Abstract
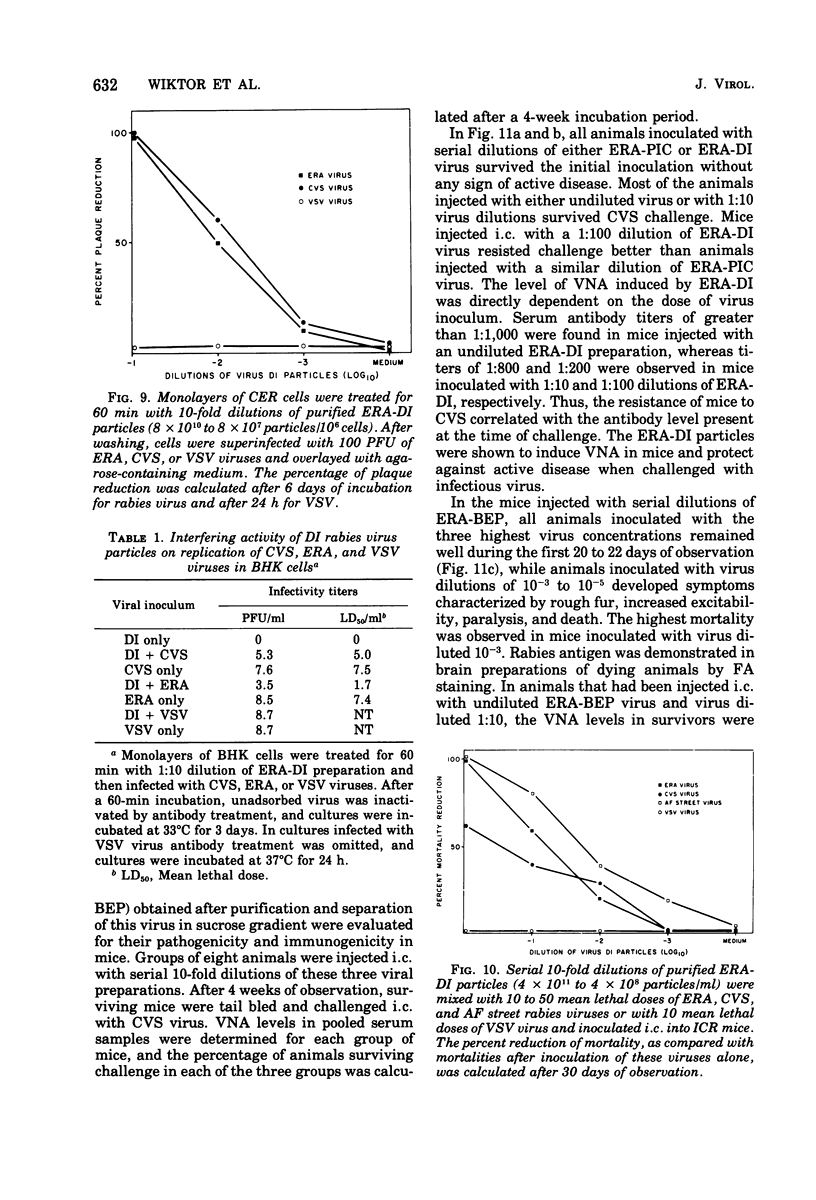
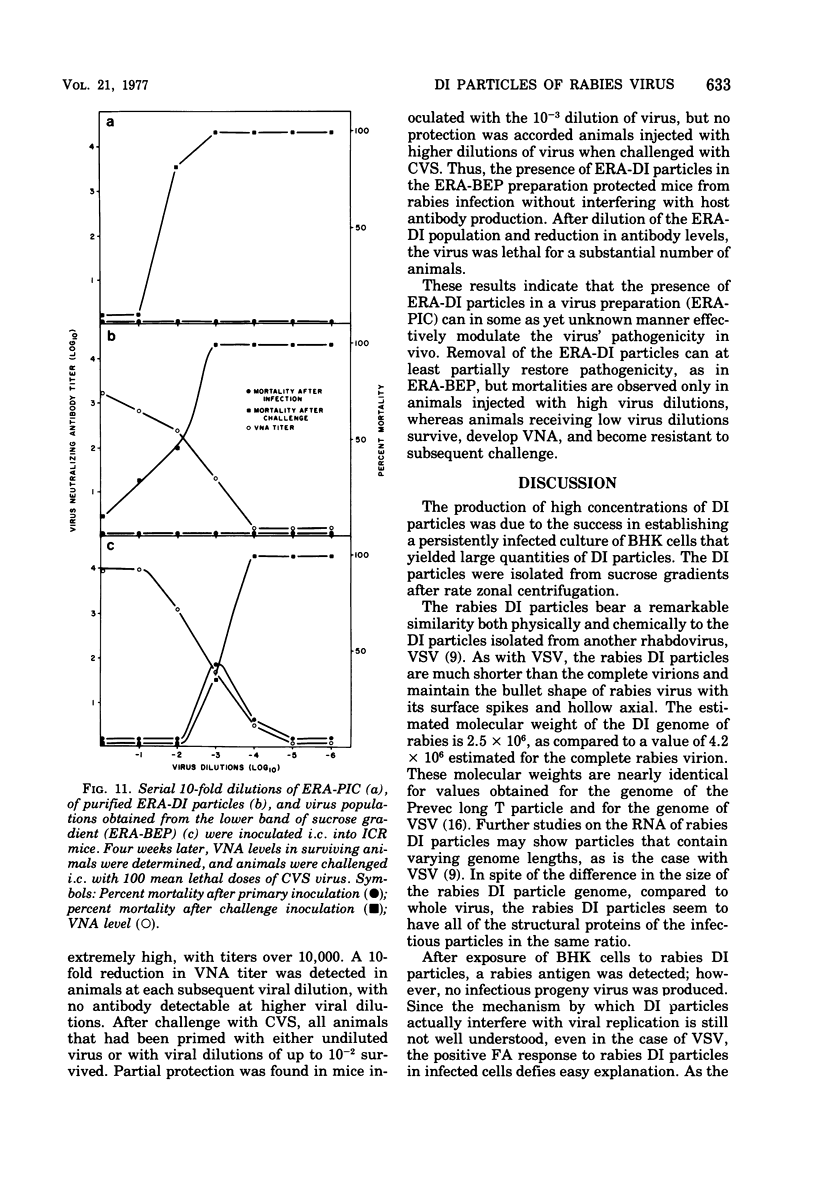
A method for obtaining large quantities of defective interfering (DI) rabies virus particles that fulfill all the criteria delineated by Huang and Baltimore (1970) is described. The purified rabies DI virion was found to be much shorter (60 to 80 nm) than the complete virion (180 nm) and to have a viral genome of about half the size of normal rabies RNA but with all of the structural proteins of standard virions. Rabies DI virions were noninfectious for both cells in culture and for animals. As determined by in vitro and in vivo techniques, interference with the replication of standard virus was specific to rabies virus. The possible role of rabies DI virion in the pathogenicity of rabies virus infection and in the establishment of attenuated strains for use as live rabies vaccines is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaslestad H. G., Clark H. F., Bishop D. H., Koprowski H. Comparison of the ribonucleic acid polymerases of two rhabdoviruses, Kern Canyon virus and vesicular stomatitis virus. J Virol. 1971 Jun;7(6):726–735. doi: 10.1128/jvi.7.6.726-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark H. F., Ohtani S. Temperature-sensitive mutants of rabies virus in mice: a mutant (ts2) revertant mixture selectively pathogenic by the peripheral route of inoculation. Infect Immun. 1976 May;13(5):1418–1425. doi: 10.1128/iai.13.5.1418-1425.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M., Holland J. J. Prophylaxis and immunization in mice by use of virus-free defective T particles to protect against intracerebral infection by vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2105–2108. doi: 10.1073/pnas.70.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDES M. V., WIKTOR T. J., KOPROWSKI H. ENDOSYMBIOTIC RELATIONSHIP BETWEEN ANIMAL VIRUSES AND HOST CELLS. A STUDY OF RABIES VIRUS IN TISSUE CULTURE. J Exp Med. 1964 Dec 1;120:1099–1116. doi: 10.1084/jem.120.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Doyle M. Attempts to detect homologous autointerference in vivo with influenza virus and vesicular stomatitis virus. Infect Immun. 1973 Apr;7(4):526–531. doi: 10.1128/iai.7.4.526-531.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPROWSKI H. Biological modification of rabies virus as a result of its adaptation to chicks and developing chick embryos. Bull World Health Organ. 1954;10(5):709–724. [PMC free article] [PubMed] [Google Scholar]
- Kawai A., Matsumoto S., Tanabe K. Characterization of rabies viruses recovered from persistently infected BHK cells. Virology. 1975 Oct;67(2):520–533. doi: 10.1016/0042-6822(75)90452-3. [DOI] [PubMed] [Google Scholar]
- Kuwert E. Laboratory techniques in rabies: the complement fixation test. Monogr Ser World Health Organ. 1973;(23):124–134. [PubMed] [Google Scholar]
- Kuwert E., Wiktor T. J., Sokol F., Koprowski H. Hemagglutination by rabies virus. J Virol. 1968 Dec;2(12):1381–1392. doi: 10.1128/jvi.2.12.1381-1392.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leamnson R. N., Reichmann M. E. The RNA of defective vesicular stomatitis virus particles in relation to viral cistrons. J Mol Biol. 1974 Jan 5;85(4):551–568. doi: 10.1016/0022-2836(74)90315-5. [DOI] [PubMed] [Google Scholar]
- Madore H. P., England J. M. Selective suppression of cellular protein synthesis in BHK-21 cells infected with rabies virus. J Virol. 1975 Nov;16(5):1351–1354. doi: 10.1128/jvi.16.5.1351-1354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Kawai A. Comparative studies on development of rabies virus in different host cells. Virology. 1969 Nov;39(3):449–459. doi: 10.1016/0042-6822(69)90093-2. [DOI] [PubMed] [Google Scholar]
- Schlumberger H. D., Wiktor T. J., Koprowski H. Antigenic and immunogenic properties of components contained in rabies virus-infected tissue culture fluids. J Immunol. 1970 Aug;105(2):291–298. [PubMed] [Google Scholar]
- Sokol F., Kuwert E., Wiktor T. J., Hummeler K., Koprowski H. Purification of rabies virus grown in tissue culture. J Virol. 1968 Aug;2(8):836–849. doi: 10.1128/jvi.2.8.836-849.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol F., Stancek D., Koprowski H. Structural proteins of rabies virus. J Virol. 1971 Feb;7(2):241–249. doi: 10.1128/jvi.7.2.241-249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIKTOR T. J., FERNANDES M. V., KOPROWSKI H. CULTIVATION OF RABIES VIRUS IN HUMAN DIPLOID CELL STRAIN WI-38. J Immunol. 1964 Sep;93:353–366. [PubMed] [Google Scholar]
- Wiktor T. J., Clark H. F. Chronic rabies virus infection of cell cultures. Infect Immun. 1972 Dec;6(6):988–995. doi: 10.1128/iai.6.6.988-995.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J. Laboratoty techniques in rabies: tissue culture methods. Monogr Ser World Health Organ. 1973;(23):101–123. [PubMed] [Google Scholar]
- Wiktor T. J., Sokol F., Kuwert E., Koprowski H. Immunogenicity of concentrated and purified rabies vaccine of tissue culture origin. Proc Soc Exp Biol Med. 1969 Jul;131(3):799–805. doi: 10.3181/00379727-131-33981. [DOI] [PubMed] [Google Scholar]
- Yoshino K., Taniguchi S., Arai K. Autointerference of rabies virus in chick embryo fibroblasts. Proc Soc Exp Biol Med. 1966 Nov;123(2):387–392. doi: 10.3181/00379727-123-31496. [DOI] [PubMed] [Google Scholar]