Abstract
Impaired attentional processing is prevalent in numerous neuropsychiatric disorders and may negatively impact other cognitive and functional domains. Nicotine – a nonspecific nicotinic acetylcholine receptor (nAChR) agonist – improves vigilance in healthy subjects and schizophrenia patients as measured by continuous performance tests (CPTs), but the nAChR mediating this effect remains unclear. Here we examine the effects of: a) nicotine; b) the selective α7 nAChR agonist PNU 282987; and c) the selective α4β2 nAChR agonist ABT-418 alone and in combination with scopolamine-induced disruption of mouse 5-choice (5C-)CPT performance. This task requires the inhibition of responses to non-target stimuli as well as active responses to target stimuli, consistent with human CPTs.
C57BL/6N mice were trained to perform the 5C-CPT. Drug effects were examined in extended session and variable stimulus-duration challenges of performance. Acute drug effects on scopolamine-induced disruption in performance were also investigated.
Nicotine and ABT-418 subtly but significantly improved performance of normal mice and attenuated scopolamine-induced disruptions in the 5C-CPT. PNU 282–987 had no effects on performance.
The similarity of nicotine and ABT-418 effects provides support for an α4β2 nAChR mechanism of action for nicotine-induced improvement in attention/vigilance. Moreover, the data provide pharmacological predictive validation for the 5C-CPT because nicotine improved and scopolamine disrupted normal performance of the task, consistent with healthy humans in the CPT. Future studies using more selective agonists may result in more robust improvements in performance.
Keywords: nicotine, continuous performance test, attention, impulsivity, nicotinic acetylcholine receptors, scopolamine
1. Introduction
Patients suffering from numerous neuropsychiatric disorders exhibit impaired attentional functioning. These disorders include schizophrenia, bipolar disorder, Alzheimer's disease, and attention deficit hyperactivity disorder. While the precise mechanisms of attentional dysfunction in each of these disorders have not been elucidated clearly, there is evidence linking cholinergic mechanisms to attentional functioning [1, 2].
Acetylcholine (ACh) acts on both muscarinic ACh receptors (mAChRs) and nAChRs. nAChRs have been linked with schizophrenia due to high smoking rates in patients compared to the general population, suggesting that patients may be self-medicating with nicotine [3–5]. Moreover, there is evidence implicating the α7 nAChR as a susceptibility gene for schizophrenia [6] with reduced protein levels in post-mortem brains of patients with schizophrenia linked to the degree of cognitive dysfunction [7]. mAChRs have also been implicated in the pathology of schizophrenia, where patients have lower levels of M1/M4 mAChRs [8, 9], with specific evidence for reduced expression of M1 mAChR (see [2]). Positron emission tomography studies in medication-free patients confirm reduced mAChR levels in the cortex, basal ganglia, and thalamus [10]. It may be that patients with reduced mAChR levels form a relatively homogenous subgroup of schizophrenia [11].
The concomitant cholinergic abnormalities and cognitive disruptions observed in schizophrenia have prompted suggestions that cholinergic agonists may improve cognition in schizophrenia [12, 13]. For example, nicotine-induced improvement in vigilance has been observed using the continuous performance test (CPT) in both healthy volunteers [14–16] and patients with schizophrenia [17], which may exert downstream beneficial effects on other cognitive domains [18]. The negative effects of nicotine, such as nausea and addiction, make it an undesirable therapeutic however [19, 20]. Thus, more selective nAChR agonists have been developed, such as ABT-418 for α4β2 nAChRs [21, 22], and PNU 282987 (PNU) for α7 nAChRs [23–25]. The efficacy of these selective agonists at improving cognition similarly to nicotine warrants testing.
In mice, the mAChR antagonist scopolamine impairs several cognitive domains with relevance to schizophrenia [25–29]. Opposite to nicotine, scopolamine disrupts vigilance in healthy humans as measured by the CPT [30]. While scopolamine disrupts [28, 29, 31] and nicotine improves [31, 32] sustained attention in the 5-choice serial reaction-time task (5CSRTT), this task requires only responses to target stimuli [33, 34]. In contrast, human CPTs also include non-targets to which subjects must inhibit responding [35]. Non-target trials in human CPTs activate distinct brain regions from target trials [36, 37], the combination of which contributes to the characteristic impaired attention in neuropsychiatric patients such as schizophrenia [37, 38]. Hence, we modified the 5CSRTT to include non-target trials, calling it the 5-choice (5C-)CPT, and demonstrated that rodents can perform the task, that it is sensitive to inbred strain differences, and that performance on the task has similarities to human CPTs [39]. The 5C-CPT has been supported for further development as a cross-species test of top-down control of attention by the NIH-funded CNTRICS initiative [40].
While we have demonstrated that dopamine D1 receptor activation can improve rat 5C-CPT [41], PCP-withdrawal can impair rat 5C-CPT [42], and genetic reduction of dopamine D4 receptor expression increases non-target responses with affecting target responses [43], we have yet to examine the effects of cholinergic manipulations on mouse 5C-CPT performance. Here, we examined what effects nicotine, the selective α7 nAChR agonist PNU, and the selective α4β2 nAChR agonist ABT-418 would have on mouse 5C-CPT performance alone and in combination with scopolamine. We hypothesized that these agonists would improve normal performance and exhibit modest attenuation of scopolamine-induced vigilance deficits, consistent with nicotine-induced improvements in patients with schizophrenia.
2. Methods
Male C57BL/6N mice (20–30 g) were obtained from Jackson laboratories at approximately 3 months of age. Mice were housed separately in groups of maximum 4/cage and maintained at 85% of free-feeding weight, with water available ad libitum, and housed in a vivarium on a reversed day-night cycle (lights off at 0800, on at 2000 h). Mice were brought to the laboratory 30 min before testing between 0900 and 1800 h. All behavioral testing procedures were approved by the UCSD Institutional Animal Care and Use Committee. All mice were maintained in an animal facility that meets all federal and state requirements for animal care and was approved by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
2.1. Apparatus
Training and testing took place in four 5-hole operant chambers (25×25× 25 cm, Med Associates Inc., St. Albans, VT). Each chamber consisted of an array of five square holes (2.5×2.5×2.5 cm) arranged horizontally on a curved wall 2.5 cm above the grid floor opposite a food delivery magazine (Lafayette Instruments, Lafayette, IN) at floor level and a house-light near the ceiling. The chamber was located in a sound-attenuating box, ventilated by a fan that also provided a low level of background noise. An infra-red camera installed in each chamber enabled the monitoring of performance during training and testing. Mice were trained to respond with a nose-poke to an illuminated LED recessed into the holes. Responses were detected by infrared beams mounted vertically located 3 mm from the opening of the hole. Liquid reinforcement in the form of strawberry milkshake (Nesquik® plus non-fat milk, 30 μl) was delivered by peristaltic pump (Lafayette Instruments, Lafayette, IN) to a well located in the magazine opposite the 5-hole wall. Magazine entries were monitored using an infrared beam mounted horizontally, 5 mm from the floor and recessed 6 mm into the magazine. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT) using custom programming [39].
2.2. 5C-CPT Training
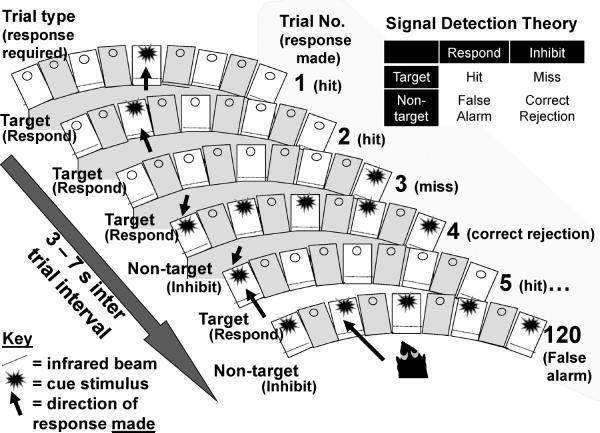
Mice were initially trained to associate the illumination of the magazine light with reward delivery. Once the magazine/reward association was acquired, mice were trained in the 5-CSR task daily, five days per week as described previously [32]. Each session lasted 30 min or 120 trials, whichever was completed first. Each trial was initiated by the mouse nose-poking, then removing its nose from the magazine. After a 5 sec ITI, a light stimulus appeared in one of the 5 apertures located opposite the magazine. A nose-poke in the lit aperture during the stimulus duration (SD) plus a 2 s limited-hold period resulted in a correct (Hit) response being registered and a reward being delivered in the magazine. A nose-poke in any other aperture over this period was registered as an incorrect response and resulted in a 4 s time-out. Failure to respond in any aperture during the SD + limited hold was registered as an omission (omission + incorrect = Miss) and also resulted in a time-out (TO). Response in any aperture during the ITI registered a premature response and triggered a TO. The next trial began when the mouse entered, then exited the magazine. The SD started at 20 s and was reduced to 10, 8, and 4 s after the attainment of each criterion (a mean correct latency less than half the current SD for two consecutive days) across sessions. At this point, mice were transferred to a variable ITI (3–7 s). Once performance stabilized (approximately 3 days), the mice were then transferred to the 5C-CPT (Fig. 1). For the 5C-CPT, 100 trials were target trials, identical to trials described in the 5CSR task where a cue stimulus appeared in any 1 of the 5 apertures, and 20 trials were non-target trials, unique to the 5C-CPT, in which all 5 apertures were illuminated and the mouse was required to inhibit from responding. Training took approximately 45 training sessions from 5-CSR task to criterion in the 5C-CPT. Consistent with human CPTs [35], successful inhibition of a response to a non-target stimulus resulted in a correct rejection (CR) being recorded and reward delivered. Responding to a non-target stimulus however, resulted in a false alarm (FA) being registered and a TO occurring. These non-target stimuli were interspersed pseudo-randomly within the 100 target trials (maximum of 3 sequential non-target trials). False alarm latency was also recorded.
Fig. 1.
Schematic of the 5C-CPT: Examples of trial-types and their responses are provided. How these responses are used for signal detection theory calculations is illustrated. Target trials are represented by singly lit holes and require a response (trials 1, 2, 3, and 5). When a response is made to a target stimulus, it is registered as a hit (trials 1, 2, and 5). A failure to respond to a target is registered as a miss (trial 3). Non-target stimuli are represented by all 5 holes being lit (trials 4 and 120). Inhibiting a response to a non-target is registered as a correct rejection (trial 4), while responding in any hole during a non-target is registered as a false alarm (trial 120). The combinations of hits, misses, correct rejections, and false alarms are analyzed using signal detection theory to identify the capability of the subject to differentiate their responses from target to non-target stimuli, consistent with human continuous performance tests. A variable 3 – 7 s interval is spaced between the trials to limit the subject's ability to use a temporal mediating strategy to predict stimulus onset.
For all three tasks, the mean correct latency (MCL) was calculated along with the following parameters:
Based upon these basic parameters, signal detection indices [44, 45] were then calculated to assess both d' and responsivity index bias (RI). The d' was calculated using the following formula:
d' provides a parametric assessment of distance between signal and noise responses [44, 45]. The non-parametric response bias measure RI [46] was chosen to provide a measure of the “tendency to respond” [46–48]. Both d' and RI are appropriate for use with single choice procedures (respond or not [47]).
2.3. Experimental design
Agonists were administered subchronically, consistent with previous studies of nicotine-induced 5CSRTT improvement in mice [31, 32] and to avoid its initial motor-inhibiting effects as reported in rats [49]. The initial nicotine study (1a) was conducted as a within-subjects design over four weeks, while experiments 1b and 1c were between-subjects designs consistent with previous studies of nicotine-induced 5CSR task improvement in mice using these two designs [31, 32]. Repeated testing of the same mice were conducted consistent with previous nicotine studies in mice [31] and rats [50, 51], and in line with the policy of re-using subjects where possible. Prior to drug treatment, mice were administered vehicle for 3 consecutive training sessions to acclimate them to being injected. During drug treatment, mice were tested in an extended session (250 trials or 60 min, whichever was attained first) variable stimulus duration (vSD; 0.75, 1.25, or 2 s) challenge. This challenge was chosen based upon previous studies identifying separable genotype effects of the α7 nAChR in the 5-CSR task [52] and the dopamine D4 receptor in the 5C-CPT [43]. Mice were given a two-week washout between studies with regular training sessions Monday-Friday. During the two-week washout stability of performance was established and experimental cohorts were counter-balanced prior to testing based on d, RI, MCL, testing run, and previous drug exposure.
2.4. Drugs
Nicotine bitartrate, scopolamine hydrobromide, PNU, and ABT-418 were purchased from Sigma-Aldrich (St Louis, MO). Each drug was dissolved in 0.9% saline, with nicotine solutions being subsequently neutralized with sodium hydroxide solution. Each drug was administered at 5 ml/kg, with nicotine administered s.c. at a 10 min pre-injection time, PNU and ABT-418 administered i.p. at a 10 min pre-injection time, and scopolamine administered i.p. at a 15 min pre-injection time. Nicotine doses and pre-injection times were chosen based upon reports that 3–4 day treatment regimens in mice produced positive effects of nicotine [31, 32] that were not observed using acute doses [31, 32, 53]. The scopolamine dose and pre-injection times were based on previous reports of scopolamine-induced disruptions of attention and novelty seeking in mice [25, 31]. PNU doses were chosen based on PNU-induced reversal of scopolamine-induced novelty preference in mice [25]. ABT-418 doses were chosen based on ABT-418-induced improvement of place learning in mice [22].
2.5.1. Experiment 1a: Subchronic nicotine effects on mouse 5C-CPT performance
C57BL/6N mice (n=16) were trained to a stable level of performance in the 5C-CPT and matched on baseline performance into 4 cohorts. After acclimation to injection, each cohort received their allocated dose (vehicle or nicotine 1, 10, or 100 μg/kg) on Tuesday-Friday 10 min prior to testing in the challenge session. The subsequent week, mice again received saline on Monday and then their allocated dose Tuesday-Friday using the same task challenge. This pattern was repeated in weeks 3 and 4.
2.5.2. Experiment 1b: Subchronic PNU 282987 effects on mouse 5C-CPT performance
A second group of C57BL/6N mice (n=43) were trained to a stable performance in the 5C-CPT. These mice were matched on baseline performance into 4 cohorts and after acclimation to injection, received PNU (vehicle, 3, 10, or 30 mg/kg; n = 11, 11, 11, and 10 respectively; i.p. 10 min preinjection time) on Tuesday-Friday prior to testing in the challenge session.
2.5.3. Experiment 1c: Subchronic ABT-418 effects on mouse 5C-CPT performance
After their two-week washout with standard training sessions, mice from experiment 1b were again matched into 4 cohorts based on performance and counterbalanced by previous treatment. After re-acclimation to injections, these mice received their allocated dose of ABT-418 (vehicle, 12, 40, and 120 μg/kg; i.p. 10 min pre-injection time) on Tuesday-Friday prior to testing in the challenge session.
2.6.1. Experiment 2a: Acute nicotine and scopolamine effects on mouse 5C-CPT performance
After their two-week washout with standard training sessions, mice from experiment 1c were again matched into 5 cohorts based on performance and counterbalanced by previous treatments. These mice received 1 of 5 drug combinations (vehicle + vehicle, vehicle + scopolamine 1 mg/kg, scopolamine 1 mg/kg + nicotine 3 μg/kg, scopolamine 1 mg/kg + nicotine 30 μg/kg, and scopolamine 1 mg/kg + nicotine 300 μg/kg) in a between-subjects design on Friday and tested in the challenge session.
2.6.2. Experiment 2b: Acute PNU 282987 and scopolamine effects on mouse 5C-CPT performance
After their two-week washout with standard training sessions, mice from experiment 2a were again matched into 6 cohorts based on performance and counterbalanced by previous treatments. These mice received 1 of 6 drug combinations (vehicle + vehicle, vehicle + scopolamine 1 mg/kg, scopolamine 1 mg/kg + nicotine 300 μg/kg, scopolamine 1 mg/kg + PNU 3 mg/kg, scopolamine 1 mg/kg + PNU 10 mg/kg, and scopolamine 1 mg/kg + PNU 30 mg/kg) in a between-subjects design on Friday and tested in the challenge session.
2.6.3. Experiment 2c: Acute ABT-418 and scopolamine effects on mouse 5C-CPT performance
After their two-week washout with standard training sessions, mice from experiment 2b were again matched into 6 cohorts based on performance and counterbalanced by previous treatments. These mice received 1 of 6 drug combinations (vehicle + vehicle, vehicle + scopolamine 1 mg/kg, scopolamine 1 mg/kg + ABT-418 12 μg/kg, scopolamine 1 mg/kg + ABT-418 40 μg/kg, and scopolamine 1 mg/kg + ABT-418 120 μg/kg) in a between-subjects design on Friday and tested in the challenge session.
2.7. Statistical Analyses
For experiment 1a, performance was analyzed using a three-factor repeated measures ANOVA with day, drug, and stimulus duration as within-subjects factors. In experiments 1a–c, performance was analyzed using a three-factor repeated measures ANOVA, with day and stimulus duration as within-subject factors and drug as a between subject factor. The effects of each drug were compared on day 1 in experiments 1a–c to determine whether acute effects of the drugs would be observed. Given that single drugs were used in experiments 1a–c, Tukey post hoc analyses were performed on significant main effects. For experiment 2a–c, performance was analyzed using a two-way repeated measures ANOVA with drug combination as a between-subjects factor and stimulus duration as a within subjects factor. Given the number of post-hoc analyses in experiments 2a–c, Bonferroni analyses were used on significant main effects. To determine the test-retest reliability of mice in the 5C-CPT, we examined the stability performance of mice from each experiment on primary and secondary outcome measures using intraclass correlation coefficient analyses with single factor ANOVAs. Data were analyzed using SPSS 19.0 (Chicago, IL).
3. Results
3.1.1. Experiment 1a: The effects of subchronic dosing of nicotine (1, 10, and 100 μg/kg) on 5C-CPT performance of C57BL/6N mice
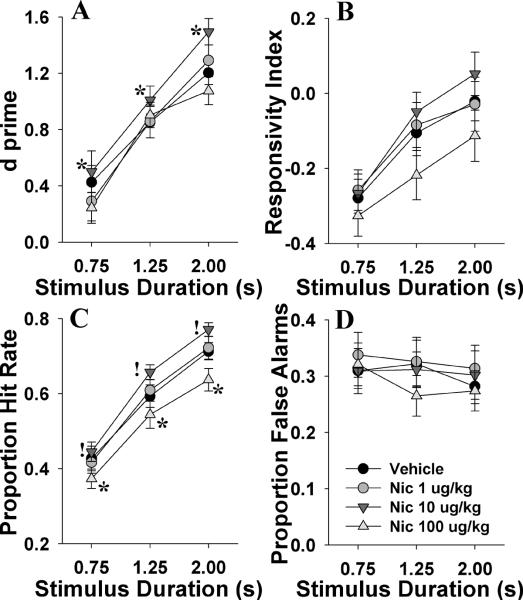
We examined whether nicotine could improve 5C-CPT performance in normal-performing mice using a subchronic within-subject design. Nicotine improved overall 5C-CPT performance as measured by increased d' (F(3,42)=3.2, p<0.05; Fig. 2A), with post hoc analysis revealing improvement at the 10 μg/kg dose when compared to vehicle (p<0.05). d' improved with longer stimuli (F(2,28)=204, p<0.0001), which did not interact with nicotine (F<1.5, NS) or drug or day (F<1, ns). Bias (RI) was unaffected by nicotine (F(3,42)=2.3, NS; Fig 2B), and while mice were more likely to respond with longer stimuli (F(2,28)=110, p<0.0001), this effect did not interact with nicotine dose. Nicotine increased responses to target stimuli (P[HR]; F(3,42)=5.1, p<0.005; Fig. 2C) at 10 μg/kg (p<0.1) while the highest dose lowered P[HR] compared to vehicle-treated mice (p<0.05). P[HR] increased with longer stimuli (F(2,28)=480, p<0.0001), but did not interact with nicotine dose, or day (F<1.9, NS). Responses to non-target stimuli (P[FA]) were unaffected by drug, day by drug, or drug by stimulus duration (F<1, NS; Fig. 2D). P[FA] also did not vary by stimulus duration (F(2,28)=2.3, NS). Data for other measures are provided in table 1.1. When day 1 data were analyzed alone to assess for acute effects of nicotine, no effect of drug was observed for any measure. No significant effect of dosing order or interaction with order was observed for any measure (F<1.8, NS).
Fig. 2.
The effects of subchronic nicotine (Nic, 1, 10, and 100 μg/kg) on mouse performance of the 5CCPT as measured using signal detection theory. Nicotine enhanced the performance of mice in the 5CCPT at 10 μg/kg as confirmed by the main effect of this dose on d⍰(A). Mice were more liberal in responding as the stimulus duration lengthened as indicated by the responsivity index, irrespective of nicotine administration (B). Correct target detection as measured by the proportion of hit rate also increased with the stimulus duration, and was reduced by nicotine at 100 μg/kg but tended to increase at 10 μg/kg irrespective of stimulus duration (C). No effect of nicotine or stimulus duration was observed on the ability of mice to inhibit from responding to non-target stimuli (D). Data presented as mean ± s.e.m, * denotes p<0.05 compared to vehicle, ! denotes p<0.1 compared to vehicle.
Table 1.1.
Effects of subchronic nicotine (1, 10, and 100 μg/kg) on 5C-CPT performance in a within subject design (n=15).
| Measure | Treatment | Mean | s.e.m. | d.f. | F | p value | |
|---|---|---|---|---|---|---|---|
| Premature Responses | Vehicle | 3.6 | 0.73 | Drug | (3,42) | 1.7 | Ns |
| 1 μg/kg | 4.5 | 1.51 | Drug × vSD | (6,84) | 1.2 | Ns | |
| 10 μg/kg | 4.9 | 1.41 | vSD | (2,28) | <1 | Ns | |
| 100 μg/kg | 3.2 | 0.76 | Day × Drug | (9,126) | <1 | Ns | |
|
|
|||||||
| Accuracy | Vehicle | 0.945 | 0.009 | Drug | (3,42) | <1 | Ns |
| 1 μg/kg | 0.945 | 0.007 | Drug × vSD | (6,84) | <1 | Ns | |
| 10 μg/kg | 0.942 | 0.011 | vSD | (2,28) | 30.6 | <0.0001 | |
| 100 μg/kg | 0.917 | 0.025 | Day × Drug | (9,126) | <1 | Ns | |
|
|
|||||||
| % Omissions | Vehicle | 38.9 | 3.05 | Drug | (3,42) | 4.2 | <0.05 |
| 1 μg/kg | 38.6 | 2.91 | Drug × vSD | (6,84) | 1.6 | Ns | |
| 10 μg/kg | 34.3 | 2.43† | vSD | (2,28) | 369 | <0.0001 | |
| 100 μg/kg | 40.9 | 2.74 | Day × Drug | (9,126) | 1.6 | Ns | |
|
|
|||||||
| Mean Correct Latency | Vehicle | 799.9 | 22.0 | Drug | (3,42) | 2.5 | 0.076 |
| 1 μg/kg | 831.3 | 21.5 | Drug × vSD | (6,84) | <1 | Ns | |
| 10 μg/kg | 807.2 | 14.7 | vSD | (2,28) | 181 | <0.0001 | |
| 100 μg/kg | 836.5 | 20.6 | Day × Drug | (9,126) | <1 | Ns | |
|
|
|||||||
| Mean False Alarm Latency | Vehicle | 589.1 | 45.6 | Drug | (3,42) | 1.3 | Ns |
| 1 μg/kg | 629.8 | 17.9 | Drug × vSD | (6,84) | 1.3 | Ns | |
| 10 μg/kg | 656.7 | 26.7 | vSD | (2,28) | <1 | Ns | |
| 100 μg/kg | 600.0 | 28.4 | Day × Drug | (9,126) | <1 | Ns | |
|
|
|||||||
| Total Trials | Vehicle | 211.7 | 8.5 | Drug | (3,42) | 2.3 | <0.1 |
| 1 μg/kg | 215.4 | 7.7 | Drug × vSD | (6,84) | 8.1 | <0.01 | |
| 10 μg/kg | 211.4 | 7.8 | vSD | (2,28) | <1 | Ns | |
| 100 μg/kg | 199.0 | 9.2† | Day × Drug | (9,126) | 3.3 | <0.05 | |
denote p<0.05 when compared to vehicle,
denotes that p<0.1 when compared to vehicle, Ns denotes non significant
3.1.2. Experiment 1b: The effects of subchronic dosing of the α7 nAChR full agonist PNU 282987 (3, 10, and 30 mg/kg) on 5C-CPT performance of C57BL/6N mice
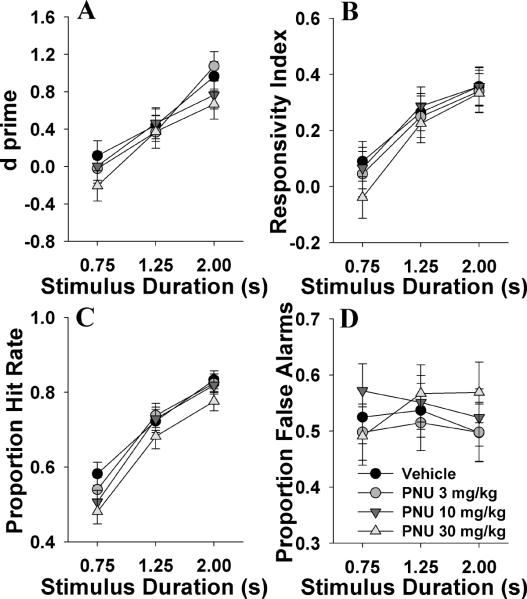
We examined whether the full α7 nAChR agonist PNU could mimic the observed effects of nicotine on mice. PNU did not affect d', bias, target, or non-target responding (F<1.9, NS; Fig. 3A, B, C, and D respectively). No drug by day interactions were observed for any of these measures (F<1, NS). With longer stimulus durations, d' improved (F(2,70)=126, p<0.0001), mice were more responsive (F(2,70)=161, p<0.0001), and target responses increased (F(2,70)=573, p<0.0001), while non-target responses were unaffected (F(2,70)=2.1, NS). A stimulus by drug effect was observed for numerous measures including d (F(2,70)=2.4, p<0.05), target responses (P[HR]; F(6,70)=3.0, p<0.05), non-target responses (P[FA]; F(6,70)=2.4, p<0.05), and a trend effect on RI (F(6,70)=2.0, p<0.1). Post hoc analyses revealed only limited effects however, with the only significant effect being 30 mg/kg PNU-induced increase in P[FA] at the 2 s stimulus duration when compared to vehicle (p<0.05). Data for other measures are provided in table 1.2. When day 1 data were analyzed alone to assess for acute effects of PNU 282987, no effect of the drug was observed for any measure.
Fig. 3.
The effects of subchronic administration of the α7 nAChR agonist PNU 282987 (PNU, 2, 10, and 30 mg/kg) on mouse performance of the 5C-CPT as measured using signal detection theory. PNU 282987 did not affect d⍰(A), bias as measured by responsivity index (B), the probability of responding to a target signal (C), or the probability of responding to a non-target signal (D). Consistent with earlier data and previous reports however, a main effect of signal duration was observed for every measure bar false alarms. Data presented as mean ± s.e.m.
Table 1.2.
Effects of subchronic PNU 282987 (3, n=11; 10, n=11; 30 mg/kg; n=10) on 5C-CPT performance.
| Measure | Treatment | Mean | s.e.m. | d.f. | F | p value | |
|---|---|---|---|---|---|---|---|
| Premature Responses | Vehicle | 2.5 | 0.61 | Drug | (3,35) | 1.2 | Ns |
| 3 mg/kg | 3.1 | 0.69 | Drug × vSD | (6,70) | 1.2 | Ns | |
| 10 mg/kg | 2.1 | 0.64 | vSD | (2,70) | <1 | Ns | |
| 30 mg/kg | 3.7 | 0.64 | Day × Drug | (9,105) | <1 | Ns | |
|
|
|||||||
| Accuracy | Vehicle | 0.963 | 0.007 | Drug | (3,35) | 1.0 | Ns |
| 3 mg/kg | 0.963 | 0.008 | Drug × vSD | (6,70) | <1 | Ns | |
| 10 mg/kg | 0.968 | 0.008 | vSD | (2,70) | 42.0 | <0.0001 | |
| 30 mg/kg | 0.952 | 0.008 | Day × Drug | (9,105) | <1 | Ns | |
|
|
|||||||
| % Omissions | Vehicle | 27.0 | 2.66 | Drug | (3,35) | 1.4 | Ns |
| 3 mg/kg | 27.8 | 2.97 | Drug × vSD | (6,70) | 1.8 | Ns | |
| 10 mg/kg | 30.6 | 2.75 | vSD | (2,70) | 562 | <0.0001 | |
| 30 mg/kg | 34.3 | 2.78 | Day × Drug | (9,105) | <1 | Ns | |
|
|
|||||||
| Mean Correct Latency | Vehicle | 802 | 29 | Drug | (3,35) | <1 | Ns |
| 3 mg/kg | 822 | 33 | Drug × vSD | (6,70) | <1 | Ns | |
| 10 mg/kg | 833 | 30 | vSD | (2,70) | 252.0 | <0.0001 | |
| 30 mg/kg | 845 | 30 | Day × Drug | (9,105) | <1 | Ns | |
|
|
|||||||
| Mean False Alarm Latency | Vehicle | 723 | 32.7 | Drug | (3,35) | <1 | Ns |
| 3 mg/kg | 730 | 32.7 | Drug × vSD | (6,70) | 1.5 | Ns | |
| 10 mg/kg | 731 | 30.8 | vSD | (2,70) | 1.4 | Ns | |
| 30 mg/kg | 730 | 34.3 | Day × Drug | (9,105) | <1 | Ns | |
|
|
|||||||
| Total Trials | Vehicle | 230.8 | 6.6 | Drug | (3,35) | 2.9 | <0.05 |
| 3 mg/kg | 230.2 | 6.7 | Drug × vSD | (6,70) | <1 | Ns | |
| 10 mg/kg | 227.2 | 6.6 | vSD | (2,70) | <1 | Ns | |
| 30 mg/kg | 206.3 | 7.0* | Day × Drug | (9,105) | <1 | Ns | |
denotes p<0.05 when compared to vehicle (n=11),
denotes that p<0.1 when compared to vehicle, Ns denotes notsignificant
3.1.3. Experiment 1c: The effects of subchronic dosing of the α4β2 agonist ABT-418 (12, 40, and 120 μg/kg) on 5C-CPT performance of C57BL/6N mice
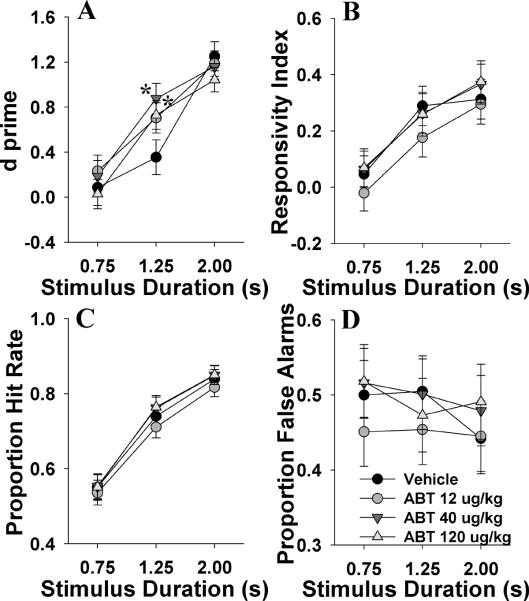
We examined whether the selective full α4β2 nAChR agonist could improve 5C-CPT performance in mice in a manner consistent with nicotine. Subchronic ABT-418 treatment did not exert a main effect on performance as measured by d', RI, P[HR], or P[FA] (all F<1, NS; Fig. 4A, B, C, and D respectively). A drug by stimulus duration effect was observed for d' (F(2,54)=2.8, p<0.05; Fig. 4A), whereby 40 and 120 μg/kg improved d' at the 1.25 s stimulus duration when compared to vehicle (p<0.05), with no other effects observed (p>0.1). No drug by stimulus duration interaction was observed for RI, P[HR], or P[FA] (F<1.8, NS). As above, lengthening stimulus durations increased d (F(2,54)=148, p<0.0001), bias (F(2,54)=133, p<0.0001), and P[HR] (F(2,54)=496, p<0.0001), with a minor effect on P[FA] (F(2,54)=3.1, p<0.1). No drug by day effect was observed for any of these measures (F<1.5, NS). Data for other measures are provided in table 1.3. When day 1 data were analyzed alone to assess for acute effects of ABT-418, no effect was observed for any measure.
Fig. 4.
The effects of subchronic administration of the α4β2 nAChR agonist ABT-418 (ABT, 12, 40, and 120 μg/kg) on mouse performance of the 5C-CPT as measured using signal detection theory. ABT-418 at 12 and 40 μg/kg improved vigilance as measured by d⍰at the 1.25 s stimulus duration (A). ABT-418 did not affect bias as measured by responsivity index (B), the probability of responding to a target signal (C), or the probability of responding to a non-target signal (D). Consistent with earlier data and previous reports a main effect of signal duration was observed for every measure bar false alarms. Data presented as mean ± s.e.m. * denotes p<0.05 when compared to vehicle.
Table 1.3.
Effects of subchronic ABT-418 (12, n= 11; 40, n=11; 120 μg/kg n = 10) on 5C-CPT performance.
| Measure | Treatment | Mean | s.e.m. | d.o.f. | F | p value | |
|---|---|---|---|---|---|---|---|
| Premature Responses | Vehicle | 2.59 | 0.85 | Drug | (3,27) | 1.9 | Ns |
| 12 μg/kg | 2.16 | 0.74 | Drug × vSD | (6,54) | 1.7 | Ns | |
| 40 μg/kg | 4.55 | 0.74 | vSD | (2,54) | 1.9 | Ns | |
| 120 μg/kg | 3.24 | 0.70 | Day × Drug | (9,81) | 1.8 | Ns | |
|
|
|||||||
| Accuracy | Vehicle | 0.976 | 0.008 | Drug | (3,27) | 1.8 | Ns |
| 12 μg/kg | 0.964 | 0.007 | Drug × vSD | (6,54) | <1 | Ns | |
| 40 μg/kg | 0.954 | 0.007 | vSD | (2,54) | 45.5 | <0.0001 | |
| 120 μg/kg | 0.959 | 0.006 | Day × Drug | (9,81) | <1 | Ns | |
|
|
|||||||
| % Omissions | Vehicle | 28.55 | 3.3 | Drug | (3,27) | <1 | Ns |
| 12 μg/kg | 28.67 | 2.9 | Drug × vSD | (6,54) | <1 | Ns | |
| 40 μg/kg | 26.16 | 2.9 | vSD | (2,54) | 423 | <0.0001 | |
| 120 μg/kg | 27.49 | 2.4 | Day × Drug | (9,81) | 1.2 | Ns | |
|
|
|||||||
| Mean Correct Latency | Vehicle | 787 | 32 | Drug | (3,27) | <1 | Ns |
| 12 μg/kg | 787 | 28 | Drug × vSD | (6,54) | <1 | Ns | |
| 40 μg/kg | 824 | 28 | VSD | (2,54) | 224 | <0.0001 | |
| 120 μg/kg | 788 | 26 | Day × Drug | (9,81) | <1 | Ns | |
|
|
|||||||
| Mean False Alarm Latency | Vehicle | 685 | 33.2 | Drug | (3,27) | <1 | Ns |
| 12 μg/kg | 717 | 31.7 | Drug × vSD | (6,54) | 1.5 | Ns | |
| 40 μg/kg | 729 | 31.7 | vSD | (2,54) | 1.7 | Ns | |
| 120 μg/kg | 746 | 31.7 | Day × Drug | (9,81) | <1 | Ns | |
|
|
|||||||
| Total Trials | Vehicle | 232.1 | 7.8 | Drug | (3,27) | <1 | Ns |
| 12 μg/kg | 219.4 | 7.4 | Drug × vSD | (6,54) | <1 | Ns | |
| 40 μg/kg | 231.5 | 7.4 | vSD | (2,54) | <1 | Ns | |
| 120 μg/kg | 233.5 | 7.4 | Day × Drug | (9,81) | <1 | Ns | |
denotes p<0.05 when compared to vehicle,
denotes that p<0.1 when compared to vehicle (n=11), Ns denotes not significant.
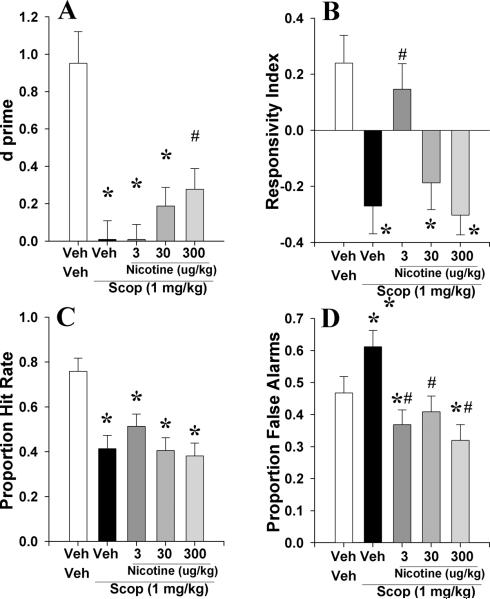
3.2.1. Experiment 2a: To examine whether acute nicotine (3, 30, and 300 μg/kg) could reverse scopolamine (1 mg/kg)-induced deficits of C57BL/6N mice in the 5C-CPT
Consistent with previous reports, scopolamine impaired 5C-CPT performance as measured by d' (F(4,32)=7.7, p<0.0001; Fig. 5A), as well as altering RI (F(4,32)=6.2, p<0.001; Fig. 5B), P[HR] (F(4,32)=6.5, p<0.001; Fig. 5C), and P[FA] (F(4,32)=5.6, p<0.005; Fig. 5D). Post hoc analyses revealed that impaired d compared with veh+veh was observed for scop+veh, scop+nic_3μg/kg, and scop+nic_30μg/kg (p<0.05) but not scop+nic_300μg/kg (p>0.05). Moreover, scop+nic_300μg/kg treated mice exhibited significantly higher d compared with scop+veh treated mice (p<0.05). For bias, each treatment group exhibited lowered RI compared with veh+veh (p<0.05) except scop+nic_3μg/kg (p>0.05). Target responding (P[HR]) was reduced by scop+nic treatment at every dose (p<0.05), while the scop+nic groups did not differ from each other (p>0.05). Non-target responses (P[FA]) was not affected by any treatment group in comparison to veh+veh or scop+veh groups (p>0.05). Data for other measures are provided in table 2.1.
Fig. 5.
The effects of co-administration of nicotine (Nic, 3, 30, and 300 μg/kg) and scopolamine (1 mg/kg) on mouse performance of the 5C-CPT as measured using signal detection theory. Scopolamine impaired vigilance in mice as measured by d⍰, an effect that was attenuated by co-administration of nicotine at 300 μg/kg (A). Scopolamine lowered the responsivity index of mice, which was attenuated by 3 μg/kg of nicotine (B). Scopolamine lowered the hit rate of mice irrespective of dose of co-administered nicotine (C), while every dose of nicotine attenuated the scopolamine-induced increases in responses to non-target signals, and even lowered such responses below vehicle-treated mice (D). Data presented as mean + s.e.m., * denotes p<0.05 when compared to vehicle, # denotes p<0.05 when compared with scopolamine.
Table 2.1.
Effects of nicotine treatment (3, n=9; 30, n=9; and 300 μg/kg, n=8) on scopolamine (1 mg/kg, n=8) pretreatment on mouse performance of the 5C-CPT.
| Measure | Treatment | Mean | s.e.m. | d.o.f. | F | p value | |
|---|---|---|---|---|---|---|---|
| Premature Responses | veh+veh | 3.56 | 2.48 | Drug | (4,32) | 4.2 | <0.01 |
| scop+veh | 13.91 | 2 48* | Drug × vSD | (8,64) | 1.4 | Ns | |
| scop+nic_3μg/kg | 16.74 | 2.28* | vSD | (2,64) | 1.4 | Ns | |
| scop+nic_30μg/kg | 12.47 | 2.40* | |||||
| scop+nic_300μg/kg | 13.17 | 2.40* | |||||
|
|
|||||||
| Accuracy | veh+veh | 0.966 | 0.030 | Drug | (4,32) | 4.5 | <0.01 |
| scop+veh | 0.858 | 0.030* | Drug × vSD | (8,64) | 1.6 | Ns | |
| scop+nic_3μg/kg | 0869 | 0.027* | vSD | (2,64) | 19.5 | <0.0001 | |
| scop+nic_30μg/kg | 0.859 | 0.029* | |||||
| scop+nic_300μg/kg | 0.791 | 0.029* | |||||
|
|
|||||||
| % Omissions | veh+veh | 21.8 | 5.8 | Drug | (4,32) | 5.7 | <0.01 |
| scop+veh | 52.3 | 5.8* | Drug × vSD | (8,64) | 2.0 | <0.1 | |
| scop+nic_3μg/kg | 42.1 | 5.3 | vSD | (2,64) | 90.9 | <0.0001 | |
| scop+nic_30μg/kg | 53.7 | 5.6* | |||||
| scop+nic_300μg/kg | 53.7 | 5.6* | |||||
|
|
|||||||
| Mean Correct Latency (ms) | veh+veh | 988 | 138 | Drug | (4,32) | <1 | Ns |
| scop+veh | 959 | 138 | Drug × vSD | (8,64) | 1.7 | Ns | |
| scop+nic_3μg/kg | 881 | 126 | vSD | (2,64) | 2.8 | <0.0001 | |
| scop+nic_30μg/kg | 895 | 133 | |||||
| scop+nic_300μg/kg | 933 | 133 | |||||
|
|
|||||||
| Mean False Alarm Latency (ms) | veh+veh | 778 | 74 | Drug | (4,32) | <1 | Ns |
| scop+veh | 900 | 69 | Drug × vSD | (8,64) | <1 | Ns | |
| scop+nic_3μg/kg | 789 | 65 | vSD | (2,64) | <1 | Ns | |
| scop+nic_30μg/kg | 801 | 65 | |||||
| scop+nic_300μg/kg | 804 | 74 | |||||
|
|
|||||||
| Total Trials | veh+veh | 246.1 | 6.8 | Drug | (4,32) | 2.5 | <0.1 |
| scop+veh | 231.6 | 6.3 | Drug × vSD | (8,64) | 1.5 | Ns | |
| scop+nic_3μg/kg | 243.8 | 6.0 | vSD | (2,64) | <1 | Ns | |
| scop+nic_300μg/kg | 225.7 | 6.8* | |||||
| scop+nic_300μg/kg | 225.7 | 6.8* | |||||
denotes p<0.05 when compared to vehicle (veh+veh, n=8), Ns denotes not sgnificant.
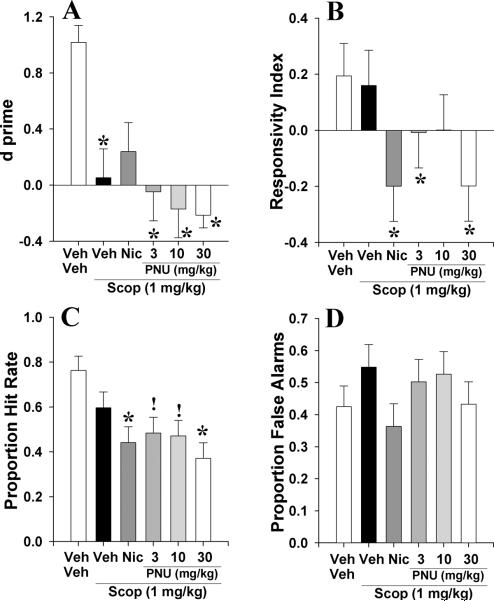
3.2.2. Experiment 2b: To examine whether acute PNU 282987 (3, 10, and 30 mg/kg) could reverse scopolamine (1 mg/kg)-induced deficits of C57BL/6N mice in the 5C-CPT
In order to determine whether the α7 nAChR agonist PNU could reverse disrupted performance in the 5C-CPT, mice pretreated with scopolamine (1 mg/kg) were treated with nicotine (300 μg/kg) or PNU (3, 10, and 30 mg/kg) prior to testing in the 5C-CPT. Scopolamine impaired performance as measured by d (F(5,31)=5.5, p<0.005; Fig. 6A). This effect was not attenuated by treatment with PNU at any dose (p<0.05), but was attenuated by nicotine (p>0.05) compared to veh+veh treated mice. RI was unaffected by drug group (F<1.9, NS; Fig. 6B). Scopolamine reduced P[HR] (F(5,31)=4.4, p<0.005; Fig. 6C), even with nicotine or PNU at 30 mg/kg (p<0.05), but this effect was only a trend when mice were co-administered 3 and 10 mg/kg PNU (p<0.1). Scopolamine did not affect non-target responses (P[FA]; F<1.0, NS; Fig. 6D). Data for other measures are provided in table 2.2.
Fig. 6.
The effect of co-administration of PNU 282987 (PNU, 3, 10, and 30 mg/kg) and scopolamine (1 mg/kg) on mouse performance of the 5C-CPT as measured using signal detection theory. Scopolamine impaired vigilance in mice as measured by d⍰, an effect that was somewhat attenuated by co-administration of nicotine at 300 μg/kg – co-administration of PNU was without effect on scopolamine-induced disruption in performance however (A). Scopolamine + nicotine and PNU 282987 (3 and 30 mg/kg) lowered the responsivity index of mice which was not seen in scopolamine treatment alone or in co-administration of scopolamine and 10 mg/kg of PNU 282987 (B). Scopolamine + all doses of nicotinic agonists reduced the proportion hit rate that was not observed in mice administered scopolamine alone (C). Although no treatment affected the proportion of responses to non-target signals, scopolamine-induced increases with co-administration of nicotine-induced attenuation was observed as before (D). Data presented as mean + s.e.m., * denotes p<0.05 when compared to vehicle, ! denotes p<0.1 when compared with vehicle.
Table 2.2.
Effects of nicotine (300 μg/kg, n=7) and PNU 282987 (3, n=7; 10, n=7; and 30 mg/kg, n=7) treatment on the effects of scopolamine (1 mg/kg) pretreatment on mouse performance of the 5C-CPT.
| Measure | Treatment | Mean | s.e.m. | d.o.f. | F | p value | |
|---|---|---|---|---|---|---|---|
| Premature Responses | veh+veh | 2.88 | 2.47 | Drug | (5,31) | 1.7 | Ns |
| scop+veh | 9.29 | 2.69 | Drug × vSD | (10,62) | 2.5 | <0.05 | |
| scop+nic_300μg/kg | 7.47 | 2.69 | vSD | (2,62) | 1.2 | Ns | |
| scop+pnu_3mg/kg | 10.49 | 2.69 | |||||
| scop+pnu_10mg/kg | 11.67 | 2.69 | |||||
| scop+pnu_30mg/kg | 11.21 | 2.69 | |||||
|
|
|||||||
| Accuracy | veh+veh | 0.979 | 0.036 | Drug | (5,31) | 2.8 | <0.05 |
| scop+veh | 0.915 | 0.039 | Drug × vSD | (10,62) | 1.4 | Ns | |
| scop+nic_300μ/kg | 0.873 | 0.039 | vSD | (2,62) | 20.5 | <0.05 | |
| scop+pnu_3mg/kg | 0.867 | 0.039 | |||||
| scop+pnu_10mg/kg | 0.878 | 0.039 | |||||
| scop+pnu_30mg/kg | 0.789 | 0.039* | |||||
|
|
|||||||
| % Omissions | veh+veh | 22.27 | 6.24 | Drug | (5,31) | 3.5 | <0.05 |
| scop+veh | 36.42 | 6.74 | Drug × vSD | (10,62) | 1.8 | <0.1 | |
| scop+nic_300μg/kg | 50.99 | 6.74* | vSD | (2,62) | 184 | <0.0001 | |
| scop+pnu_3mg/kg | 45.52 | 6.74 | |||||
| scop+pnu_10mg/kg | 48.12 | 6.74 | |||||
| scop+pnu_30mg/kg | 55.38 | 6.74* | |||||
|
|
|||||||
| Mean Correct Latency (ms) | veh+veh | 805 | 79 | Drug | (5,31) | 2.45 | <0.1 |
| scop+veh | 893 | 85 | Drug × vSD | (10,62) | 1.5 | Ns | |
| scop+nic_300μg/kg | 993 | 85 | vSD | (2,62) | 31.4 | <0.0001 | |
| scop+pnu_3mg/kg | 1161 | 85 | |||||
| scop+pnu_10mg/kg | 880 | 85 | |||||
| scop+pnu_30mg/kg | 1060 | 85 | |||||
|
|
|||||||
| Mean False Alarm Latency (ms) | veh+veh | 705 | 92 | Drug | (5,31) | 1.2 | Ns |
| scop+veh | 721 | 92 | Drug × vSD | (10,62) | 1.8 | <0.1 | |
| scop+nic_300μg/kg | 804 | 100 | vSD | (2,62) | 4.1 | <0.05 | |
| scop+pnu_3mg/kg | 802 | 100 | |||||
| scop+pnu_10mg/kg | 869 | 100 | |||||
| scop+pnu_30mg/kg | 991 | 100 | |||||
|
|
|||||||
| Total Trials | veh+veh | 242.2 | 11.4 | Drug | (5,31) | 2.0 | <0.1 |
| scop+veh | 231.4 | 11.4 | Drug × vSD | (10,62) | <1 | Ns | |
| scop+nic_300μg/kg | 221.5 | 12.4 | vSD | (2,62) | <1 | Ns | |
| scop+pnu_3mg/kg | 233.3 | 12.4 | |||||
| scop+pnu_10mg/kg | 240.0 | 12.4 | |||||
| scop+pnu_30mg/kg | 195.0 | 12.4* | |||||
denotes p<0.05 when compared to vehicle (n=8), Ns denotes not significant.
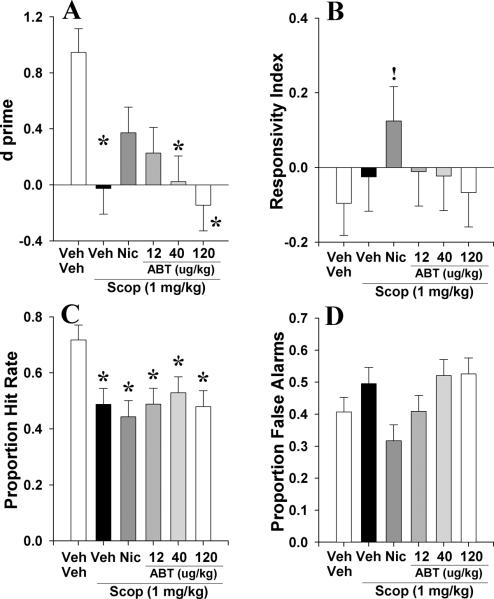
3.2.3. Experiment 2c: To examine whether acute ABT-418 (12, 40, and 120 μg/kg) could reverse scopolamine (1 mg/kg)-induced deficits in C57BL/6N mice in the 5C-CPT
To examine whether the α4β2 nAChR agonist ABT-418 could reverse disrupted performance in the 5C-CPT, mice pretreated with scopolamine (1 mg/kg) were treated with nicotine (300 μg/kg) or ABT-418 (12, 40, and 120 μg/kg) prior to testing in the 5C-CPT. Scopolamine again disrupted performance as measured by d (F(5,31)=3.6, p<0.005; Fig. 7A), bias (RI; F(5,31)=2.7, p<0.05; Fig. 7B), target responses (P[HR]; F(5,31)=3.3, p<0.05; Fig. 7C), and non-target responses (P[FA]; F(5,31)=2.7, p<0.05; Fig. 7D). Scop+veh, scop+abt_40μg/kg, and scop+abt_120μg/kg treatments impaired d (p<0.05) when compared with veh+veh treated mice. Treatment with scop+nic and scop+abt_12μg/kg did not affect d (p>0.1) compared with veh+veh treated mice however. Scopolamine pretreatment produced a trend toward reducing P[HR] irrespective of treatment dose when compared to veh+veh treated mice (p<0.1), except in mice treated with ABT-418 at 40 μg/kg (p>0.1). Despite the main effect of drug on RI and P[FA], post hoc analyses did not reveal any group that differed from veh+veh treated mice, (p>0.1), except for a trend toward increased responsivity in scop+nic (p<0.1). Data for other measures are provided in table 2.3.
Fig. 7.
The effect of co-administration of ABT-418 (ABT, 12, 40, and 120 μg/kg) and scopolamine (1 mg/kg) on mouse performance of the 5C-CPT as measured using signal detection theory. Scopolamine impaired vigilance in mice as measured by d⍰, an effect that was attenuated by co-administration of nicotine at 300 μg/kg and ABT at 12 μg/kg, but not at 40 or 120 μg/kg (A). Scopolamine + nicotine tended to increase responsivity compared to veh+veh alone, an effect that was not observed in other doses (B). Scopolamine administration reduced the proportion of hits to target signals that was unaffected by co-administration of nicotine or ABT-418 at any dose (C). Although no treatment affected the proportion of responses to non-target signals, scopolamine-induced increased while co-administration of nicotine- decreased such responses (D). Data presented as mean + s.e.m., * denotes p<0.05 when compared to vehicle, ! denotes p<0.1 when compared with vehicle.
Table 2.3.
Effects of nicotine (300 μg/kg, n=7) and ABT-418 (12, n=7; 40, n=7; and 120 μg/kg, n=7) treatment on the effects of scopolamine (1 mg/kg) pretreatment on mouse performance of the 5C-CPT.
| Measure | Treatment | Mean | s.e.m. | d.f. | F | p value | |
|---|---|---|---|---|---|---|---|
| Premature Responses | veh+veh | 2.79 | 2.70 | Drug | (5,31) | 5.0 | <0.005 |
| scop+veh | 22.17 | 2.91* | Drug × vSD | (10,62) | <1 | Ns | |
| scop+nic_300μg/kg | 10.22 | 2.91 | vSD | (2,62) | <1 | Ns | |
| scop+abt_12μg/kg | 8.82 | 2.91† | |||||
| scop+abt_40μg/kg | 13.11 | 2.91 | |||||
| scop+abt_120μg/kg | 12.60 | 2.91 | |||||
|
|
|||||||
| Accuracy | veh+veh | 0.966 | 0.021 | Drug | (5,31) | 3.7 | <0.05 |
| scop+veh | 0.854 | 0.023* | Drug × vSD | (10,62) | <1 | Ns | |
| scop+nic_300μg/kg | 0.858 | 0.023* | vSD | (2,62) | 18.3 | <0.0001 | |
| scop+abt_12μg/kg | 0.875 | 0.023* | |||||
| scop+abt_40μg/kg | 0.904 | 0.023 | |||||
| scop+abt_120μg/kg | 0.905 | 0.023 | |||||
|
|
|||||||
| % Omissions | veh+veh | 25.75 | 5.13 | Drug | (5,31) | 2.6 | <0.05 |
| scop+veh | 43.65 | 5.54 | Drug × vSD | (10,62) | 185 | <0.0001 | |
| scop+nic_300μg/kg | 48.54 | 5.54 | vSD | (2,62) | <1 | Ns | |
| scop+abt_12μg/kg | 45.97 | 5.54 | |||||
| scop+abt_40μg/kg | 42.90 | 5.54 | |||||
| scop+abt_120μg/kg | 47.26 | 5.54 | |||||
|
|
|||||||
| Mean Correct Latency (ms) | veh+veh | 935 | 57 | Drug | (5,31) | <1 | Ns |
| scop+veh | 940 | 62 | Drug × vSD | (10,62) | 55.8 | <0.0001 | |
| scop_nic_300μg/kg | 972 | 62 | vSD | (2,62) | <1 | Ns | |
| scop+abt_12μg/kg | 920 | 62 | |||||
| scop+abt_40μg/kg | 956 | 62 | |||||
| scop+abt_120μg/kg | 888 | 62 | |||||
|
|
|||||||
| Mean False Alarm Latency (ms) | veh+veh | 720 | 70 | Drug | (5,31) | 1.4 | Ns |
| scop+veh | 950 | 75 | Drug × vSD | (10,62) | 1.8 | <0.1 | |
| scop+nic_300μg/kg | 889 | 89 | vSD | (2,62) | <1 | Ns | |
| scop+abt_12μg/kg | 791 | 75 | |||||
| scop+abt_40μg/kg | 761 | 75 | |||||
| scop+abt_120μg/kg | 753 | 75 | |||||
|
|
|||||||
| Total Trials | veh+veh | 229.9 | 9.2 | Drug | (5,31) | 1.6 | Ns |
| scop+veh | 241.0 | 9.9 | Drug × vSD | (10,62) | 1.3 | Ns | |
| scop+nic_300μg/kg | 221.4 | 11.7 | vSD | (2,62) | 1.5 | Ns | |
| scop+abt_12μg/kg | 227.3 | 9.9 | |||||
| scop+abt_40μg/kg | 228.7 | 9.9 | |||||
| scop+abt_120μg/kg | 236.7 | 9.9 | |||||
denotes p<0.05 when compared to vehicle treated mice (n=8), Ns denotes not significant.
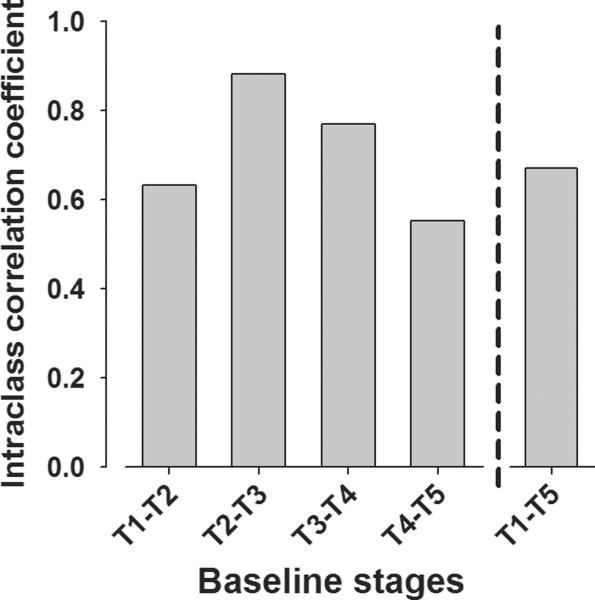
3.3. Test-retest reliability of mice in the 5C-CPT
The test-retest reliability in mice in the 5C-CPT was examined over the course of experiments 1b–2c using intraclass correlation coefficient analyses. Correlation coefficient analyses for the primary measure of vigilance (d') were always significant (F(1,85) ranging from 13.7 – 76.0, ps<0.01) with intraclass correlation coefficients ranging from fair to substantial (0.553 – 0.882; Fig. 8), in accordance with established guidelines [54]. Thus, during periods of stable performance, the primary measures of performance remained consistent with previous baseline levels. When examined over the longer period between experiments 1b and 2c, a significant correlation of d' was also observed (r=0.78, p<0.005). Intraclass correlation coefficients of secondary outcome measures also remained strong (e.g. for bias, correlations ranged from 0.66–0.88, for mean correct latency correlations ranged from 0.71–0.98, for accuracy correlations ranged from 0.68–0.92).
Fig. 8.
Intraclass coefficient correlation of performance across test stages. The reliability of performance of the primary outcome measure (d) was compared across the five experiments at baseline (T1–T5). The correlation of 5C-CPT performance was always highly significant, with fair to substantial correlations between each test and from initial baseline (T1) to 13 weeks later (T5). These data support the reliability of 5C-CPT performance over time and the premise that the same cohort could be tested repeatedly over time.
4. Discussion
Cholinergic manipulation of mice performing the 5C-CPT in these studies produced similar effects to that of humans performing CPTs. Specifically, nicotine subtly improved, while scopolamine greatly disrupted, attentional CPT performance [14, 30, 55, 56]. Importantly, we also observed modestly improved vigilance in mice administered the α4β2 nAChR selective agonist ABT-418. Moreover, both nicotine and ABT-418 attenuated scopolamine-induced disruption in performance. No effect of the α7 nAChR agonist PNU was observed in either normal performance or scopolamine-induced disruption in performance.
These findings demonstrate that nicotine can improve attention/vigilance as measured by the 5C-CPT in mice, consistent with healthy human subjects in the Connors CPT [14]. Because both tasks utilize target and non-target trials, the distinct brain regions mediating human CPT performance to these two trial types are likely to be required in the 5C-CPT [36–38]. Thus, by utilizing non-target stimuli in conjunction with target stimuli in the 5C-CPT, it is clear that the mechanism by which nicotine improved mouse performance was consistent with humans – increased target responses without affecting non-target responses [14]. While one could hypothesize that this effect is attributable to nicotine-induced increases in target responses (reduced omissions) in mice in the 5CSRTT [31, 32], these data are the first to demonstrate that the nicotine-induced increase in target responses was not accompanied by an increase in non-target responses, thus confirming the selectivity of responding. Hence, this result supports the cross-species translational validity of the 5C-CPT in terms of its pharmacological predictive validity, an important tenet for drug development [57–59].
In previous 5CSRTT studies in mice [31, 32] and rats [50, 51], nicotine improved accuracy, an effect not observed here presumably because of the concomitant use of non-target stimuli. Previous reports support a nAChR agonist-induced improvement in attention via increasing the detection of target stimuli [60, 61]. Increased target detection may result from increases in transient choline spikes after the detection of a target, utilizing a network including the prefrontal cortex, mediodorsal nucleus, and basal forebrain [61]. This network is hypothesized to be mediated in part by α4β2 nAChRs, which could explain why the more selective α4β2 nAChRs agonist (ABT-418 compared to nicotine) also enhanced the accuracy of responding in scopolamine-induced disrupted performance in mice, an effect that was not observed for nicotine. Moreover, the network for target detection likely also includes mAChRs in the prefrontal cortex, which may mediate scopolamine-induced disruption in performance in the 5C-CPT [61]. When co-administered with scopolamine, the primary effect of the attenuating dose of nicotine (300 μg/kg) was actually a reduction of responses to non-target stimuli. The reason for the altered effective dose and mechanism of effect remains unclear. The network that subserves non-target responding has yet to be elucidated, but may include a dopamine D4 mechanism [43]. The present data are consistent with an interaction between nAChR and mAChRs.
ABT-418-induced improvement in normal and scopolamine-induced disruption of 5C-CPT performance supports an α4β2 nAChR-mediated mechanism of attentional enhancement. The effect of ABT-418 was more subtle than nicotine however, with improvements being observed only at the 1.25 s stimulus duration in the variable stimulus duration challenge and could relate to the limited potency of ABT-418 for α4β2 nAChR compared with other high affinity nAChRs. Despite the lack of sizable effects however, these findings support earlier preclinical studies of subtle ABT-418-induced improvement in attention only in a poor-performing strain of rats [62], with no effect in good performers [63]. In the 5CSRTT, ABT-418 subtly increased accuracy in rats but only in the first 10 min [51]. To observe clearer effects in the mouse 5C-CPT, perhaps a poorer-performing strain should be used, such as DBA/2 mice [39]. The use of such a high-performing strain in the present study could explain the limited effect of nicotine and ABT-418 as well as the lack of dose-dependent findings. Alternatively, a smaller dose range could be investigated [32]. Importantly, neither nicotine nor ABT-418 affected bias in the task, suggesting that the improvement in d' with these drugs reflected enhanced vigilance [47, 64].
Unlike the selective α4β2 nAChR agonist, PNU did not improve 5C-CPT performance but only increased responses to non-target stimuli at the highest dose. The lack of an attention-enhancing effect of an α7 nAChR agonist is consistent with human CPT [65] and rat 5CSRTT studies [50, 51, 66], although this study is the first to test PNU. Mice with a null mutation of the α7 nAChR exhibit impaired 5CSRTT performance as measured by reduced target responses (increased omissions) [32, 52, 53]. The lack of correspondence between genetic and pharmacological manipulations of the α7 nAChR on measures of attention warrants further investigation [67]. To date, the 5C-CPT performance of α7 nAChR null mutants has not been assessed. With the inclusion of non-target trials, these mice may not exhibit the same deficit in omissions as observed in the 5CSRTT. Alternatively, nicotine- and ABT-418-induced improvements in performance were in the μg/kg range, with higher doses of PNU utilized. It is possible that the optimal dose of PNU was not used. Current doses were based on reversal of scopolamine-induced effects on novel environment preference in mice [25] and reversal of subchronic phencyclidine-induced deficits of novel object recognition and cued reversal learning in rats [68]. While the effects of scopolamine on performance were somewhat inconsistent between the PNU and the other studies, the fact that PNU did not improve normal performance may indicate that α7 nAChR have effects in domains other than attention, such as learning and memory [68–70]. Testing novel and more selective α7 nAChR compounds with good blood brain barrier permeability, such as ABT-107 [71, 72] or SSR180711 [73], may also be required given such poor permeability of other α7 nAChR compounds [74, 75]. Future studies examining wider dose ranges, novel compounds, and other cognitive domains are warranted [76].
While its applicability to a particular disease remains to be understood, scopolamine-induced disruption of mouse 5C-CPT performance could be regarded as a mouse model of impaired vigilance. Scopolamine reduced target responses and modestly increased non-target responding, a pattern of errors consistent with healthy humans administered scopolamine in the CPT [77]. Beyond the signal detection theory measures of performance used in human CPT studies, scopolamine also impaired accuracy and increased premature responses in mice, differences not observed in patients with schizophrenia in the human 5C-CPT [78]. This generalized deficit in responding induced by scopolamine is also observed when administered to mice in the 5CSRTT, albeit with some strain differences and no measure of non-target responding [28, 29, 31]. The lack of scopolamine-induced effects on latency measures suggests that these effects are not confounded by alterations in activity levels however. The present data add to these observations by indicating that scopolamine also modestly increases non-target responses. Thus, by utilizing non-target stimuli in the 5C-CPT, it is clear that scopolamine administration does not simply reduce responding levels in rodents, but impairs their ability to respond appropriately.
Co-administration of nicotine attenuated the effects of scopolamine on performance. While a full nicotine-induced attenuation of the disruptive effects of scopolamine was not evident, it is important to note that a modest improvement was consistently observed in d' across the three experiments. The subtlety of effect could be because scopolamine administration resulted in chance levels of performance. Interestingly, nicotine modestly improves CPT performance in patients with schizophrenia [17] and ADHD [20] as well as the aforementioned healthy subjects, all of which perform at above chance levels. Nicotine-induced attenuation of the disruptive effects of scopolamine was complicated by the fact that it was observed at 300 μg/kg in scopolamine-treated mice, while improvements in normal performance were observed at 10 μg/kg. Moreover, differences in response bias further complicate the interpretation of these data. While it would be simplest to indeed claim that nicotine attenuated the scopolamine-induced disruption of vigilance and that a larger dose was required because of the disruptive effects of scopolamine, this remains unclear. Co-administration of nicotine (300 μg/kg) with scopolamine treatment proved to be useful as a positive control however in the further 2 studies, attenuating the effects of scopolamine in all 3 studies. Consistent with their effects on normal mice performing the 5C-CPT, PNU did not attenuate scopolamine-induced disruption in performance, while ABT-418 exhibited a very modest attenuation of the effects of scopolamine. While ABT-418 treatment of scopolamine-induced deficits did not improve performance significantly beyond that of scopolamine alone, its co-treatment no longer differed significantly from vehicle treatment which was in contrast with scopolamine effects alone. Hence, consistent with its effects over 4 days, ABT-418 produced very subtle effects on performance. The effects of treatment on RI were less clear in the PNU and ABT-418 studies. The lack of effects of nicotine or ABT-418 on bias suggests that their improvement in performance and attenuation of the disruptive effects of scopolamine were likely to be attentive in nature [47]. Thus, while these studies are beneficial, they are limited by a lack of a full factorial design whereby treatments would be administered both alone and in combination with scopolamine. A factorial design would help to determine whether nicotine and ABT-418 partially blocked the effects of scopolamine or were beneficial alone. Future studies should use such a factorial design. Importantly for the drug discovery process [40], the consistent performance of the mice tested in these studies was always strong. Thus, the psychometric test-retest reliability of performance in the 5C-CPT provided support that the numerous consistencies observed between studies - such as various stimulus duration effects or nicotine-induced attenuation of scopolamine deficits - were reliable and that a single cohort can be tested repeatedly, consistent with other studies in mice [31], and rats [50, 51].
5. Conclusion
In conclusion, the present studies demonstrate that it is possible to observe nicotine-induced improvement in the 5C-CPT, consistent with the effects of nicotine on healthy humans in the CPT [14]. Furthermore, scopolamine impairs vigilance in mice as it does in man [30]. These data extend similar work in the 5CSRTT [31, 79], since they detail the effects of these drugs on non-target responding also, similarly to human CPTs. Thus, the patterns of effects of nicotine and scopolamine in mice in the 5C-CPT were consistent with healthy human subjects in CPTs, supporting the pharmacological predictive validity of the 5C-CPT. The availability of the 5CCPT in mice enabled the assessment of more selective nAChR agonists than nicotine, specifically the α4β2 nAChR agonist ABT-418 and the α7 nAChR agonist PNU. ABT-418 exhibited modest improvements in vigilance in mice while PNU was without effect at the doses tested. As well as improving vigilance in normal mice, nicotine and ABT-418 modestly attenuated scopolamine-induced disruption in vigilance - consistent with modest nicotine-induced improvements in patients with schizophrenia [17] and ADHD [20] - while again PNU was without effect. These studies support an α4β2 nAChR-mechanism of action for nicotine-induced improvement in vigilance. These findings remain far from conclusive however, and future studies will examine different doses of PNU as well as more selective α7 and α4β2 nAChR agonists. Furthermore, the availability of this task in mice will enable the assessment of nAChR knockout mice in the task, whereby it can be examined whether nicotine-induced improvement in performance can be observed in these mice.
Research Highlights
Nicotine improves mouse attention/vigilance in the 5C-CPT
ABT-418, the α4β2 nAChR agonist also improves attention/vigilance
Nicotine and ABT-418 attenuate scopolamine-induced 5C-CPT deficits
The α7 nAChR agonist PNU 282987 did not affect 5C-CPT performance
Mouse 5C-CPT performance is reliable across time
Acknowledgements
We thank Dr. Gregory Light, Richard Sharp, and Mahálah Buell for their support. This study was supported by NIH grants R21-MH085221, and R01-MH071916, as well as by the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–46. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- [2].Scarr E, Dean B. Role of the cholinergic system in the pathology and treatment of schizophrenia. Expert Rev Neurother. 2009;9:73–86. doi: 10.1586/14737175.9.1.73. [DOI] [PubMed] [Google Scholar]
- [3].Segarra R, Zabala A, Eguiluz JI, Ojeda N, Elizagarate E, Sanchez P, et al. Cognitive performance and smoking in first-episode psychosis: the self-medication hypothesis. Eur Arch Psychiatry Clin Neurosci. 2010 doi: 10.1007/s00406-010-0146-6. [DOI] [PubMed] [Google Scholar]
- [4].Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatry. 2010;23:112–9. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- [5].Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–34. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- [6].Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–92. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Martin-Ruiz CM, Haroutunian VH, Long P, Young AH, Davis KL, Perry EK, et al. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol Psychiatry. 2003;54:1222–33. doi: 10.1016/s0006-3223(03)00348-2. [DOI] [PubMed] [Google Scholar]
- [8].Zavitsanou K, Katsifis A, Mattner F, Huang XF. Investigation of m1/m4 muscarinic receptors in the anterior cingulate cortex in schizophrenia, bipolar disorder, and major depression disorder. Neuropsychopharmacology. 2004;29:619–25. doi: 10.1038/sj.npp.1300367. [DOI] [PubMed] [Google Scholar]
- [9].Deng C, Huang XF. Decreased density of muscarinic receptors in the superior temporal gyrusin schizophrenia. J Neurosci Res. 2005;81:883–90. doi: 10.1002/jnr.20600. [DOI] [PubMed] [Google Scholar]
- [10].Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, et al. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160:118–27. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- [11].Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B. Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry. 2009;14:1017–23. doi: 10.1038/mp.2008.28. [DOI] [PubMed] [Google Scholar]
- [12].Friedman JI. Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology (Berl) 2004;174:45–53. doi: 10.1007/s00213-004-1794-x. [DOI] [PubMed] [Google Scholar]
- [13].Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- [14].Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, et al. Transdermal nicotine effects on attention. Psychopharmacology (Berl) 1998;140:135–41. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- [15].Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–67. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- [16].Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210:453–69. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, et al. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology. 2006;31:637–43. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- [18].Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem. 2004;4:267–82. doi: 10.2174/1568026043451401. [DOI] [PubMed] [Google Scholar]
- [19].White HK, Levin ED. Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer's disease. Psychopharmacology (Berl) 1999;143:158–65. doi: 10.1007/s002130050931. [DOI] [PubMed] [Google Scholar]
- [20].Conners CK, Levin ED, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine and attention in adult attention deficit hyperactivity disorder (ADHD) Psychopharmacol Bull. 1996;32:67–73. [PubMed] [Google Scholar]
- [21].Garvey DS, Wasicak JT, Decker MW, Brioni JD, Buckley MJ, Sullivan JP, et al. Novel isoxazoles which interact with brain cholinergic channel receptors have intrinsic cognitive enhancing and anxiolytic activities. J Med Chem. 1994;37:1055–9. doi: 10.1021/jm00034a002. [DOI] [PubMed] [Google Scholar]
- [22].Decker MW, Curzon P, Brioni JD, Arneric SP. Effects of ABT-418, a novel cholinergic channel ligand, on place learning in septal-lesioned rats. Eur J Pharmacol. 1994;261:217–22. doi: 10.1016/0014-2999(94)90323-9. [DOI] [PubMed] [Google Scholar]
- [23].Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hajos M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, et al. The selective alpha7 nicotinic acetylcholine receptor agonist PNU-282987 [N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J Pharmacol Exp Ther. 2005;312:1213–22. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- [25].Redrobe JP, Nielsen EO, Christensen JK, Peters D, Timmermann DB, Olsen GM. Alpha7 nicotinic acetylcholine receptor activation ameliorates scopolamine-induced behavioural changes in a modified continuous Y-maze task in mice. Eur J Pharmacol. 2009;602:58–65. doi: 10.1016/j.ejphar.2008.09.035. [DOI] [PubMed] [Google Scholar]
- [26].Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009;122:150–202. doi: 10.1016/j.pharmthera.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Riedel G, Kang SH, Choi DY, Platt B. Scopolamine-induced deficits in social memory in mice: reversal by donepezil. Behav Brain Res. 2009;204:217–25. doi: 10.1016/j.bbr.2009.06.012. [DOI] [PubMed] [Google Scholar]
- [28].de Bruin NM, Fransen F, Duytschaever H, Grantham C, Megens AA. Attentional performance of (C57BL/6Jx129Sv)F2 mice in the five-choice serial reaction time task. Physiol Behav. 2006;89:692–703. doi: 10.1016/j.physbeh.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [29].Humby T, Laird FM, Davies W, Wilkinson LS. Visuospatial attentional functioning in mice: interactions between cholinergic manipulations and genotype. Eur J Neurosci. 1999;11:2813–23. doi: 10.1046/j.1460-9568.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- [30].Koller G, Satzger W, Adam M, Wagner M, Kathmann N, Soyka M, et al. Effects of scopolamine on matching to sample paradigm and related tests in human subjects. Neuropsychobiology. 2003;48:87–94. doi: 10.1159/000072883. [DOI] [PubMed] [Google Scholar]
- [31].Pattij T, Janssen MC, Loos M, Smit AB, Schoffelmeer AN, van Gaalen MM. Strain specificity and cholinergic modulation of visuospatial attention in three inbred mouse strains. Genes Brain Behav. 2007;6:579–87. doi: 10.1111/j.1601-183X.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- [32].Young JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, et al. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]
- [33].Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–80. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- [34].Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–80. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- [35].Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol. 2002;17:235–72. [PubMed] [Google Scholar]
- [36].Eyler LT, Dawes SE, Asgaard GL, Young JW. Abnormalities of brain responses during vigilance and inhibition in bipolar disorder. Bipolar Disorders. 2011;13:42. [Google Scholar]
- [37].Fallgatter AJ. Electrophysiology of the prefrontal cortex in healthy controls and schizophrenic patients: a review. J Neural Transm. 2001;108:679–94. doi: 10.1007/s007020170045. [DOI] [PubMed] [Google Scholar]
- [38].Kleinlogel H, Strik W, Begre S. Increased NoGo-anteriorisation in first-episode schizophrenia patients during Continuous Performance Test. Clin Neurophysiol. 2007;118:2683–91. doi: 10.1016/j.clinph.2007.08.022. [DOI] [PubMed] [Google Scholar]
- [39].Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS ONE. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lustig C, Kozak R, Sarter M, Young JW, Robbins TW. CNTRICS final animal model task selection: Control of attention. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Barnes SA, Young JW, Neill JC. D(1) receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Barnes SA, Young JW, Neill JC. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–92. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Green DM, Swets JA. Signal Detection Theory and Psychophysics. Wiley & Sons; New York: 1966. [Google Scholar]
- [45].McNicol D. A primer of signal detection theory. George Allen & Unwin; London: 1972. [Google Scholar]
- [46].Frey PW, Colliver JA. Sensitivity and responsibility measures for discrimination learning. Learning and Motivation. 1973;4:327–42. [Google Scholar]
- [47].Marston HM. Analysis of cognitive function in animals, the value of SDT. Brain Res Cogn Brain Res. 1996;3:269–77. doi: 10.1016/0926-6410(96)00012-2. [DOI] [PubMed] [Google Scholar]
- [48].Sahgal A. Some limitations of indices derived from signal detection theory: evaluation of an alternative index for measuring bias in memory tasks. Psychopharmacology (Berl) 1987;91:517–20. doi: 10.1007/BF00216022. [DOI] [PubMed] [Google Scholar]
- [49].Hahn B, Shoaib M, Stolerman IP. Nicotine-induced enhancement of attention in the five-choice serial reaction time task: the influence of task demands. Psychopharmacology (Berl) 2002;162:129–37. doi: 10.1007/s00213-002-1005-6. [DOI] [PubMed] [Google Scholar]
- [50].Grottick AJ, Higgins GA. Effect of subtype selective nicotinic compounds on attention as assessed by the five-choice serial reaction time task. Behav Brain Res. 2000;117:197–208. doi: 10.1016/s0166-4328(00)00305-3. [DOI] [PubMed] [Google Scholar]
- [51].Hahn B, Sharples CG, Wonnacott S, Shoaib M, Stolerman IP. Attentional effects of nicotinic agonists in rats. Neuropharmacology. 2003;44:1054–67. doi: 10.1016/s0028-3908(03)00099-6. [DOI] [PubMed] [Google Scholar]
- [52].Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, et al. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–55. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [53].Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl) 2006;189:211–23. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Shrout PE. Measurement reliability and agreement in psychiatry. Statistical Methods in Medical Research. 1998;7:301–17. doi: 10.1177/096228029800700306. [DOI] [PubMed] [Google Scholar]
- [55].Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, et al. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–89. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- [56].Wesnes K, Revell A. The separate and combined effects of scopolamine and nicotine on human information processing. Psychopharmacology (Berl) 1984;84:5–11. doi: 10.1007/BF00432014. [DOI] [PubMed] [Google Scholar]
- [57].Floresco SB, Geyer MA, Gold LH, Grace AA. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr Bull. 2005;31:888–94. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- [58].Jones DNC, Garlton JE, Minassian A, Perry W, Geyer MA. Developing new drigs for schizophrenia: From animals to the clinic. In: McArthur R, Borsini F, editors. Animal and translational models for CNS drug discovery: Psychiatric disorders. Elsevier Inc.; New York: 2008. pp. 199–262. [Google Scholar]
- [59].Young JW, Zhou X, Geyer MA. Animal models of schizophrenia. In: Swerdlow NR, editor. Behavioral Neurobiology of Schiozphrenia and tis Treatment. Springer; Berlin: 2010. pp. 391–433. [DOI] [PubMed] [Google Scholar]
- [60].Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, et al. Enhancement of attentional performance by selective stimulation of alpha4beta2(*) nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sarter M, Parikh V, Howe WM. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem Pharmacol. 2009;78:658–67. doi: 10.1016/j.bcp.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psychopharmacology (Berl) 1999;144:175–82. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- [63].Turchi J, Holley LA, Sarter M. Effects of nicotinic acetylcholine receptor ligands on behavioral vigilance in rats. Psychopharmacology (Berl) 1995;118:195–205. doi: 10.1007/BF02245840. [DOI] [PubMed] [Google Scholar]
- [64].Dudchenko P, Paul B, Sarter M. Dissociation between the effects of benzodiazepine receptor agonists on behavioral vigilance and responsitivity. Psychopharmacology (Berl) 1992;109:203–11. doi: 10.1007/BF02245501. [DOI] [PubMed] [Google Scholar]
- [65].Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–7. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Grottick AJ, Haman M, Wyler R, Higgins GA. Reversal of a vigilance decrement in the aged rat by subtype-selective nicotinic ligands. Neuropsychopharmacology. 2003;28:880–7. doi: 10.1038/sj.npp.1300102. [DOI] [PubMed] [Google Scholar]
- [67].Young JW, Powell SB, Geyer MA. Mouse pharmacological models of cognitive disruption relevant to schizophrenia. Neuropharmacology. 2012;62:1381–90. doi: 10.1016/j.neuropharm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McLean SL, Grayson B, Idris NF, Lesage AS, Pemberton DJ, Mackie C, et al. Activation of alpha7 nicotinic receptors improves phencyclidine-induced deficits in cognitive tasks in rats: implications for therapy of cognitive dysfunction in schizophrenia. Eur Neuropsychopharmacol. 2011;21:333–43. doi: 10.1016/j.euroneuro.2010.06.003. [DOI] [PubMed] [Google Scholar]
- [69].Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett. 2010;471:114–8. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- [70].Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA. Delayed procedural learning in alpha7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav. 2011;10:720–33. doi: 10.1111/j.1601-183X.2011.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Malysz J, Anderson DJ, Gronlien JH, Ji J, Bunnelle WH, Hakerud M, et al. In vitro pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107. J Pharmacol Exp Ther. 2010;334:863–74. doi: 10.1124/jpet.110.167072. [DOI] [PubMed] [Google Scholar]
- [72].Othman AA, Lenz RA, Zhang J, Li J, Awni WM, Dutta S. Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective alpha7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J Clin Pharmacol. 2011;51:512–26. doi: 10.1177/0091270010370460. [DOI] [PubMed] [Google Scholar]
- [73].Thomsen MS, Christensen DZ, Hansen HH, Redrobe JP, Mikkelsen JD. alpha(7) Nicotinic acetylcholine receptor activation prevents behavioral and molecular changes induced by repeated phencyclidine treatment. Neuropharmacology. 2009;56:1001–9. doi: 10.1016/j.neuropharm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- [74].Turek JW, Kang CH, Campbell JE, Arneric SP, Sullivan JP. A sensitive technique for the detection of the alpha 7 neuronal nicotinic acetylcholine receptor antagonist, methyllycaconitine, in rat plasma and brain. J Neurosci Methods. 1995;61:113–8. doi: 10.1016/0165-0270(95)00032-p. [DOI] [PubMed] [Google Scholar]
- [75].Cilia J, Cluderay JE, Robbins MJ, Reavill C, Southam E, Kew JN, et al. Reversal of isolation-rearing-induced PPI deficits by an alpha7 nicotinic receptor agonist. Psychopharmacology (Berl) 2005;182:214–9. doi: 10.1007/s00213-005-0069-5. [DOI] [PubMed] [Google Scholar]
- [76].Levin ED. alpha7-Nicotinic receptors and cognition. Curr Drug Targets. 2012;13:602–6. doi: 10.2174/138945012800398937. [DOI] [PubMed] [Google Scholar]
- [77].Duka T, Ott H, Rohloff A, Voet B. The effects of a benzodiazepine receptor antagonist beta-carboline ZK-93426 on scopolamine-induced impairment on attention, memory and psychomotor skills. Psychopharmacology (Berl) 1996;123:361–73. doi: 10.1007/BF02246647. [DOI] [PubMed] [Google Scholar]
- [78].Young JW, Geyer MA, Rissling AJ, Eyler LT, Asgaard GL, Light GA. Impaired vigilance but not accuracy of patients with schziophrenia in a human version of the rodent 5-choice continuous performance test. Schizophrenia Bulletin. 2011;37:236. [Google Scholar]
- [79].Jones DN, Higgins GA. Effect of scopolamine on visual attention in rats. Psychopharmacology (Berl) 1995;120:142–9. doi: 10.1007/BF02246186. [DOI] [PubMed] [Google Scholar]