Abstract
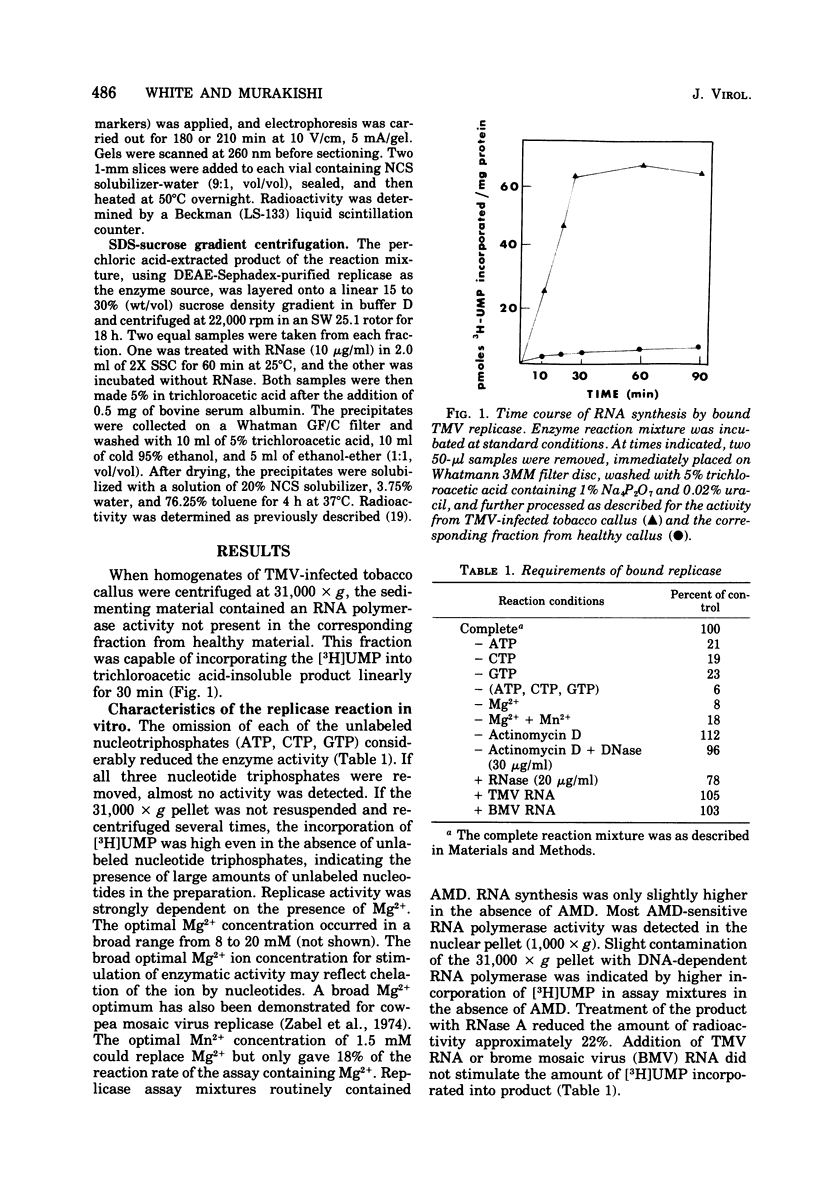
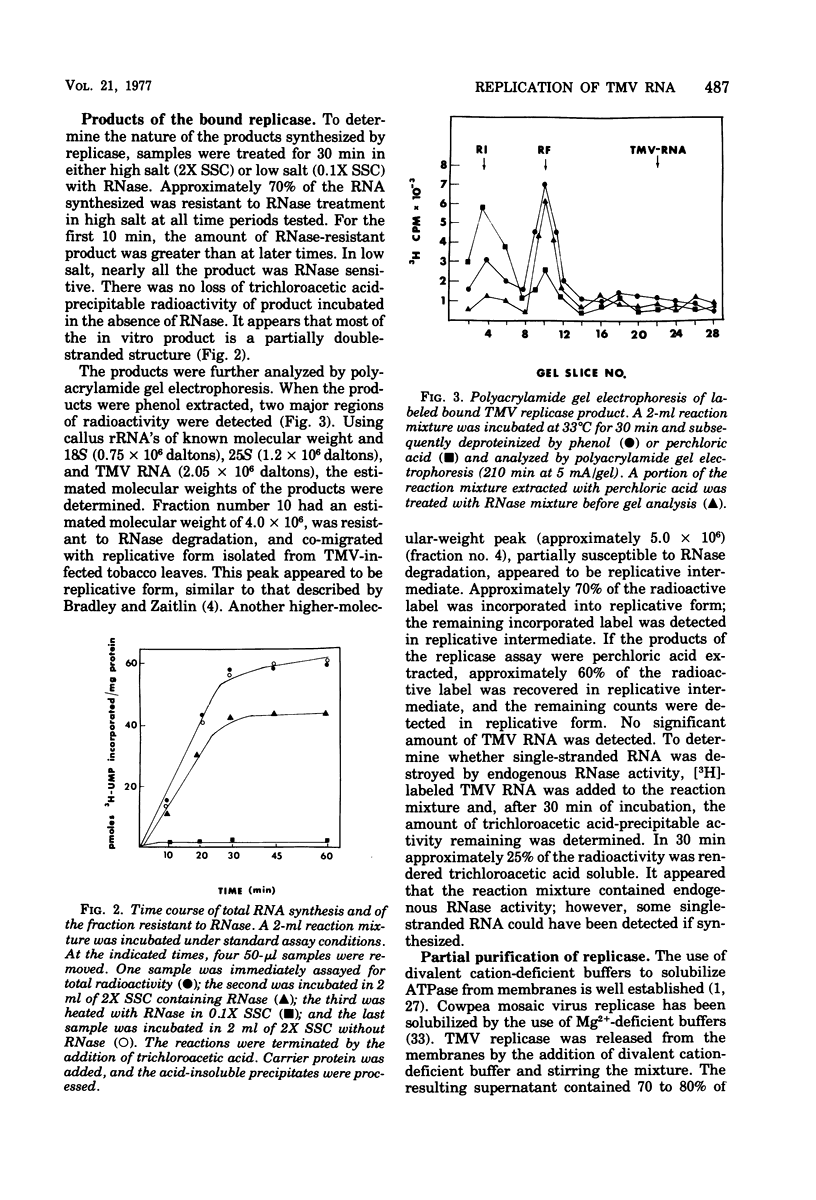
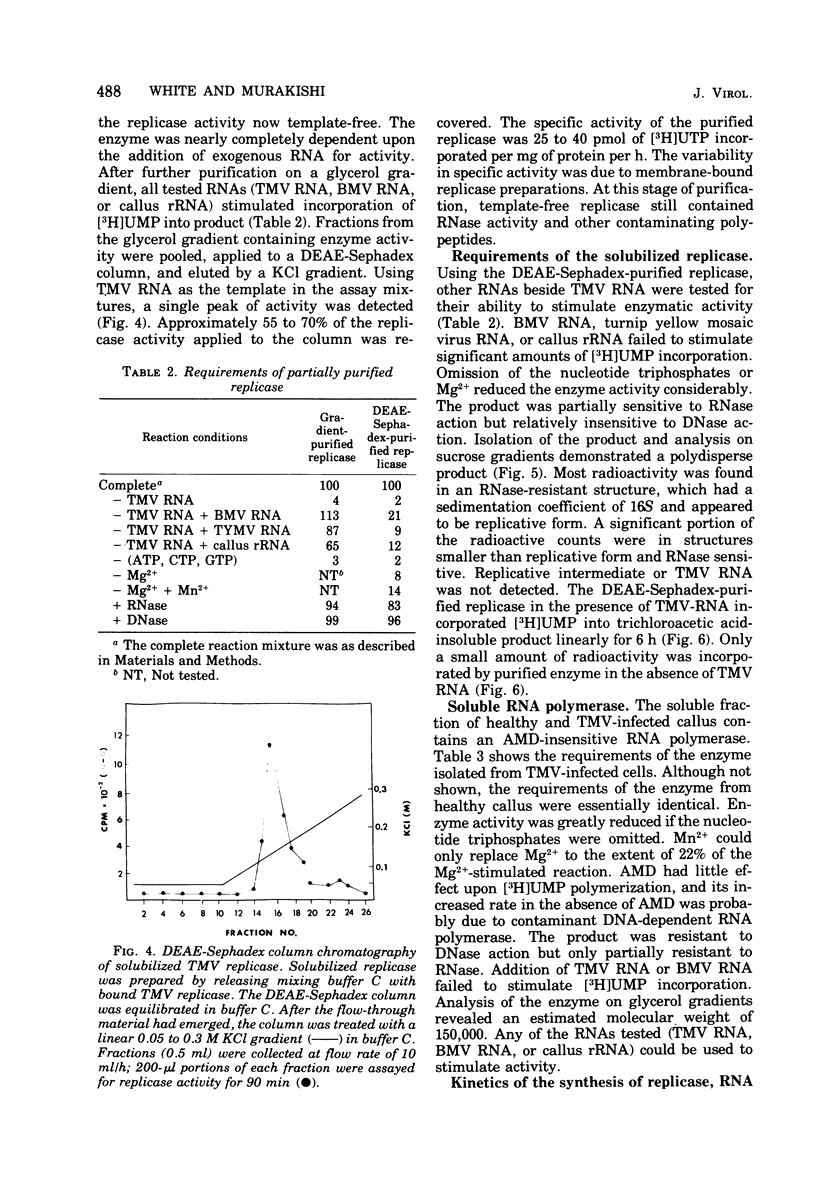
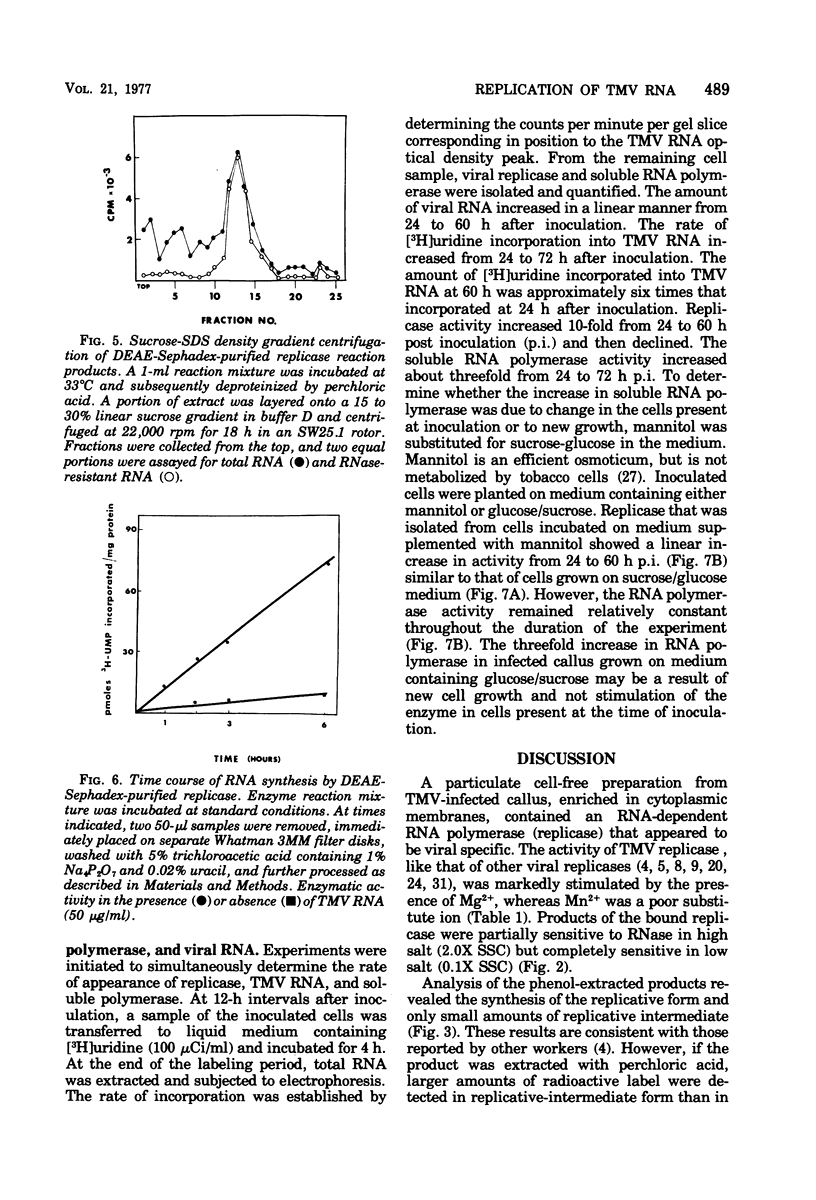
A fraction containing membrane-bound tobacco mosaic virus RNA replicase was isolated form tobacco mosaic virus-infected tobacco callus cultures. The replicase activity reached a maximum 60 h after inoculation and then declined. The enzyme activity was insensitive to actinomycin D and DNase. The corresponding fraction from healthy callus contained essentially no activity. The viral RNA synthesis in vitro proceeded linearly for 30 min and required the four nucleotide triphosphates and Mg2+ ions. Mn2+ was a poor substitute for Mg2+. During RNA synthesis the product was at least 70% resistant to RNase in 2X SSC (0.15 M NaCl plus 0.015 M sodium citrate), but completely digested by RNase in 0.1X SSC. Analysis of the product by polns) that appeared to be replicative form and a partially RNase-resistant structure similar to replicative intermediate form. Washing the membrane-bound replicase with Mg2+-deficient buffer solubilized enzyme. The solubulized enzyme was further purified by DEAE-Sephadex column chromatography. The DEAE-purified enzyme was nearly completely dependent upon tobacco mosaic virus RNA for activity. Analysis of the product on a sucrose gradient revealed a double-stranded RNA with sedimentation of 16S and smaller heterogeneous RNase-sensitive products.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A. The release of bound adenosine triphosphatase from isolated bacterial membranes and the properties of the solubilized enzyme. J Biol Chem. 1965 Sep;240(9):3675–3681. [PubMed] [Google Scholar]
- Arlinghaus R. B., Polatnick J. The isolation of two enzyme-ribonucleic acid complexes involved in the synthesis of foot-and-mouth disease virus ribonucleic acid. Proc Natl Acad Sci U S A. 1969 Mar;62(3):821–828. doi: 10.1073/pnas.62.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astier-Manifacier S., Cornuet P. RNA-dependent RNA polymerase in Chinese cabbage. Biochim Biophys Acta. 1971 Mar 25;232(3):484–493. doi: 10.1016/0005-2787(71)90602-2. [DOI] [PubMed] [Google Scholar]
- Bradley D. W., Zaitlin M. Replication of tobacco mosaic virus. II. The in vitro synthesis of high molecular weight virus-specific RNAs. Virology. 1971 Jul;45(1):192–199. doi: 10.1016/0042-6822(71)90126-7. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Duda C. T., Zaitlin M., Siegel A. In vitro synthesis of double-stranded RNA by an enzyme system isolated from tobacco leaves. Biochim Biophys Acta. 1973 Aug 10;319(1):62–71. doi: 10.1016/0005-2787(73)90041-5. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld E., Maizel J. V., Summers D. F. Soluble RNA polymerase complex from poliovirus-infected HeLa cells. Virology. 1970 Apr;40(4):840–846. doi: 10.1016/0042-6822(70)90129-7. [DOI] [PubMed] [Google Scholar]
- HORTON E., LIU S. L., DALGARNO L., MARTIN E. M., WORK T. S. DEVELOPMENT OF RIBONUCLEIC ACID POLYMERASE IN CELLS INFECTED WITH ENCEPHALOMYOCARDITIS VIRUS AND THE SYNTHESIS OF SINGLE- AND DOUBLE-STRANDED RNA BY THE ISOLATED POLYMERASE. Nature. 1964 Oct 17;204:247–250. doi: 10.1038/204247a0. [DOI] [PubMed] [Google Scholar]
- Hadidi A., Fraenkel-Conrat H. Characterization and specificity of soluble RNA polymerase of brome mosaic virus. Virology. 1973 Apr;52(2):363–372. doi: 10.1016/0042-6822(73)90331-0. [DOI] [PubMed] [Google Scholar]
- Kamen R. A new method for the purification of Q RNA-dependent RNA polymerase. Biochim Biophys Acta. 1972 Feb 23;262(1):88–100. doi: 10.1016/0005-2787(72)90221-3. [DOI] [PubMed] [Google Scholar]
- Kondo M., Gallerani R., Weissmann C. Subunit structure of Q-beta replicase. Nature. 1970 Nov 7;228(5271):525–527. doi: 10.1038/228525a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin E. M., Sonnabend J. A. Ribonucleic acid polymerase catalyzing synthesis of double-stranded arbovirus ribonucleic acid. J Virol. 1967 Feb;1(1):97–109. doi: 10.1128/jvi.1.1.97-109.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouches C., Bove C., Bove J. M. Turnip yellow mosaic virus-RNA replicase: partial purification of the enzyme from the solubilized enzyme-template complex. Virology. 1974 Apr;58(2):409–423. doi: 10.1016/0042-6822(74)90076-2. [DOI] [PubMed] [Google Scholar]
- Murakishi H. H., Hartmann J. X., Beachy R. N., Pelcher L. E. Growth curve and yield of tobacco mosaic virus in tobacco callus cells. Virology. 1971 Jan;43(1):62–68. doi: 10.1016/0042-6822(71)90224-8. [DOI] [PubMed] [Google Scholar]
- Murakishi H. H., Hartmann J. X., Pelcher L. E., Beachy R. N. Improved inoculation of cultured plant cells resulting in high virus titer and crystal formation. Virology. 1970 Jun;41(2):365–367. doi: 10.1016/0042-6822(70)90089-9. [DOI] [PubMed] [Google Scholar]
- Pelcher L. E., Murakishi H. H., Hartmann J. X. Kinetics of TMV-RNA synthesis and its correlation with virus accumulation and crystalline viral inclusion formation in tobacco tissue culture. Virology. 1972 Mar;47(3):787–796. doi: 10.1016/0042-6822(72)90570-3. [DOI] [PubMed] [Google Scholar]
- Ralph R. K., Bullivant S., Wojcik S. J. Cytoplasmic membranes, a possible site of tobacco mosaic virus RNA replication. Virology. 1971 Mar;43(3):713–716. doi: 10.1016/0042-6822(71)90295-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., Diskin B., Oron L., Traub A. Isolation and subunit structure of polycytidylate-dependent RNA polymerase of encephalomyocarditis virus. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3815–3819. doi: 10.1073/pnas.69.12.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai F., Takebe I. A non-coat protein synthesized in tobacco mesophyll protoplasts infected by tobacco mosaic virus. Mol Gen Genet. 1972;118(1):93–96. doi: 10.1007/BF02428336. [DOI] [PubMed] [Google Scholar]
- Sela I., Hauschner A. Isolation and characterization of a TMV-RNA dependent enzyme from TMV-infected tobacco leaves. Virology. 1975 Mar;64(1):284–288. doi: 10.1016/0042-6822(75)90102-6. [DOI] [PubMed] [Google Scholar]
- Wilcockson J., Hull R. The rapid isolation of plant virus RNAs using sodium perchlorate. J Gen Virol. 1974 Apr;23(1):107–111. doi: 10.1099/0022-1317-23-1-107. [DOI] [PubMed] [Google Scholar]
- Wilcockson J. The use of sodium perchlorate in deproteinization during the preparation of nucleic acids. Biochem J. 1973 Nov;135(3):559–561. doi: 10.1042/bj1350559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., Van Krammen A. In vitro replication of cowpea mosaic virus RNA. II. Solubilization of membrane-bound replicase and the partial purification of the solubilized enzyme. J Virol. 1976 Mar;17(3):679–685. doi: 10.1128/jvi.17.3.679-685.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Weenen-Swaans H., van Kammen A. In vitro replication of cowpea mosaic virus RNA: I. Isolation and properties of the membrane-bound replicase. J Virol. 1974 Nov;14(5):1049–1055. doi: 10.1128/jvi.14.5.1049-1055.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitlin M., Duda C. T., Petti M. A. Replication of tobacco mosaic virus. V. Properties of the bound and solubilized replicase. Virology. 1973 Jun;53(2):300–311. doi: 10.1016/0042-6822(73)90207-9. [DOI] [PubMed] [Google Scholar]
- Zaitlin M., Hariharasubramanian V. A gel electrophoretic analysis of proteins from plants infected with tobacco mosaic and potato spindle tuber viruses. Virology. 1972 Feb;47(2):296–305. doi: 10.1016/0042-6822(72)90265-6. [DOI] [PubMed] [Google Scholar]


