure 3). After some generations, the final average reaction norm of those populations where ER occurred can be compared with that of the ancestral population (or of populations that went extinct). Plasticity is expected to have increased if autocorrelation increased, but to have decreased if autocorrelation was reduced (as in figure 3). An experiment along these lines was previously conducted on the evolution of plasticity [77], but not in the context of ER.
It is important to note that in such experiments, the source of stress is the pattern of variation in the environment, rather than its state. Because the environment is experienced by all individuals in the population, its fluctuations cause stochasticity (randomness) regardless of population size [78], in contrast to other sources of stochasticity (e.g. life histories, mutation events, etc. [79]), so its impact on population growth can be large. Furthermore, extinction of a given population may occur because of an unfortunate series of environments, even when its long-term growth rate is positive, such that ER would be expected on average [78]. In practice, this means that what needs to be analysed is the average of even more replicates than in other ER experiments. This is becoming more and more feasible with the use of robots [54].
(c). What is the genetic basis of phenotypic plasticity evolution?
Current quantitative genetic predictions for ER with evolving phenotypic plasticity are based on models that assume constant genetic (co)variances of reaction norm parameters, but this assumption may be violated under strong stress, or when mutations affecting population growth are rare or recombine little. Understanding reaction norm evolution requires knowing more about the mutational input, and in particular whether genes affecting plasticity differ from those affecting the trait in a reference environment, which has important evolutionary consequences [80]. Besides, if phenotypic plasticity evolved during the rescue, did it imply mutations of large effects, or was ER instead caused by the cumulative effect of multiple small mutations (as addressed theoretically for non-plastic traits by Gomulkiewicz [81])?
(i). Experiment 1
Measure the mutational variance of reaction norm parameters, by placing genotypes that differ by a controlled number of mutations over a range of environments. This can be done for single mutants in bacteria and viruses [82]. For multicellular eucaryotes, the most common approach is to use the so-called ‘mutation accumulation lines’, where spontaneous mutations are preserved by maintaining a low effective population size that reduces the efficiency of natural selection (reviewed in [83]).
(ii). Experiment 2
Measure the heritable component of the reaction norm for genotypes sampled at several time points along the trajectory of ER. This allows investigating whether reaction norms changed gradually or suddenly, and which aspect (plasticity or intercept in a reference environment) changed at different time points. This is easier for microbes, because they allow resurrecting frozen samples for replicate lines that went extinct in the experiments, or that were eventually rescued.
Both these experiments can be combined with the sequencing of whole genomes [84], or the genotyping of numerous SNPs throughout the genome [85], to identify genes involved in the evolution of plasticity. This can allow investigating finer aspects of the genetic architecture of phenotypic plasticity. For instance, is plasticity due to the turning on/off of genes by the environment, to a more gradual regulation of gene expression, or simply to the environmental sensitivity of specific alleles [19, ch. 3]? If genes of major effect on plasticity are identified, these can then be knocked out, to directly assess the influence of phenotypic plasticity on population persistence in experiments such as those described in §5a,b. Furthermore, molecular polymorphism and divergence at loci next to these genes can be used to seek molecular signatures of natural selection [86,87].
(d). Are there demographic constraints on the evolution of phenotypic plasticity?
Recently, Gomulkiewicz & Houle [88] emphasized that, by allowing only certain populations to persist conditional on their evolutionary dynamics, ER operates a filter on evolutionary trajectories and on their determinants (i.e. genetic (co)variance of adaptive traits), thus constraining evolution. Here, an important question is whether purely demographic properties of a population (initial population size N0, or intrinsic growth rate when well-adapted rmax) affect whether, and to what extent, the evolution of plasticity contributes to adaptation and ER.
(i). Experiment
Compare reaction norms of populations that were genetically rescued to those that were not, under a given level of environmental stress (same selective pressure), but with different rmax, or starting from different N0. For microbes, rmax can be set by changing the dilution rate (more frequent or stronger bottlenecks for batch culture, or faster dilution in a chemostat), causing higher mortality independently of the environmental challenge.
(e). How do costs of phenotypic plasticity affect the outcome of the rescue?
Where costs of plasticity have been measured, they were generally weak [39], but even small costs can make the difference between survival and extinction in a changed environment [13,14]. Theory predicts that ER is mostly due to the evolution of plasticity if the environmental change is large enough that plastic responses allow overcoming costs of phenotypic plasticity [13]. This can be tested empirically.
(i). Experiment
Costs of plasticity are measured by regressing the residual of the relationship between phenotype and fitness (in one or multiple environments) against plasticity, in a multiple regression framework [37,38]. By measuring these costs in a collection of genotypes before exposing them to various levels of environmental stress, one may predict to what extent plasticity is expected to contribute to ER. This can then be verified by comparing reaction norms of evolved and ancestral populations.
(f). How does density dependence influence evolutionary rescue under abiotic stress?
When starting from large population sizes, absolute fitness is initially reduced by a combination of two factors: the extrinsic stress that triggered the extinction risk in the first place, and density-dependent competition with conspecifics [13]. As population density decreases when the population crashes, the intensity of intraspecific competition goes down. This may slow down the decline of the population, which may even reach a new equilibrium size before it starts increasing again when evolution restores higher fitness. In this scenario, the population dynamics would have the typical U shape of ER. However, if the mutation supply is limited, the first phase of the apparent rescue (reaching r ∼ 0, and the minimum population size) can occur without genetic evolution, instead only resulting from density-dependence of population growth (figure 4). This can be seen as a form of ER caused in part by phenotypic plasticity, if density dependence of population growth is mediated by sensitivity to density of underlying traits affecting fitness. For instance, stem elongation in response to shading by conspecifics is a common form of density-dependent plasticity in plants, which probably underlies the effects of competition on population growth [91].
Figure 4.
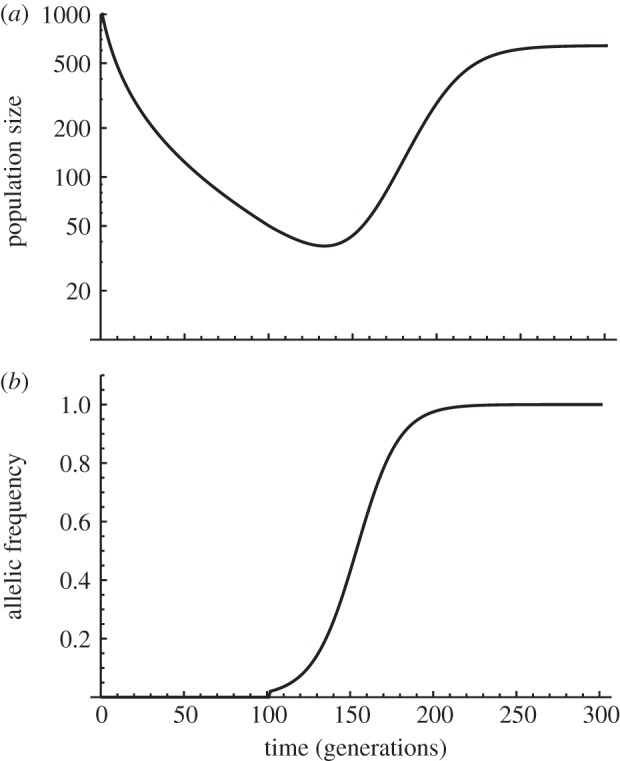
Competition and evolutionary rescue. (a) Demographic dynamics with negative density-dependence of population growth is represented, together with (b) the evolutionary dynamics of a single mutation causing evolutionary rescue. Density regulation follows the Ricker model (discrete time analogue to logistic growth [89]). Selection, in contrast, is density-independent, such that both the environmental stress and the beneficial mutation only affect the intrinsic rate of increase r0 of the population, while the density-dependent component of population growth does not evolve (as described in [13,90]). After the onset of stress (generation 0), the population starts declining because the lowered r0 no longer allows maintenance of a large equilibrium population size. A beneficial mutant restoring higher r0 appears in one copy in generation 100, causing the population to increase back to a high equilibrium size. Note that the first phase of the apparent population recovery (until the population size stabilizes at a lower value) occurs without any genetic evolution. Parameters are, before the onset of stress: intrinsic rate of increase r0 = 0.1, carrying capacity K = 1000; stress-induced reduction in r0: s0 = −0.11; selection coefficient of the rescue mutation: s = 0.077.
Some species instead are subject to positive density dependence at low numbers (Allee effects), where fitness declines as population size decreases. This heightens the risk of extinction by setting up a vicious positive feedback between shrinking numbers and lowered fitness. Plastic responses could play a crucial role in mitigating these Allee effects. For instance, a plant that normally outcrosses might suffer reproductive failure when numbers become low because of the scarcity of pollen. A plastic ability to self, or divert resources from flower and reproduction to vegetative growth, might help it persist.
(i). Experiment 1
Sample different time points along the trajectory of an ER, and experimentally place each sampled population at several densities to measure their mean fitness. This would allow assessment of how much fitness is affected by competition, and to what extent density dependence is what was originally causing the population to decline.
(ii). Experiment 2
Manipulate the competition intensity during ER, independently of the environmental stress. This can be done by dynamically tuning resource availability to population density (i.e. per capita), which is straightforward using optical density in microbes. Results can be compared with those where a constant amount of food is provisioned for the whole population.
(iii). Experiment 3
Regarding Allee effects, experiment similar to the one with Mimulus guttatus described in [59] (see §4a) might be extended to examine the evolution of density-dependent plasticity of selfing in the rescued populations.
6. Choosing the right model organism
The prototype experiments described above are all somewhat idealized, and their actual implementation will depend on the specificities of the chosen model species. The choice of the appropriate model organism to experimentally study the effect of phenotypic plasticity on ER is not straightforward. In particular, there is a clear dichotomy of approaches and constraints between microbes and multicellular organisms.
On the one hand, microorganisms seem quite appropriate for studying ER. Because fitness/population growth per se is generally the main trait of interest in studies with microbes, and is also used to quantify stress, they are inherently well-suited to work on evolutionary demography. Their short generation times, large population sizes and scope for extensive replication of treatments, allow monitoring of population dynamics over many generations across a range of environments [53]. This enables the observation of rare events such as de novo mutations restoring positive growth rate. The possibility of freezing most microbes makes it possible to compete evolved strains against their ancestor, and transformation with fluorescent markers allows fine measurement of the dynamics of selection [92]. Various techniques are also available to magnify mutagenesis (site-directed mutagenesis or random mutations via error-prone polymerases, transposition, chemicals, UV, etc.), facilitating analysis of the role of mutational variance in ER. Finally, their relatively small genomes, and the development of new sequencing technologies, enable whole genome sequencing for a decent price, allowing identification and tracking of adaptive mutations [84].
On the other hand, understanding how plasticity might contribute to the persistence of multicellular eucaryotes is a theme of potentially great importance in conservation [14], while the numbers and geographical spread of micro-organisms in natural systems are great enough that there is no reason to worry about extinction: there is not yet a ‘conservation microbiology’. Besides, the inheritance and phenotypic plasticity of adaptive traits is commonly measured in multicellular eucaryotes, while current analyses with microbes often are rather removed from a mechanistic understanding of the phenotypic traits that determine fitness. In particular, sexual animals and plants allow investigation of the mechanistic underpinnings of adaptation mediated by complex (integrative) traits with multiple recombining loci responding simultaneously to selection, consistent with what is observed for wild populations with conservation issues. Experimental evolution over multiple generations has been carried out with Drosophila [85], Caenorhabditis [93], mice [66] and plants [59], for instance, suggesting that insightful ER experiments could also be performed with multicellular organisms, despite their relatively longer generation time, provided that the evolutionary dynamics per generation are relatively rapid. Potential lags between an environmental cue and selection on the expressed plastic trait also may be easier to measure or manipulate in multicellular organisms than in microbes (even though such lags have already been measured precisely with the latter [72]). A caveat is that the larger body size of multicellular eucaryotes implies that fewer individuals can be reared per space unit. This necessarily limits replication, and restricts attention to events that are not too rare, turning the focus from mutational input to standing genetic variation.
Despite these differences, it could be argued that the reasons why phenotypes of multicellular organisms are more studied than those of microorganisms are mostly historical. Until relatively recent technological improvements, multicellular eukaryotes were easier to manipulate and observe individually than microbes, but this is rapidly changing. One can now measure, for instance, the cell biovolume and inner pH of thousands of individual bacteria in few seconds with a flow cytometer and a GFP marker [94]. It is also possible to measure the number of proteins on the cell surface [95], or even the number of proteins [96] and mRNAs [97] inside the cytoplasm of a single bacterium. The study of micro-colonies under a microscope [98] is a promising technique to follow the inheritance of traits and of their plasticity along lineages akin to pedigrees. The next challenge is to identify meaningful adaptive traits, as has been done, for instance, by Dykhuizen & Dean [99], who studied metabolic pathways in E. coli and related fitness to underlying traits (expression of lactose permease, and β-galactosidase).
Ultimately, in order to reach robust conclusions, one should combine and compare results from microorganisms and multicellular eucaryotes. Two conceptual tools to connect them are the adaptive landscape, depicting fitness against traits or genotypes, and the ecological niche, which in effect is the reaction norm of fitness against environments. Adaptive landscapes have been used to predict both the evolution of traits and the distribution of fitness effects of mutations across environments [100,101]. Indeed predictions for fitness evolution generally rely on adaptation models that include underlying traits, even when fitness is the only trait actually measured (e.g. for most microbes).
7. Perspectives and conclusions
(a). Other topics of interest
We have highlighted above some of the questions that are both biologically important and reasonably amenable to experimental investigation. We now list some other aspects that would also be worth addressing, but are either more specific or most difficult to study in the laboratory.
First, the effect of random genetic drift on the evolution of plasticity has received little attention, and most theory is deterministic for its genetic aspects (even though the environment might change stochastically). Investigating the effect of genetic drift on the efficiency of selection on plasticity is particularly relevant in the context of ER, where incursions at low densities could result in several generations of mostly neutral evolution of plasticity, together with reduced variance in plasticity and the trait. This can be studied with individual-based simulations, as was done for ER without phenotypic plasticity by, e.g. Holt et al. [102], and more recently for evolving plasticity (but without demography leading to extinction) by Scheiner & Holt [35].
We have here described ER caused by evolution and plasticity of a single trait, but ER may also involve multiple genetically correlated traits (as modelled without plasticity in [88]). A more general approach would thus investigate multi-trait phenotypic plasticity, where the environmental stress may cause correlated plastic responses by many characters (plasticity integration [103,104]). Whether multi-trait plasticity facilitates or hampers ER should depend on the interaction of patterns of selection with the genetic covariances of reaction norms for multiple traits. A particularly interesting case of multi-trait plasticity is that where traits involved in adaptation to the abiotic environment are plastically correlated to traits that mediate interactions with other species (e.g. lowering of immune functions in response to abiotic stress). Plasticity of interspecific interactions could then determine the likelihood of ER in the face of abiotic environmental challenge. It would also be useful to understand if costs of phenotypic plasticity cumulatively add up among characters (such that having more traits that are plastic imposes a greater total cost), or if instead there is ‘superadditivity’, where costs of multiple trait plasticity exceed those predicted from a simple addition of costs for each trait considered singly.
Some examples of ER probably involve frequency-dependent selection, and genetic evolution of the environment by ‘niche-construction’ [105] or ecological facilitation [106]. This occurs when a given genotype, while increasing in frequency under selection, progressively makes the environment more suitable for others, thus alleviating the stress and stopping the population decline. For instance, some mutant genotype may release molecules that make nutrients available to others, e.g. invertases hydrolysing glucose in the outer medium in yeast [107], or siderophores capturing iron from the host in bacteria [108]. In this case, it is the environment itself that changes (possibly in response to natural selection), rather than how phenotypes respond to it.
We here mostly described temporal environmental change, but the evolution of plasticity can also be fostered by spatial variation along an environmental gradient, with shifts in the local optimum. Dispersal favours plasticity in a spatially heterogeneous environment [109], and the relative importance of plasticity and local adaptation over an environmental gradient is influenced notably by the rate of dispersal, and the steepness of the gradient [35,110]. How this process interacts with demography and a species geographical range in a temporally constant environment was recently analysed [110], but theory has not specifically investigated how the geographical range limits evolve after a sudden increase in steepness of the gradient, as can be studied empirically in an experiment similar to Bell & Gonzalez's [57].
It would also be useful to consider the behavioural dimension of plasticity, and its interaction with ecology. Mechanisms such as habitat selection, foragers sampling, and learning about patch or prey item quality, have been the focus of a rich literature in behavioural ecology [111], and provide avenues by which many animals can potentially persist in novel environments [112]. Experimental evolution of learning has been performed in the laboratory [113]. Therefore, experimental tests of the contribution of phenotypic plasticity to ER should also involve studies of behaviour and learning.
Finally, it would be worth investigating experimentally how phenotypic plasticity may evolve from simpler forms of phenotypic switches independently of the environment, such as slow growing ‘persister’ phenotypes in E. coli [114,115]. Kussell & Leibler [116] have shown theoretically that plastic changes tuned to environmental variation (‘responsive switching’) should evolve if the cost of sensing the environment is low, and the environment fluctuates on the time-scale of a generation, while random switching is favoured otherwise. These predictions have not yet been tested empirically, to our knowledge.
(b). Conclusion
We have provided an overview of key issues relating phenotypic plasticity and its evolution to ER, and have illustrated how experimental evolution may be used to answer arising questions on this topic. The importance of these questions is highlighted by recent studies indicating that observed phenotypic change in the wild often includes a substantial component of phenotypic plasticity [7,8,117,118], which affects population growth [119,120], and thus potentially persistence in changed environments. The tools to investigate those questions experimentally in the laboratory are available in a variety of models organisms, with contrasted life-history and genetic properties. Of particular interest for the study of ER is the possibility of (i) storing ancestral samples, allowing comparisons with even replicate lines that went extinct; and (ii) performing many replicates using robots [54], allowing investigation of highly stochastic processes such as rare mutations, and randomly fluctuating environments.
Acknowledgements
We are grateful to J. N. Jasmin and S. M. Scheiner for useful discussions and comments on this manuscript. L.-M. Chevin is supported by the grant ‘ContempEvol’ from the ANR ‘retour post-doc’ programme. S.F. was supported by ANR-09-PEXT-011 and ANR 2010 BLANC 1715 02. R.G. was supported by US National Science Foundation grant DEB-0919376.
References
- 1.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 2.Davis MB, Shaw RG, Etterson JR. 2005. Evolutionary responses to changing climate. Ecology 86, 1704–1714 10.1890/03-0788 (doi:10.1890/03-0788) [DOI] [Google Scholar]
- 3.Caswell H. 2001. Matrix population models: construction, analysis, and interpretation. Sunderland, MA: Sinauer Associates [Google Scholar]
- 4.Charlesworth B. 1994. Evolution in age-structured populations, 2nd edn Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Gomulkiewicz R, Holt RD. 1995. When does evolution by natural selection prevent extinction. Evolution 49, 201–207 10.2307/2410305 (doi:10.2307/2410305) [DOI] [PubMed] [Google Scholar]
- 6.Lynch M, Lande R. 1993. Evolution and extinction in response to environmental change. In Biotic interactions and global change (eds Kareiva P, Kingsolver J, Huey R.), pp. 234–250 Sunderland, MA: Sinauer [Google Scholar]
- 7.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LE, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 10.1126/science.1157174 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 8.Reale D, McAdam AG, Boutin S, Berteaux D. 2003. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. Lond. B 270, 591–596 10.1098/rspb.2002.2224 (doi:10.1098/rspb.2002.2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van de Pol M, Osmond HL, Cockburn A. 2011. Fluctuations in population composition dampen the impact of phenotypic plasticity on trait dynamics in superb fairy-wrens. J. Anim. Ecol. 81, 411–422 10.1111/j.1365-2656.2011.01919.x (doi:10.1111/j.1365-2656.2011.01919.x) [DOI] [PubMed] [Google Scholar]
- 10.Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J. 2008. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 17, 167–178 10.1111/j.1365-294X.2007.03413.x (doi:10.1111/j.1365-294X.2007.03413.x) [DOI] [PubMed] [Google Scholar]
- 11.Scheiner S. 1993. Genetics and evolution of phenotypic plasticity. Annu. Rev. Ecol. Syst. 24, 35–68 10.1146/annurev.es.24.110193.000343 (doi:10.1146/annurev.es.24.110193.000343) [DOI] [Google Scholar]
- 12.Baldwin JM. 1896. A new factor in evolution. Am. Nat. 30, 441–451 10.1086/276408 (doi:10.1086/276408) [DOI] [Google Scholar]
- 13.Chevin LM, Lande R. 2010. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated population? Evolution 64, 1143–1150 10.1111/j.1558-5646.2009.00875.x (doi:10.1111/j.1558-5646.2009.00875.x) [DOI] [PubMed] [Google Scholar]
- 14.Chevin LM, Lande R, Mace GM. 2010. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLoS Biol. 8, e1000357. 10.1371/journal.pbio.1000357 (doi:10.1371/journal.pbio.1000357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT. 2010. Phenotypic plasticity and population viability: the importance of environmental predictability. Proc. R. Soc. B 277, 3391–3400 10.1098/rspb.2010.0771 (doi:10.1098/rspb.2010.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chevin LM, Collins S, Lefevre F. In press. Phenotypic plasticity and evo-demographic responses to climate change: taking theory out to the field. Funct. Ecol. 10.1111/j.1365-2435.2012.02043.x (doi:10.1111/j.1365-2435.2012.02043.x) [DOI] [Google Scholar]
- 17.Vander Wal E, Garant D, Festa-Bianchet M, Pelletier F. 2012. Evolutionary rescue in vertebrates: evidence, applications and uncertainty. Phil. Trans. R. Soc. B 368, 20120090. 10.1098/rstb.2012.0090 (doi:10.1098/rstb.2012.0090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pigliucci M. 2001. Phenotypic plasticity: beyond nature and nurture. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 19.Schlichting C, Pigliucci M. 1998. Phenotypic evolution: a reaction norm perspective. Sunderland, MA: Sinauer [Google Scholar]
- 20.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 21.Woltereck R. 1913. Weitere experimentelle untersuchungen über Aränderung, speziell über das Wesen quantitativer Artunterschiede bei Daphniden. Mol. Gen. Genet. 9, 146 [Google Scholar]
- 22.Lande R. 1982. A quantitative genetic theory of life-history evolution. Ecology 63, 607–615 10.2307/1936778 (doi:10.2307/1936778) [DOI] [Google Scholar]
- 23.van Tienderen P. 2000. Elasticities and the link between demographic and evolutionary dynamics. Ecology 81, 666–679 10.1890/0012-9658(2000)081[0666:EATLBD]2.0.CO;2 (doi:10.1890/0012-9658(2000)081[0666:EATLBD]2.0.CO;2) [DOI] [Google Scholar]
- 24.Barfield M, Holt RD, Gomulkiewicz R. 2011. Evolution in stage-structured populations. Am. Nat. 177, 397–409 10.1086/658903 (doi:10.1086/658903). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roff D. 2002. Life history evolution. Sunderland, MA: Sinauer Associates [Google Scholar]
- 26.Gilchrist GW. 1995. Specialists and generalists in changing environments. I. Fitness landscapes of thermal sensitivity. Am. Nat. 146, 252–270 10.1086/285797 (doi:10.1086/285797) [DOI] [Google Scholar]
- 27.Lynch M, Gabriel W. 1987. Environmental tolerance. Am. Nat. 129, 283–303 10.1086/284635 (doi:10.1086/284635) [DOI] [Google Scholar]
- 28.Holt RD. 2009. Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl Acad. Sci. USA 106, 19 659–19 665 10.1073/pnas.0905137106 (doi:10.1073/pnas.0905137106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavrilets S, Scheiner SM. 1993. The genetics of phenotypic plasticity. V. Evolution of reaction norm shape. J. Evol. Biol. 6, 31–48 10.1046/j.1420-9101.1993.6010031.x (doi:10.1046/j.1420-9101.1993.6010031.x) [DOI] [Google Scholar]
- 30.de Jong G. 1999. Unpredictable selection in a structured population leads to local genetic differentiation in evolved reaction norms. J. Evol. Biol. 12, 839–851 10.1046/j.1420-9101.1999.00118.x (doi:10.1046/j.1420-9101.1999.00118.x) [DOI] [Google Scholar]
- 31.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435–1446 10.1111/J.1420-9101.2009.01754.X (doi:10.1111/J.1420-9101.2009.01754.X). [DOI] [PubMed] [Google Scholar]
- 32.Levins R. 1963. Theory of fitness in a heterogeneous environment. II. Developmental flexibility and niche selection. Am. Nat. 97, 75–90 10.1086/282258 (doi:10.1086/282258) [DOI] [Google Scholar]
- 33.Moran NA. 1992. The evolutionary maintenance of alternative phenotypes. Am. Nat. 139, 971–989 10.1086/285369 (doi:10.1086/285369) [DOI] [Google Scholar]
- 34.Tufto J. 2000. The evolution of plasticity and nonplastic spatial and temporal adaptations in the presence of imperfect environmental cues. Am. Nat. 156, 121–130 10.1086/303381 (doi:10.1086/303381) [DOI] [PubMed] [Google Scholar]
- 35.Scheiner SM, Holt RD. 2012. The genetics of phenotypic plasticity. X. Variation versus uncertainty. Ecol. Evol. 2, 751–767 10.1002/ece3.217 (doi:10.1002/ece3.217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghalambor C, McKay J, Carroll S, Reznick D. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407 10.1111/j.1365-2435.2007.01283.x (doi:10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 37.Dewitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81 10.1016/S0169-5347(97)01274-3 (doi:10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 38.Van Tienderen P. 1991. Evolution of generalists and specialists in spatially heterogeneous environments. Evolution 45, 1317–1331 10.2307/2409882 (doi:10.2307/2409882) [DOI] [PubMed] [Google Scholar]
- 39.Van Buskirk J, Steiner UK. 2009. The fitness costs of developmental canalization and plasticity. J. Evol. Biol. 22, 852–860 10.1111/j.1420-9101.2009.01685.x (doi:10.1111/j.1420-9101.2009.01685.x) [DOI] [PubMed] [Google Scholar]
- 40.Auld JR, Agrawal AA, Relyea RA. 2010. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503. 10.1098/rspb.2009.1355 (doi:10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falconer D. 1952. The problem of environment and selection. Am. Nat. 86, 293–298 10.1086/281736 (doi:10.1086/281736) [DOI] [Google Scholar]
- 42.Via S, Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39, 505–522 10.2307/2408649 (doi:10.2307/2408649) [DOI] [PubMed] [Google Scholar]
- 43.Gomulkiewicz R, Kirkpatrick M. 1992. Quantitative genetics and the evolution of reaction norms. Evolution 46, 390–411 10.2307/2409860 (doi:10.2307/2409860) [DOI] [PubMed] [Google Scholar]
- 44.de Jong G. 1995. Phenotypic plasticity as a product of selection in a variable environment. Am. Nat. 145, 493. 10.1086/285752 (doi:10.1086/285752) [DOI] [PubMed] [Google Scholar]
- 45.Huey R, Hertz P. 1984. Is a jack-of-all-temperatures a master of none? Evolution 38, 441–444 10.2307/2408502 (doi:10.2307/2408502) [DOI] [PubMed] [Google Scholar]
- 46.Gilchrist GW. 1996. A quantitative genetic analysis of thermal sensitivity in the locomotor performance curve of Aphidius ervi. Evolution 50, 1560–1572 10.2307/2410892 (doi:10.2307/2410892) [DOI] [PubMed] [Google Scholar]
- 47.Izem R, Kingsolver JG. 2005. Variation in continuous reaction norms: quantifying directions of biological interest. Am. Nat. 166, 277–289 10.1086/431314 (doi:10.1086/431314) [DOI] [PubMed] [Google Scholar]
- 48.Knies JL, Kingsolver JG, Burch CL. 2009. Hotter is better and broader: thermal sensitivity of fitness in a population of bacteriophages. Am. Nat. 173, 419–430 10.1086/597224 (doi:10.1086/597224) [DOI] [PubMed] [Google Scholar]
- 49.Crow JF, Kimura M. 1970. An introduction to population genetics theory. New York, NY: Harper & Row [Google Scholar]
- 50.Holt RD. 1990. The microevolutionary consequences of climate change. Trends Ecol. Evol. 5, 311–315 10.1016/0169-5347(90)90088-U (doi:10.1016/0169-5347(90)90088-U) [DOI] [PubMed] [Google Scholar]
- 51.Paenke I, Sendhoff B, Kawecki TJ. 2007. Influence of plasticity and learning on evolution under directional selection. Am. Nat. 170, E47–E58 10.1086/518952 (doi:10.1086/518952) [DOI] [PubMed] [Google Scholar]
- 52.Buckling A, Craig Maclean R, Brockhurst MA, Colegrave N. 2009. The Beagle in a bottle. Nature 457, 824–829 10.1038/nature07892 (doi:10.1038/nature07892) [DOI] [PubMed] [Google Scholar]
- 53.Elena SF, Lenski RE. 2003. Evolution experiments with microorganisms: the dynamics and genetic bases of adaptation. Nat. Rev. Genet. 4, 457–469 10.1038/nrg1088 (doi:10.1038/nrg1088) [DOI] [PubMed] [Google Scholar]
- 54.Bell G, Gonzalez A. 2009. Evolutionary rescue can prevent extinction following environmental change. Ecol. Lett. 12, 942–948 10.1111/j.1461-0248.2009.01350.x (doi:10.1111/j.1461-0248.2009.01350.x) [DOI] [PubMed] [Google Scholar]
- 55.Orr HA, Unckless RL. 2008. Population extinction and the genetics of adaptation. Am. Nat. 172, 160–169 10.1086/589460 (doi:10.1086/589460) [DOI] [PubMed] [Google Scholar]
- 56.Martin G, Aguilée R, Ramsayer J, Kaltz O, Ronce O. 2012. The probability of evolutionary rescue: towards a quantitative comparison between theory and evolution experiments. Phil. Trans. R. Soc. B 368, 20120088. 10.1098/rstb.2012.0088 (doi:10.1098/rstb.2012.0088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bell G, Gonzalez A. 2011. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science 332, 1327–1330 10.1126/science.1203105 (doi:10.1126/science.1203105) [DOI] [PubMed] [Google Scholar]
- 58.Toprak E, Veres A, Michel JB, Chait R, Hartl DL, Kishony R. 2011. Evolutionary paths to antibiotic resistance under dynamically sustained drug selection. Nat. Genet. 44, 101–105 10.1038/ng.1034 (doi:10.1038/ng.1034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roels SA, Kelly JK. 2011. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution 65, 2541–2552 10.1111/j.1558-5646.2011.01326.x (doi:10.1111/j.1558-5646.2011.01326.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scheiner S. 2002. Selection experiments and the study of phenotypic plasticity. J. Evol. Biol. 15, 889–898 10.1046/j.1420-9101.2002.00468.x (doi:10.1046/j.1420-9101.2002.00468.x) [DOI] [Google Scholar]
- 61.Garland T, Jr, Kelly SA. 2006. Phenotypic plasticity and experimental evolution. J. Exp. Biol. 209, 2344–2361 10.1242/jeb.02244 (doi:10.1242/jeb.02244) [DOI] [PubMed] [Google Scholar]
- 62.Scheiner S, Lyman R. 1991. The genetics of phenotypic plasticity. II. Response to selection. J. Evol. Biol. 4, 23–50 10.1046/j.1420-9101.1991.4010023.x (doi:10.1046/j.1420-9101.1991.4010023.x) [DOI] [Google Scholar]
- 63.Reboud X, Bell G. 1997. Experimental evolution in Chlamydomonas. III. Evolution of specialist and generalist types in environments that vary in space and time. Heredity 78, 507–514 10.1038/hdy.1997.79 (doi:10.1038/hdy.1997.79) [DOI] [Google Scholar]
- 64.Scheiner SM. 1998. The genetics of phenotypic plasticity. VII. Evolution in a spatially-structured environment. J. Evol. Biol. 11, 303–320 [Google Scholar]
- 65.Falconer DS. 1990. Selection in different environments—effects on environmental sensitivity (reaction norm) and on mean performance. Genet. Res. 56, 57–70 10.1017/S0016672300028883 (doi:10.1017/S0016672300028883) [DOI] [Google Scholar]
- 66.Kelly SA, Czech PP, Wight JT, Blank KM, Garland T. 2006. Experimental evolution and phenotypic plasticity of hindlimb bones in high-activity house mice. J. Morphol. 267, 360–374 10.1002/jmor.10407 (doi:10.1002/jmor.10407) [DOI] [PubMed] [Google Scholar]
- 67.Czesak ME, Fox CW, Wolf JB. 2006. Experimental evolution of phenotypic plasticity: how predictive are cross-environment genetic correlations? Am. Nat. 168, 323–335 10.1086/506919 (doi:10.1086/506919) [DOI] [PubMed] [Google Scholar]
- 68.Bennett AF, Lenski RE. 2007. An experimental test of evolutionary trade-offs during temperature adaptation. Proc. Natl Acad. Sci. USA 104, 8649–8654 10.1073/pnas.0702117104 (doi:10.1073/pnas.0702117104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hughes BS, Cullum AJ, Bennett AF. 2007. Evolutionary adaptation to environmental pH in experimental lineages of Escherichia coli. Evolution 61, 1725–1734 10.1111/j.1558-5646.2007.00139.x (doi:10.1111/j.1558-5646.2007.00139.x) [DOI] [PubMed] [Google Scholar]
- 70.Nussey DH, Postma E, Gienapp P, Visser ME. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 10.1126/science.1117004 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 71.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 10.1098/rspb.2007.0997 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitchell A, Pilpel Y. 2011. A mathematical model for adaptive prediction of environmental changes by microorganisms. Proc. Natl Acad. Sci. USA 108, 7271–7276 10.1073/pnas.1019754108 (doi:10.1073/pnas.1019754108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hammill E, Rogers A, Beckerman A. 2008. Costs, benefits and the evolution of inducible defences: a case study with Daphnia pulex. J. Evol. Biol. 21, 705–715 10.1111/j.1420-9101.2008.01520.x (doi:10.1111/j.1420-9101.2008.01520.x) [DOI] [PubMed] [Google Scholar]
- 74.Mitchell A, Romano GH, Groisman B, Yona A, Dekel E, Kupiec M, Dahan O, Pilpel Y. 2009. Adaptive prediction of environmental changes by microorganisms. Nature 460, 220–224 10.1038/nature08112 (doi:10.1038/nature08112) [DOI] [PubMed] [Google Scholar]
- 75.Tagkopoulos I, Liu YC, Tavazoie S. 2008. Predictive behavior within microbial genetic networks. Science 320, 1313–1317 10.1126/science.1154456 (doi:10.1126/science.1154456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vasseur DA, Yodzis P. 2004. The color of environmental noise. Ecology 85, 1146–1152 10.1890/02-3122 (doi:10.1890/02-3122) [DOI] [Google Scholar]
- 77.Scheiner SM, Yampolsky LY. 1998. The evolution of Daphnia pulex in a temporally varying environment. Genet. Res. 72, 25–37 10.1017/S0016672398003322 (doi:10.1017/S0016672398003322) [DOI] [Google Scholar]
- 78.Lande R, Engen S, Saether B. 2003. Stochastic population dynamics in ecology and conservation: an introduction. Oxford, UK: Oxford University Press [Google Scholar]
- 79.Lenormand T, Roze D, Rousset F. 2009. Stochasticity in evolution. Trends Ecol. Evol. 24, 157–165 10.1016/j.tree.2008.09.014 (doi:10.1016/j.tree.2008.09.014) [DOI] [PubMed] [Google Scholar]
- 80.de Jong G, Gavrilets S. 2000. Maintenance of genetic variation in phenotypic plasticity: the role of environmental variation. Genet. Res. 76, 295–304 10.1017/S0016672300004729 (doi:10.1017/S0016672300004729) [DOI] [PubMed] [Google Scholar]
- 81.Gomulkiewicz R, Holt RD, Barfield M, Nuismer SL. 2010. Genetics, adaptation, and invasion in harsh environments. Evol. Appl. 3, 97–108 10.1111/J.1752-4571.2009.00117.X (doi:10.1111/J.1752-4571.2009.00117.X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Remold SK, Lenski RE. 2004. Pervasive joint influence of epistasis and plasticity on mutational effects in Escherichia coli. Nat. Genet. 36, 423–426 10.1038/Ng1324 (doi:10.1038/Ng1324) [DOI] [PubMed] [Google Scholar]
- 83.Bataillon T. 2000. Estimation of spontaneous genome-wide mutation rate parameters: whither beneficial mutations? Heredity 84, 497–501 10.1046/j.1365-2540.2000.00727.x (doi:10.1046/j.1365-2540.2000.00727.x) [DOI] [PubMed] [Google Scholar]
- 84.Barrick JE, Yu DS, Yoon SH, Jeong H, Oh TK, Schneider D, Lenski RE, Kim JF. 2009. Genome evolution and adaptation in a long-term experiment with Escherichia coli. Nature 461, 1243–1247 10.1038/nature08480 (doi:10.1038/nature08480). [DOI] [PubMed] [Google Scholar]
- 85.Burke MK, Dunham JP, Shahrestani P, Thornton KR, Rose MR, Long AD. 2010. Genome-wide analysis of a long-term evolution experiment with Drosophila. Nature 467, 587–590 10.1038/nature09352 (doi:10.1038/nature09352) [DOI] [PubMed] [Google Scholar]
- 86.Nielsen R. 2005. Molecular signatures of natural selection. Annu. Rev. Genet. 39, 197–218 10.1146/annurev.genet.39.073003.112420 (doi:10.1146/annurev.genet.39.073003.112420) [DOI] [PubMed] [Google Scholar]
- 87.Pavlidis P, Hutter S, Stephan W. 2008. A population genomic approach to map recent positive selection in model species. Mol. Ecol. 17, 3585–3598 [DOI] [PubMed] [Google Scholar]
- 88.Gomulkiewicz R, Houle D. 2009. Demographic and genetic constraints on evolution. Am. Nat. 174, E218–E229 10.1086/645086 (doi:10.1086/645086). [DOI] [PubMed] [Google Scholar]
- 89.Kot M. 2001. Elements of mathematical ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 90.Chevin LM. 2011. On measuring selection in experimental evolution. Biol. Lett. 7, 210–213 10.1098/rsbl.2010.0580 (doi:10.1098/rsbl.2010.0580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dudley SA, Schmitt J. 1996. Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. Am. Nat. 147, 445–465 10.1086/285860 (doi:10.1086/285860) [DOI] [Google Scholar]
- 92.Gallet R, Cooper TF, Elena SF, Lenormand T. 2012. Measuring selection coefficients below 10–3: method, questions and prospects. Genetics 190, 175–186 10.1534/genetics.111.133454 (doi:10.1534/genetics.111.133454) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Estes S, Lynch M. 2003. Rapid fitness recovery in mutationally degraded lines of Caenorhabditis elegans. Evolution 57, 1022–1030 [DOI] [PubMed] [Google Scholar]
- 94.Olsen KN, Budde BB, Siegumfeldt H, Rechinger KB, Jakobsen M, Ingmer H. 2002. Noninvasive measurement of bacterial intracellular pH on a single cell level with green fluorescent protein and fluorescence ratio imaging microscopy. Appl. Environ. Microbiol. 68, 4145–4147 10.1128/AEM.68.8.4145-4147.2002 (doi:10.1128/AEM.68.8.4145-4147.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wollscheid B, Bausch-Fluck D, Henderson C, O'Brien R, Bibel M, Schiess R, Aebersold R, Watts JD. 2009. Mass spectrometric identification and relative quantification of N-linked cell surface glycoproteins. Nat. Biotechnol. 27, 378–386 10.1038/nbt.1532 (doi:10.1038/nbt.1532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang B, Wu H, Bhaya D, Grossman A, Granier S, Kobilka BK, Zare RN. 2007. Counting low copy number proteins in a single cell. Science 315, 81–84 10.1126/science.1133992 (doi:10.1126/science.1133992) [DOI] [PubMed] [Google Scholar]
- 97.Taniguchi Y, Choi PJ, Li G-W, Chen H, Babu M, Hearn J, Emili A, Xie XS. 2010. Quantifying E. coli proteome and transcriptome with single molecule sensitivity in single cells. Science 329, 533–538 10.1126/science.1188308 (doi:10.1126/science.1188308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stewart EJ, Madden R, Paul G, Taddei F. 2005. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 3, 295–300 10.1371/journal.pbio.0030295 (doi:10.1371/journal.pbio.0030295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dykhuizen DE, Dean AM. 1990. Enzyme activity and fitness: evolution in solution. Trends Ecol. Evol. 5, 257–262 10.1016/0169-5347(90)90067-N (doi:10.1016/0169-5347(90)90067-N) [DOI] [PubMed] [Google Scholar]
- 100.Lande R. 1979. Quantitative genetic analysis of multivariate evolution, applied to brain:body size allometry. Evolution 33, 402–416 10.2307/2407630 (doi:10.2307/2407630) [DOI] [PubMed] [Google Scholar]
- 101.Martin G, Lenormand T. 2006. A general multivariate extension of Fisher's geometrical model and the distribution of mutation fitness effects across species. Evolution 60, 893–907 [PubMed] [Google Scholar]
- 102.Holt RD, Barfield M, Gomulkiewicz R. 2005. Theories of niche conservatism and evolution: could exotic species be potential tests? In Species invasions: insights into ecology, evolution, and biogeography (eds Sax D, Stachowicz J, Gaines SD.), pp. 259–290 Sunderland, MA: Sinauer Associates [Google Scholar]
- 103.Parsons KJ, Robinson BW. 2006. Replicated evolution of integrated plastic responses during early adaptive divergence. Evolution 60, 801–813 [PubMed] [Google Scholar]
- 104.Tonsor SJ, Scheiner SM. 2007. Plastic trait integration across a CO2 gradient in Arabidopsis thaliana. Am. Nat. 169, E119–E140 10.1086/513493 (doi:10.1086/513493) [DOI] [PubMed] [Google Scholar]
- 105.Laland KN, Odling-Smee FJ, Feldman MW. 1999. Evolutionary consequences of niche construction and their implications for ecology. Proc. Natl Acad. Sci. USA 96, 10 242–10 247 10.1073/pnas.96.18.10242 (doi:10.1073/pnas.96.18.10242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bruno JF, Stachowicz JJ, Bertness MD. 2003. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 18, 119–125 10.1016/S0169-5347(02)00045-9 (doi:10.1016/S0169-5347(02)00045-9) [DOI] [Google Scholar]
- 107.Gore J, Youk H, van Oudenaarden A. 2009. Snowdrift game dynamics and facultative cheating in yeast. Nature 459, 253–256 10.1038/nature07921 (doi:10.1038/nature07921). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buckling A, Harrison F, Vos M, Brockhurst MA, Gardner A, West SA, Griffin A. 2007. Siderophore-mediated cooperation and virulence in Pseudomonas aeruginosa. FEMS Microbiol. Ecol. 62, 135–141 10.1111/j.1574-6941.2007.00388.x (doi:10.1111/j.1574-6941.2007.00388.x) [DOI] [PubMed] [Google Scholar]
- 109.Sultan S, Spencer H. 2002. Metapopulation structure favors plasticity over local adaptation. Am. Nat. 160, 271–283 10.1086/341015 (doi:10.1086/341015) [DOI] [PubMed] [Google Scholar]
- 110.Chevin LM, Lande R. 2011. Adaptation to marginal habitats by evolution of increased phenotypic plasticity. J. Evol. Biol. 24, 1462–1476 10.1111/j.1420-9101.2011.02279.x (doi:10.1111/j.1420-9101.2011.02279.x) [DOI] [PubMed] [Google Scholar]
- 111.Stephens DW. 2007. Models of information use. In Foraging: behavior and ecology (eds Stephens DW, Brown JS, Ydenberg RC.), pp. 31–60 Chicago, IL: University of Chicago Press [Google Scholar]
- 112.Sutter M, Kawecki TJ. 2009. Influence of learning on range expansion and adaptation to novel habitats. J. Evol. Biol. 22, 2201–2214 10.1111/j.1420-9101.2009.01836.x (doi:10.1111/j.1420-9101.2009.01836.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mery F, Kawecki TJ. 2004. The effect of learning on experimental evolution of resource preference in Drosophila melanogaster. Evolution 58, 757–767 [DOI] [PubMed] [Google Scholar]
- 114.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. 2004. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (doi:10.1126/science.1099390 [DOI] [PubMed] [Google Scholar]
- 115.Masel J, King OD, Maughan H. 2007. The loss of adaptive plasticity during long periods of environmental stasis. Am. Nat. 169, 38–46 10.1086/510212 (doi:10.1086/510212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kussell E, Leibler S. 2005. Phenotypic diversity, population growth, and information in fluctuating environments. Science 309, 2075–2078 10.1126/science.1114383 (doi:10.1126/science.1114383) [DOI] [PubMed] [Google Scholar]
- 117.Hendry AP, Farrugia TJ, Kinnison MT. 2008. Human influences on rates of phenotypic change in wild animal populations. Mol. Ecol. 17, 20–29 10.1111/J.1365-294x.2007.03428.X (doi:10.1111/J.1365-294x.2007.03428.X) [DOI] [PubMed] [Google Scholar]
- 118.Teplitsky C, Mills JA, Alho JS, Yarrall JW, Merila J. 2008. Bergmann's rule and climate change revisited: disentangling environmental and genetic responses in a wild bird population. Proc. Natl Acad. Sci. USA 105, 13 492–13 496 10.1073/pnas.0800999105 (doi:10.1073/pnas.0800999105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T. 2010. Coupled dynamics of body mass and population growth in response to environmental change. Nature 466, U482–U485 10.1038/nature09210 (doi:10.1038/nature09210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ozgul A, Tuljapurkar S, Benton TG, Pemberton JM, Clutton-Brock TH, Coulson T. 2009. The dynamics of phenotypic change and the shrinking sheep of St. Kilda. Science 325, 464–467 10.1126/science.1173668 (doi:10.1126/science.1173668) [DOI] [PMC free article] [PubMed] [Google Scholar]