Summary
The immune system is made up of a diverse collection of cells, each of which has distinct sets of triggers that elicit unique and overlapping responses. It is correctly described as a ‘system’ because its overall properties (e.g. ‘tolerance’, ‘allergy’) emerge from multiple interactions of its components cells. To mobilize a response where needed, the majority of the cells of the system are obligatorily highly motile and so must communicate with one another over both time and space. Here, we discuss the flexibility of the primary immunological synapse (IS) with respect to motility. We then consider the primary IS as an initiating module that licenses ‘immunological circuits’: the latter consisting of two or more cell-cell synaptic interactions. We discuss how two or three component immunological circuits interact might with one another in sequence and how the timing, stoichiometry, milieu, and duration of assembly of immunological circuits are likely to be key determinants in the emergent outcome and thus the system-wide immune response. An evolving consideration of immunological circuits, with an emphasis on the cell-cell modules that complement T-antigen-presenting cell interaction, provides a fundamental starting point for systems analysis of the immune response.
Keywords: immunological synapse, motility, immune circuit
Introduction
The immune system is more than just a collection of individual cells making single cell-cell contacts that define their entire fate. Lymphocytes rather engage in active and ongoing communication with one another, and these communications provide the collection of responding cells with true system-like properties. These include emergent behaviors or states, such as ‘immunity’ or ‘tolerance’.
An important property that permits robust states of the system is the amplification of the response made by one cell, through its interaction with other triggered cells. We have yet to fully understand this second level of communication or to exploit that understanding to improve our ability to purposely direct an immune system, once initiated. However, a collection of studies show that a first class of ‘primary’ triggering-interactions through antigen-receptors (T cells and B cells) and pattern receptors (innate immune cells) results in an initial program that then licenses these cells to interact with one another or other similarly triggered cells via ‘secondary’ class of interactions. We propose to formally consider these classes of events as collections of ‘modules’ to begin to understand the greater context of immune system activation.
‘Immunological circuits’ as sources of emergent behaviors
The idea that circuits of immune cell-cell interactions are primary determinants of the systemic properties of immunity is not new, although our ability to realize it has been limited until recent advances in computation and imaging. Cantor and Gershon (1) proposed a ‘circuit-based’ model for immunity over 40 years ago. However, in the intervening years, necessary focus has been placed upon the individual ‘triggering’ receptors and their biochemistry (2–5). Studies of the T-cell and B-cell receptors (TCRs and BCRs), in particular, have revealed significant details for the role that weak and/or antagonist ligands can play in modulating the nature of the ensuing proliferative response (6–9). This type of understanding has fed the contrary or at most complementary understanding whereby the capacity of a triggering receptor such as a TCR to recognize its ligand is the fundamental determinant of whether an immune reaction is permitted or will occur. The observations that TCRs are polyspecific (10, 11), support multiple degrees of triggering based on the quality of their ligands (7, 9), and broadly that T cells require ‘costimulatory’ ligands for maximal activation(12) all represent variations in the ‘single T cell-single antigen-presenting cell (APC)’ paradigm for generating an adaptive immune response. In such a view, T-cell reactivity is largely programmed on the quality of a single or the ‘best’ APC encounter.
Much data suggests that such a model is at best incomplete and lacks considerable temporal assembly and re-assembly steps. Observations of T-cell priming in the lymph node alone have shown that T cells make multiple encounters with different APCs en route to their activation (13, 14). Also in the lymph node, there is evidence for cross-talk between individual T cells (15) and evidence for cell-cell based communication between T cells of varying subsets (16, 17), primed B cells (18), and new incoming APCs (19). Most provocative, perhaps, are studies of T-cell tolerance that have demonstrated that many measures of T-cell signaling and proliferation over the first 3–4 days appear identical between conditions eliciting ‘tolerance’ and those eliciting ‘immunity’(20). Late divergence, multiple days after antigen triggering, suggests that signals beyond those generated by the TCR alone are the source of system-wide decisions for tolerance or immunity.
While much still remains to be done to understand how molecules such as the TCR are triggered, much data supports the concept that the response develops then as an emergent system, integrating the responses of many cells. We discuss the ongoing nature of primary immunological circuits that are built on the first priming events and then discuss a ‘secondary circuit’ concept with emphasis on system-wide behaviors that modulate T-cell immunity.
Structure and dynamics of primary immunological synapses
A large variety of priming for adaptive immunity takes place via a specific cell junction with similarity to a neurological synapse. This is the contact between the T cell and the APC, bearing peptide-major histocompatibility complex (pMHC) on its surface. This contact, termed the immunological synapse (IS), serves as a prototype for communication between many cell types during their initial priming. Beyond the interaction between T cell and activating APCs that initially primed T cells (21, 22), synapses are broadly used between natural killer (NK) cells and their activating myeloid cell partners (23) as well as B cells whose BCRs are frequently triggered in a synapse with follicular dendritic cells (DCs) (24). For the purposes of this review and for understanding the system-wide circuitry of immunity, we refer to these cell-cell contacts as ‘primary’ synapses to distinguish them with cell-cell contacts that take place after cells engage in a first triggering interaction. Examples of ‘secondary’ synapses may include those between activated T cells and activated B cells, involved in the delivery of ‘help’ (18, 25), or recently described synapses that form between two activated T cells (T-T) (15), to name a few.
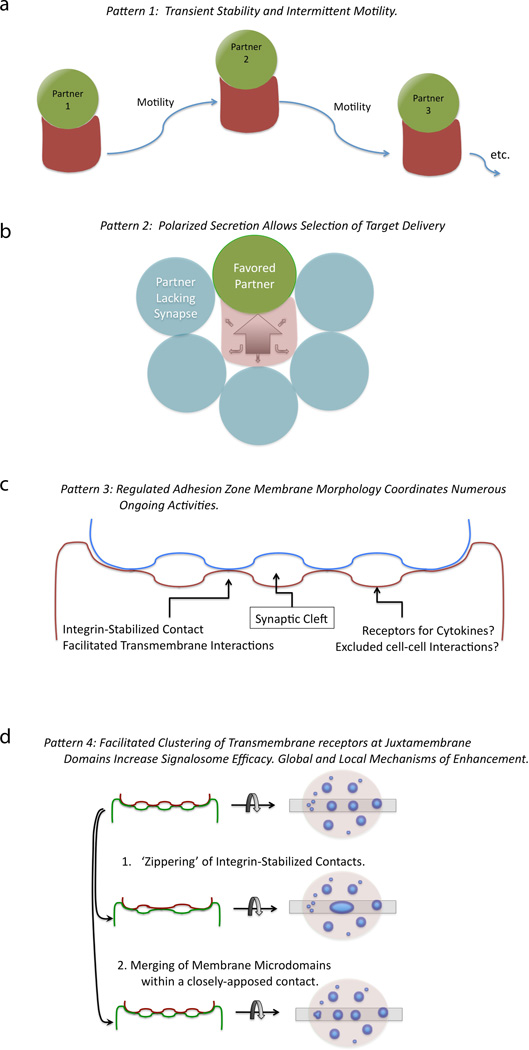
We have reviewed the ‘patterns’ of the synapse previously with regard to synapse but four ‘patterns’ that appear to be common to all IS. These are (i) impermanence, (ii) polarity modulating, (iii) generating synaptic ‘clefts’, and (iv) modulating signalosome coalescence and/or isolation.
First, T cell-APC interactions are not permanent structures. Rather, the cell-cell contacts last for seconds to hours but all ultimately result in ‘abscission’ of the T cell from the APC and possible reattachment to other partners (Fig. 1A). In vivo, there is considerable variation in the length of contact and the variability appears to be regulated by the strength of antigenic stimulation (26, 27) as well as T-cell-intrinsic factors (28).
Fig. 1. Common forms for primary synapses during initial activation of T cells and B cells.
(A) Dynamic cellular assembly and disassembly. (B) Defined but flexible polarity. (C) Close membrane-membrane juxtaposition with a synaptic cleft. (D) Aggregation and segregation of transmembrane receptors and lipids.
Second, polarity of signals generated at cell-cell contacts as well as subsequent secretion into these contacts, then, represents a second highly conserved pattern of immune cell-cell interactions. As diagrammed in Fig. 1B, this pattern permits cells to direct messages to one another while excluding bystanders. As an example, when T cells are engaging a cell presenting pMHC complexes, it has been shown that CD40L is directly accumulated at the IS, where it is available to crosslink CD40 (25). Interestingly, it has been proposed that this pattern is only true for some signals; vesicles containing interferon γ (IFNγ) appear to be more synapse localized, while internal vesicles containing other secreted products such as tumor necrosis factor (TNF) and chemokines may be more broadly directed (29). However, given the limitations on vesicle-membrane fusion, these latter molecules too may ultimately prove to be secreted largely toward the IS or a similar polarized cue. Polarized secretion permits exquisite spatial specificity for intercellular communication by immune cells.
Third, the T cell-APC ‘immunological synapse’ was first defined as a synapse by virtue of the presence of both adhesion domains and signaling domains, but it is apparent that synaptic clefts are also frequently present. Transmission electron microscopy (TEM) analysis of physiologically relevant contacts suggests that T-DC interactions (30), cytotoxic T-lymphocyte (CTL)-target contacts (31), and typically even T-B interactions (Krummel MF, unpublished observations) contain this architecture. There are frequently spatially restricted areas where cell-cell signaling may occur surrounded by membrane domains which may restrict direct membrane contact (Fig. 1C). The latter domains, however, sample synaptic spaces and provide a region for the accumulation of soluble mediators. Notably, the variable spacing of membranes around the closest point of apposition has been suggested to be important for protein organization in the IS (32–34), and MHCs with variable length extracellular domains result in altered capacities to signal (35). However, some ‘large’ molecules that are typically excluded, such as CD43, are not excluded on the basis of extracellular size alone, as tail-less forms can enter the central IS but do not interfere with signaling (36).
Finally, it is well established that all immunological synapses serve as a platform for the aggregation of receptor complexes and lipid domains (Fig. 1D). Based on observations of membrane-membrane topology by TEM, there are likely two scales of clusters and at least two methods of cluster coalescence. Small, initial ‘micro’ clusters likely provide for the formation of higher-ordering signaling arrays or ‘signalosomes’. Clusters of TCRs likely provide a high avidity lattice to capture pMHC complexes on the outside of cells and trap signaling intermediates in their active state on the inside of the membrane. It has been observed that early microclusters of TCRs are in fact highly enriched for tyrosine phosphorylation (37, 38). For example, at the far edges of the synapse, continuous membrane extension and retraction are commonly observed and, at the B-DC synapse, have been observed to be involved in accumulating new ligands for the BCR (24).
An unresolved question in the field is the way in which these larger clusters form. As shown for T cells interacting with membranes with reduced lateral protein mobility, it is likely that the formation of these large clusters hastens termination of signaling (38).To this end, the dynamics of coalescence of clusters may involve multiple mechanisms. On the one hand, flat lipid bilayers demonstrate that TCRs can move laterally along the membrane and in a centripetal manner (37–40). On the other hand, cluster coalescence in T cell-B cell or NK-APC contacts present a much less concerted effect, although a centralized supramolecular activation cluster (cSMAC) is typically still formed (41, 42). One intriguing possibility, in the confines of a cell-cell interaction, is that multiple mechanisms may act to give the final aggregated structure. While membrane movement and coalescence of microclusters in the membrane may drive cluster aggregation within a given domain (Fig. 1D, middle panel), the joining of individual membrane-membrane contacts may also be necessary to reorganize contacts in a full synaptic membrane architecture (‘zippering’)(Fig. 1D, lower panel).
In the subsequent sections, we review our evolving understanding of synapses and how we have come to understand synapses as being highly flexible in their organization and in their ability to support and co-exist with concurrent motility. This concludes with a discussion of the dichotomous or ‘continuum’ nature of motility with regard to synapses.
The stop signal revisited
Signaling at cell-cell junctions has been modeled, until very recently, as being mediated by and necessitating a fully ‘stopped’ T cell—one in which the movement of the cell body (center-of-mass motility) was reduced to effectively zero. This idea was largely supported by in vitro studies in which intracellular calcium levels were specifically varied (43, 44). In one such study, an intracellular calcium clamp showed that T-cell shape and motility are extremely sensitive to changes in intracellular calcium, resulting in high calcium-dependent immobilization and rounding. Calcium-dependent immobilization in these in vitro studies resulted in prolonged T-cell contact with an antigen-presenting B cell and buffering the calcium signal prevented the formation of stable cell pairs. Furthermore, using stimulatory anti-TCR antibodies, again on cell lines, it was apparent that cell motility arrest occurred within minutes of administration of this signal (45).
Using a pre-activated lymph node system in which the endogenous response had developed for 18 h in response to antigen plus adjuvant, Parker and coworkers (46) obtained data that largely supported the conclusion that initial arrest was at least accompanied by a rise in intracellular calcium and a concomitant slowing of cells. However, that experiment was done in the context of already primed milieu, and it remains possible that part of what was observed was a milieu effect. Nevertheless, the arrest of cells that contained greater than a threshold level (150µM) intracellular calcium was typically very robust (46).
A variety of data has refined the concept of a ‘stop’ signal and suggested that motility arrest may not be obligatory for activation. Friedl and colleagues (47) first demonstrated activation of T cells and their subsequent proliferation in an artificial collagen environment in a setting in which few if any cells underwent profound motility arrest. Some cells generated calcium influxes during transient interactions, and many proceeded to upregulate activation markers. Presuming that individual cells that arrested were not responsible for all the activation, features which were never formally addressed, one would conclude that cell-cell signaling leading to complete activation took place in the absence of a profound arrest. Advances in 2-photon imaging also permitted direct observation of T-cell behavior during an immune response in lymph nodes (LNs) and subsequent readouts of their activation, followed at various time following an immunization. Following recognition of their cognate antigen presented by a DC, T cells initially only decelerated upon contact with antigen-bearing DC partners (13, 14, 48), only fully ‘arresting’ (slowing to displacements less than about 2µm/min) about 24 h after antigen is first administered. Mempel in particular noted in these types of experiments that CD69 upregulation, an event tightly linked to TCR triggering, was evident prior to the 24 h timepoint in which profound arrest was observed. In most if not all of these studies, there remains considerable evidence for ruffling of membranes and transit of T cells around within the DC-rich milieu, and little success has been made in showing that cells remain committed to one DC throughout triggering in vivo.
In vivo data using either altered peptide ligands for T cells or using peptides at varying concentration has shown only an association between strength of activation and cellular arrest, with the latter often falling many hours after antigen is first engaged. For example, using a variation in agonist doses in vivo, Henrickson and colleagues (26) demonstrated that T cells took longer to commit to a profound arrest when less agonist was encountered; however, the initial encounter with antigen typically involved ongoing motility regardless of whether the cells ultimately arrested on the APC. Similarly, Skokos and colleagues (27) observed that only high potency antigens gave rise to rapid induction of TCR-dependent cell arrest, although lower potency demonstrably triggered TCR signaling, as evidenced by upregulation of the activation marker CD69. In the latter case, the low potency antigen responders not only typically did not arrest but also entered a state characterized as anergy, either due to the weak signal or due to other consequences surrounding or derived from the weak signal. In this latter study, the term ‘stop’ signal (45) was refined to ‘deceleration’, a careful refinement that more accurately represents the ongoing motility evident in most latter timepoints, even in responses that result in profound immune activation (27).
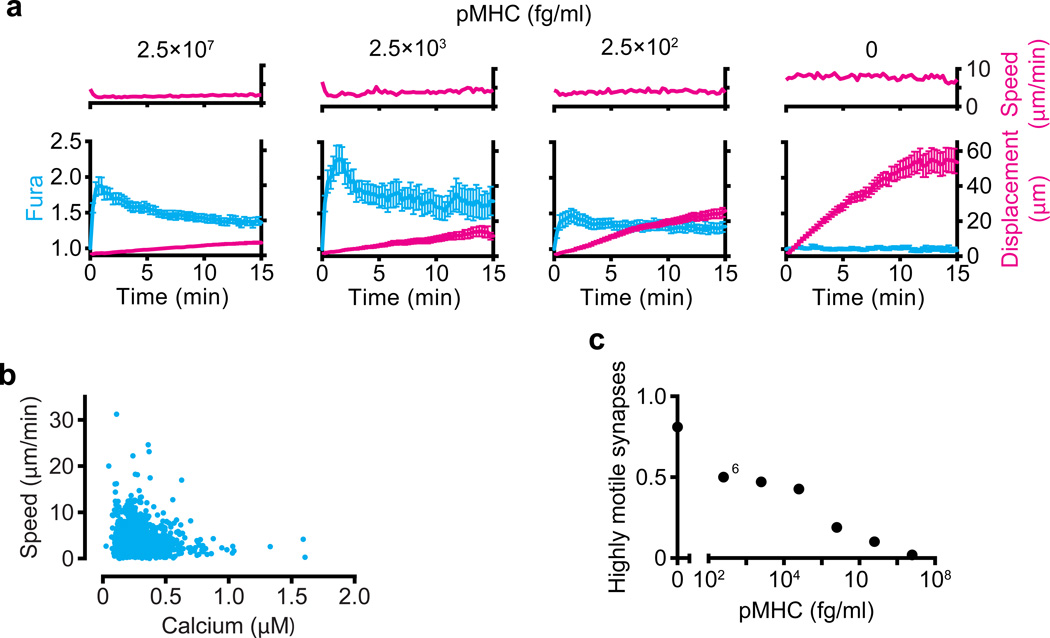
The above data suggest that T cells perhaps need a threshold of antigen, either in terms of dose or chemical composition, which itself delivers the ‘stop’ signal to T cells but that not all TCR signaling that is meaningful for proliferation requires this level and that this level may not be sustained for very long. We recently used a lipid-bilayer system to re-examine the absolute relationship between T-cell signaling, the ensuing calcium signals generated, and the corresponding motility rate. As shown in Fig. 2A, when faced with varying concentrations of agonist peptide ranging from super-physiological (panel A, far left) to physiological (dilutions), cells may exhibit profound and characteristic influxes of calcium during TCR engagement and yet continue to displace. The rate of displacement is inversely proportional to the strength of signal but only the very strongest and likely super-physiological levels of peptides give rise to the most profound arrest, and almost all cells at all levels continue to displace across the bilayer to some degree. Furthermore, when instantaneous velocities of cells that were confirmed to be forming a synapse with the bilayer were analyzed for the relationship between calcium and velocity, we found that only the very highest levels of intracellular calcium (greater than 1µM) resulted in speeds that were reliably lower than 2µm/min (Fig. 2B, far right dots). A vast majority of cells that were involved in signaling, by contrast, had intracellular calcium levels below 0.5µM and were variably motile at speeds much faster than this. Consistent with signaling generally ‘decelerating’ T-cell movement, only those cells with the lowest intracellular calcium levels typically exceed instantaneous speeds of 10um/min. These data support a ‘flexible’ model for signaling and motility where cells may move faster or slower during their response but that there is a trend towards arrest as the highest calcium signals (Fig. 2B) or highest antigen levels (Fig. 2A,C) are encountered.
Fig. 2. T cells remain motile during TCR signaling triggered by a large range of agonist doses.
(A) Speed (above) and both calcium concentration (presented as normalized Fura-2 ratio) and displacement (below) of cells (>75 per condition) interacting with bilayers loaded with various concentrations of pMHC (top). (B) Speed and cytosolic calcium concentration of cells (n = 96) interacting with bilayers loaded with pMHC at a concentration of 2.5 × 103 fg/ml. (C) Fraction of cells that formed high motility synapses (average speed, 3.8 µm/min) among the cells in a and additional cells (n = 776 cells total). Reproduced with permission from Beemiller et al. Nat Immunol 2012 (40).
A refinement of the ‘TCR stop signal’ concept should therefore be made to suggest a deceleration of motility with TCR occupancy, indeed a concept that was proposed by Skokos et al. (27) and supported by these data. The source of the most profound cell arrest (typically cutoff at <2µm/min or <2.5µm/min) in vivo and many hours after the first TCR signals, therefore, could either be very high signaling levels or additional milieu signals beyond those generated by the TCR.
Flexible synapse structures in synapses of varying motility
The prevailing model of ISs over the past 10 years has remained one based on a symmetric and concentric organization of signaling proteins within the junction. In this model, first proposed by Kupfer and colleagues (49) based on analysis of a small number of cell-cell contacts formed in a single T-cell clone, TCRs assemble into a cSMAC, which represents the site of active signaling, and correspondingly integrins coalesce into a fringing peripheral SMAC (pSMAC). Clusters were first seen to coalesce in real-time into in the center of these synapse in T-B contacts but in those real-time experiments, cSMAC formation lagged behind the onset of signaling. This strongly suggested that the latter could not be necessary for the former and that the ‘cSMAC’ was not in fact a requisite structure for signaling (41). Analysis of these dynamics in bilayers also showed that the degree of clustering closely tracked the strength of the pMHC interaction with the TCR (22). Further total internal reflection fluorescence (TIRF) microscopy analysis of these types T cells interacting with bilayers containing intercellular adhesion molecule (ICAM) and super-physiological levels of pMHC showed that TCR ‘microclusters’ in fact move centripetally into the IS, while leukocyte function-associated antigen-1 (LFA-1) clusters are squeezed out and segregated into the periphery (37, 50). Other molecules such as CD4 and CD28 transiently co-cluster with the central cluster in such symmetrical ISs (41, 51). Internalization of spent receptors is now thought to take place from the cSMAC region in this paradigm (52, 53).
Along with the result that most signaling takes place against a backdrop of a continuum of cell motility and translation, recent evidence has shown that T cells are actually quite ‘flexible’ (54) in their synapse assembly dynamics; rather than matching the ‘SMAC’ paradigm, they generate a continuum of behaviors within the IS. A specific counter-example to the highly flat and centripetally organized synapse came from serial section, electron microscopy analysis of membrane-juxtaposition at synapses between T cells and DCs (30). In that study, it was evident that most cellular junctions observed were ‘multifocal’, meaning that they consisted of many small individual contacts separated by large synaptic clefts much as was inherent in the original term use of the term ‘synapse’ (21). The fact that all published EMs show evidence of such multifocal synaptic contact suggest that the concepts of membrane-membrane juxtaposition together with single-site centralization, at least in contacts between T cells and APCs, may need to be considered rather a rarity.
In CTL synapses, Griffiths et al. (31) generated immunofluorescence data that suggested a ‘split’ central synapse in which cytotoxic granule exocytosis would occur just alongside the single cSMAC. However, more recent evidence from Sukylev’s group and others (55) has demonstrated that cells often generate multiple granule events that separately target to various portions of the synapse. The cSMAC secretion concept is also apparently at odds with the fact that most if not all EM of a cytolytic synapse also shows them to be multifocal and thus lacking a single central synapse. Studies of actin structure at the NK cytolytic structure suggest that there is ‘flexible’ generation of actin-poor secretory sites at multiple points around the cytolytic synapse (56, 57), and it seems likely that the localization of TCRs and secretion of granules take place in distinct subregions of membrane when viewed on this scale. To this extent, TCR clusters and secretory domains may indeed be distinct but be much greater in number than was previously understood.
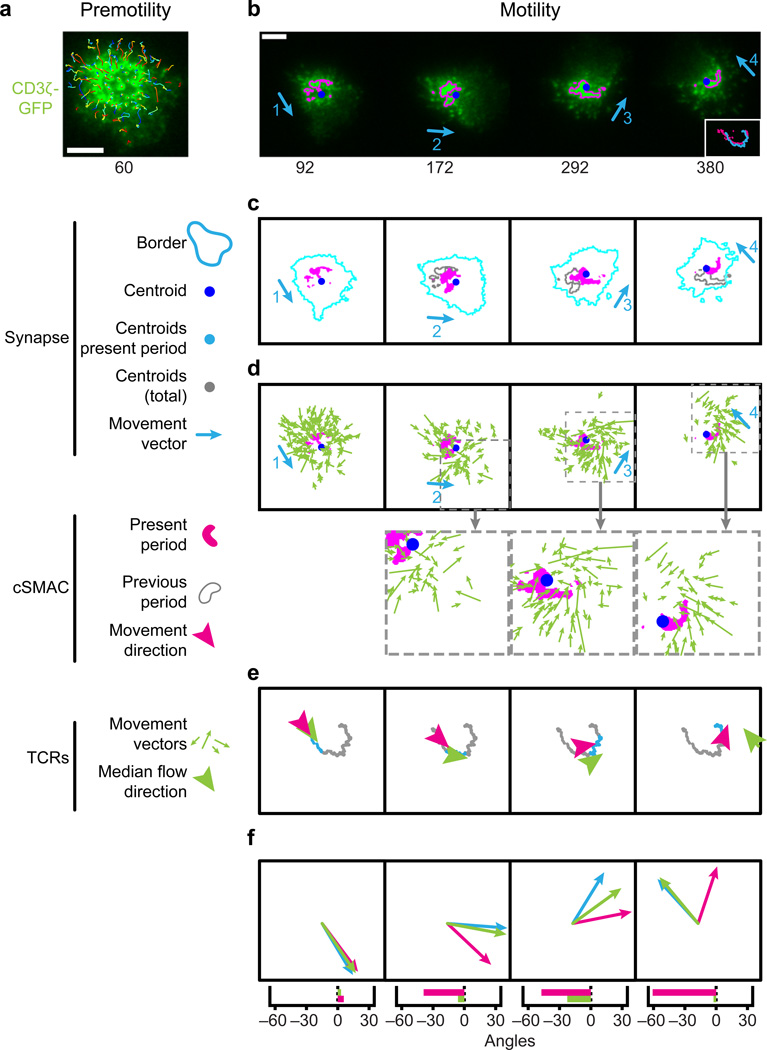
The flexibility in the gross dynamics whereby T cells activate was particularly evident from studies using a transgenic mouse strain in which the TCR was genetically tagged with the green-fluorescence protein (GFP). This labeling method allowed us to visualize the activation of naive cells as well as assessing TCR dynamics in vivo, which prior to this had been quite challenging. Tracking of naive T cells as they activated with DCs in vitro frequently demonstrated cells in which TCRs were visibly internalized while engaged with a single DC, without ever forming a single cSMAC (Fig. 3A). Fig. 3B shows that scoring of many cells interacting with many APCs consistently failed to form a cSMAC structure in a majority of the cells, even while the majority proceeded to internalize the TCR. Recognizing that that TCR internalization measures antigen recognition, this supports a pathway for TCR stimulation and subsequent processing that is not linked to a cSMAC structure. Activation leading to internalization, in the absence of a strong cSMAC, was a robust finding across many orders of magnitude of TCR stimulation (Fig. 3B,C). We subsequently performed similar imaging using 2-photon microscopy in vivo and again observed that only some of the cells formed a prototypical ‘stopped’ synapse characterized by a leading edge cSMAC distribution (Fig. 3D,E). Many cells, in contrast, provided evidence for ongoing movement during signaling as well as the ultimate internalization of receptors without ever forming a centralized cSMAC zone from which they were derived (Fig. 3F,G).
Fig. 3. Flexible synapse dynamics: TCR internalization does not require cSMAC formation.
Naive or activated OT-I–GFP T cells were incubated with DCs pulsed with the indicated concentration of SL8 peptide and imaged by epifluorescence microscopy. (A) Naive T cell-DC interactions. Contrast (top) and maximum intensity Z-projection of OT-I–GFP fluorescence (bottom) imaged by epifluorescence microscopy. The dotted line indicates DC location, and the arrows indicate internalized TCR vesicles. Scale bar = 10mm. Here, the T cell contacts the antigen-bearing DC (10ng/ml SL8), forms microclusters at the T cell-DC interface, and then rapidly internalizes the TCR without forming a cSMAC. (B) TCR dynamics. Percent of T cells in contact with DCs (contact) with a cSMAC or internalized TCR are indicated. (C) Frequency of cSMAC-independent TCR internalization. Only cells that internalized their TCR are included in (F); those that did not form a cSMAC are designated cSMAC-independent. Reproduced with permission from Friedman et al. J Exp Med, 2010. (54)
Flexible synapse assembly and the integration of actin dynamics and signaling cluster behaviors
The preceding in vivo and in vitro data provided compelling evidence that cells signaled ‘on the fly’, and this was supported by calcium data akin to what was shown in Fig. 2. The concept that T cells are much more flexible with respect to the relationship between signaling, synapse assembly, and motility was also hinted at previously, by studies of PKCθ-deficient T cells on lipid bilayers containing pMHC and ICAM1 (28). Those studies were the first to document T cells that migrated while engaging agonist pMHC complexes on bilayers, and the studies found that cells underwent phases of greater or lesser migration, where cells would ‘break symmetry’ and begin to move. In contrast, cells lacking a component of the TCR signal transduction machinery, PKCθ, were hyperstable and did not easily transition towards motility. This observation suggests that autonomous signals within the T cell are at work to encourage motility and that inhibition of PKCθ is necessary to make a stable synapse. Since TCR signaling typically activates PKCθ, it seems likely that an TCR-extrinsic signal (i.e. one from the milieu) might be one missing link (beyond very high TCR occupancy) that would be necessary to inactivate it (akin to the knockout). Such a TCR-extrinsic cue would be part of the solution to generation of <2µm/min ‘arrested’ T cells that are described in vivo, following an initial phase of ongoing motility. An alternative explanation would be a cell-intrinsic signal that inactivates PKCθ or engages a similar anti-motility signaling pathway. It is also possible that ‘stopping’ in vivo (<2µm/min) is not arrest of the acto-myosin based motility machinery at all but is in fact mediated by cells becoming ‘stuck’ to a site in the milieu.
We recently took a systematic approach toward understanding the nature of synapses and how they take place against varying degrees of cell translation (motility)(40). Using conditions based on those described in Fig. 2 above, we were able to study T cells interacting with antigen-presenting lipid-supported bilayers under varying degrees of ongoing motility. As shown in Fig. 4, this approach suggests a more unified reinterpretation of the movement of microclusters and the existence of the cSMAC. When cells are arrested, TCR microclusters have been shown to move inward into the cSMAC (37), and the presumption has been that TCR microclusters are moving toward the cSMAC (e.g. are in some way attracted to ‘join’ it). When cells are moving, however, it becomes evident that microclusters are not in fact moving toward the cSMAC. Their motility vectors are pointing toward a spot where the cSMAC soon will move. However, that cSMAC, in motile synapses, is revealed to follow behind the center of the synapse, essentially trailing into the extended uropod and not ‘in the zone’ where microclusters are headed. Microclusters will frequently join the cSMAC, presumably en route to internalization or attempted internalization. However, this joining results from microclusters that are moved toward the center of the motile synapse and then are ultimately engulfed by the cSMAC, when the latter catches up to them.
Fig. 4. Microcluster flow aligns with movement in motile synapses.
(A,B) Time-lapse TIRF images of a CD3z-GFP+ OT-I T cell during synapse formation, showing microcluster paths before cell movement (A), and synapse motility (B) divided into four periods based on the direction of movement (blue arrows); magenta indicates cSMAC borders. Inset (B), paths followed by the cSMAC and synapse centroids. Time (below) is relative to the start of TCR centralization. Scale bars are 5 mm. (C) The cSMAC (magenta), synapse border (light-blue), and cSMAC border at the end of the preceding motility period (gray). (D) Displacement vectors for microclusters formed during each motility period overlaid onto the cSMAC mask at the start of the period (magenta). Below, enlargement of areas outlined by dashed gray lines above. (E) Median microcluster flow direction (green) and direction of cSMAC movement (magenta) for each period. Arrowhead points are positioned at the mean microcluster endpoint or cSMAC position at the end of the period. (F) Cell (blue), cSMAC (magenta), and median microcluster movement vectors (green) in each period, drawn with a common origin and scaled to the same magnitude (top), angles between median microcluster movement vector and cell movement (green) and between cSMAC movement and cell movement (magenta) in each motility period (bottom). Reproduced with permission from Beemiller et al. Nat Immunol 2012.(40)
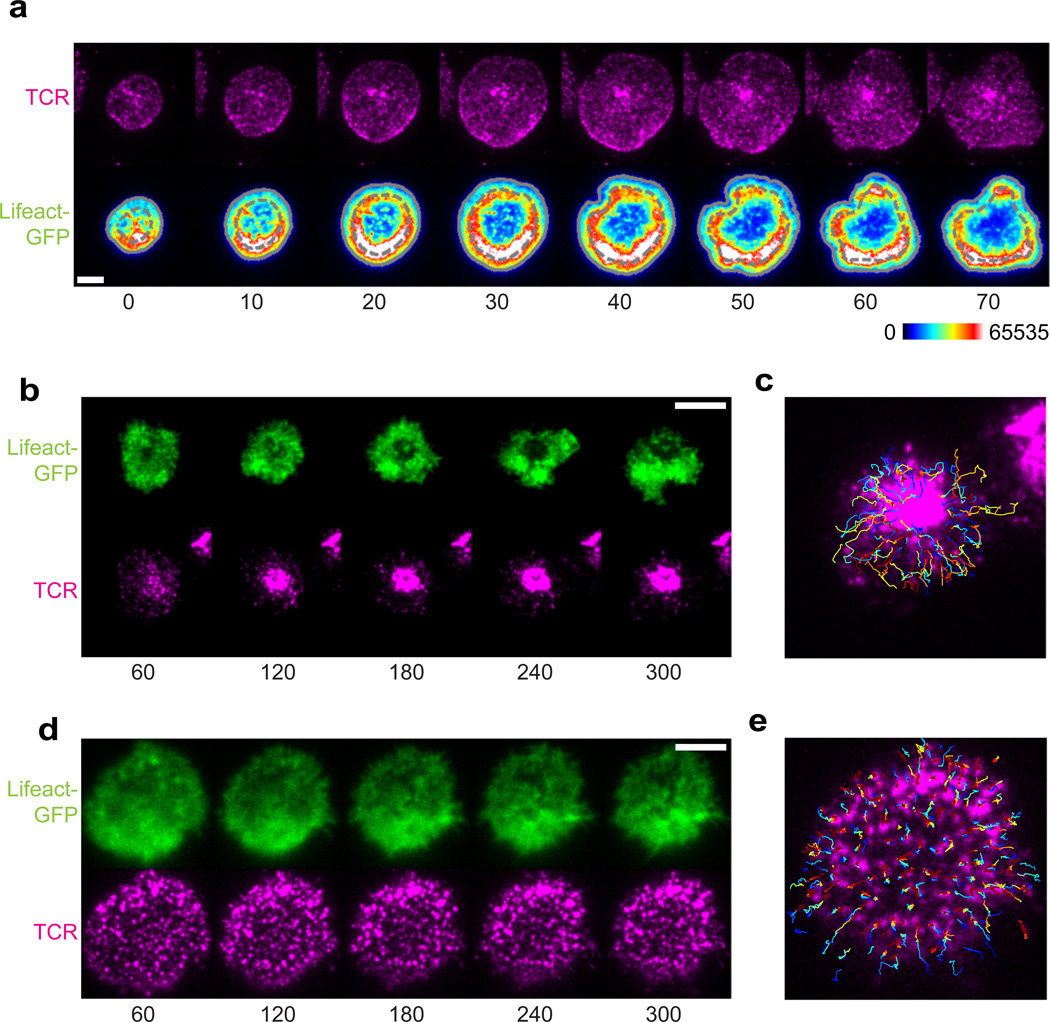
What feature underlies this central domain in the synapse into which both microclusters and cSMACs move? We extensively analyzed actin dynamics at the IS of synapses of varying degrees of motility and noted a correlation between actin-mediated collapse of edges and rapid involution of TCRs toward the center of the synapse (40). We therefore analyzed the precise dynamics of the evolution of actin structures in the IS. By analyzing polymerized actin using an f-actin binding protein-GFP fusion [Lifeact-GFP (58, 59)], it is found (Fig. 5A) that actin polymerization occurs and is enhanced at the edge of the synapse, as the cell spreads to make a synapse. However, rather than the central region remaining f-actin rich, leftover from it having been so during the spreading, the levels of f-actin there drop precipitously, providing evidence for an active depolymerase that functions there. As with the edge collapse/microcluster observations made during random-edge collapse, the movement of microclusters inward did not take place until this ‘actin void’ region had been established.
Fig. 5. The polymerization and depolymerization of actin organizes synapses.
(A) Time-lapse TIRF microscopy of a Lifeact-GFP+ OT-I T-cell blast spreading onto a stimulating bilayer (bottom), and Alexa Fluor 568–H57-597 labeled TCRs in the cell (top). Numbers below image are time in seconds (s). Scale bar, 5 mm. (B) TIRF images of Lifeact-GFP (top) and TCRs (bottom) during synapse formation by a control OT-I T-cell blast treated with DMSO; time (below) is relative to the start of spreading. Scale bar, 5 mm. (C) Microcluster paths overlaid onto images in (B). (D) TIRF images of Lifeact-GFP (top) and TCRs (bottom) during synapse formation by an OT-I T-cell blast treated with 50 nM jasplakinolide; time (below) as in (B). Scale bar, 5 mm. (E) Microcluster paths overlaid onto images in (F). Reproduced with permission from Beemiller et al. Nat Immunol 2012 (40).
We then sought to understand whether the act of depolymerization was actually regulatory for cluster movement. We compared the inward movement of TCRs in synapses that were treated with titrated concentrations of the actin depolymerization inhibitor Jasplakinomide (Jas) (Fig. 5B–E). Concentrations of this drug were carefully chosen that did not affect the spreading response to avoid blocking actin polymerization. Under these conditions, microcluster movement was severely restricted. This observation suggests that at least a part of inward movement occurs as a result of localized clearance of the ‘actin void’ domains. We hypothesize that specific depolymerizing activities are activated along or near spent TCR microclusters and facilitate their inward movement. Such a model is appealing, since it has already been appreciated that actin is undergoing continuous retrograde flow inward and yet only some TCRs move inwards (60). A full description of inward movement likely involves coordinated polymerization plus depolymerization.
What are the key factors then in microcluster dynamics in the IS? First, actin depolymerization might generate cluster movement by aligning the retrograde flow of actin microfilaments into ‘railcar’ tracks—microfilaments are extended on one end and disassembled on the other, pulling the microfilament and actin-associated factors inward. Although MyosinIIA seems to be non-essential (40), other myosin motors could contribute to this mechanism by regulating actin-receptor interactions, moving cargo along filaments or via pulling actions. Alternatively, actin depolymerization could establish a viscosity gradient to squeeze receptors inward. In this model, depolymerization regulates the local viscosity of the poroelastic cytoplasm, driving clusters towards depolymerizing activities where viscosity is reduced. A porosity or viscosity gradient would explain the observation that linking integrins into higher order complexes increases the extent to which they flow inward in synapses (61).
A railcar mechanism would seem to suggest that clusters couple to filament flow through actin-binding motifs. Intermittent release of cluster-actin interactions could modulate the rate at which receptors flow inward. In a viscosity reduction mechanism, TCRs need not be attached to actin microfilaments to flow. Actin microfilaments would corral TCRs, possibly enhancing interactions with signaling factors, until depolymerization released the cluster. Some biochemical data suggests that clusters attach directly to actin (62), but the issue is not resolved. These mechanisms, however, are not mutually exclusive, and might cooperate to move clusters or operate in different synapse regions. For example, retrograde flow of radially aligned microfilaments might pull pores of cytoplasmic material inward until the pocket reaches depolymerase activities, releasing the pockets’ contents.
A synapse/kinapse dichotomy versus a continuum of behaviors?
How to study the variations in cell arrest as compared during cell-cell contact? The generic term ‘synapse’ is both correct term to describe the closely juxtaposed interface, regardless of the level of movement or ‘symmetry’. One proposal has been to use the separate term ‘kinapse’ to define motile synapses (63). This separation aligns closely to our evolving understanding of the calcium signaling as not simply driving cells to stop but in helping to decelerate them (27, 40, 54). Only very high levels of antigen, beyond those that would accompany most non-pathological levels of activation, typically appear to be used in studies wherein the TCR signal drives cells to a complete absence of motility. To this extent, synapses that are motile to one level (those at lower concentrations of antigen) or another are perhaps the norm rather than the exception, and it may be that it has simply been easier technically to study the ‘immotile’ signaling synapse as compared to the motile ones. An application of an arbitrary cutoff of 2 or 2.5µm/min has recently been proposed as one definition of a distinct ‘synapse’ versus ‘kinapse’ activation (64), but there is no evident experimental basis for choosing this cutoff. It may be that, viewed over the continuum, that synapses and <2.5µm/min synapses as generated in vivo are a simple continuum of IS, with the position on the continuum simply related to signal strength. Certainly the actin-dependent processes underlying motile synapses at least account for the behavior of highly structured and arrested IS (40). As noted, motility rate is well correlated to peptide concentration and avidity (27, 40, 54). One possibility is that arrest mediated solely exceptional levels of TCR engagement lies at an extreme end of the activation spectrum. In that regard, centrally symmetric non-motile synapses modulated solely by TCR signaling might in fact be associated with high-dose suppression of T-cell activation (65).
Two things are clear then from the past five years of work in this area. First, motility arrest is not required for TCR signaling to take place (40, 64, 66). Second, T-cell arrest in vivo often does take place during priming due to antigens in the T-cell zone among antigen-presenting DCs and notably at times beyond those in which signaling is initiated (13, 67–69). There are mixed reports on whether activation regimens leading to anergy or activation do or do not induce differential degrees of arrest at these later stages (68–70). However, if TCR-driven activation is taking place prior to arrest, what more could arrest at later times provide? A conventional explanation is that T cells ramp up their signaling and/or integrate signal during serial engagements so that they ultimately are coerced into arresting their motility. It is proposed that large numbers of T cells in lymph nodes containing small amounts of antigen do not arrest due to competition and can be forced to arrest by addition of more antigen (71). However, it is not clear that arrest, when it occurs in vivo, is exclusively mediated by TCR signaling, and deceleration in general may be achieved by a summed TCR signaling, differentiation-induced change in cells responses to antigen and/or effects that condition the milieu to reduce motility.
In the following section, we discuss ‘secondary synapses’ as a class of interactions between already activated cells. In this light, profound T-cell deceleration and the associated ‘clustering’ of multiple T cells and other ‘primed’ cells in a small region of the lymph node or peripheral tissue, however it is achieved, favor ‘secondary immunological circuits’. These secondary circuits are conceived of as facilitated by confining cells and their cytokines to a smaller milieu and encouraging lateral interactions. To this extent, ‘primary’ activation, as is provided by APCs for T cells, is a mechanism that serves to ‘license’ secondary circuits.
Secondary immunological circuits
Recent advances in 2-photon imaging have permitted direct observation of T-cell behavior during an immune response in lymph nodes (LNs) and peripheral organs (72). Following recognition of their cognate antigen presented by a DC, primed T cells will change their migration pattern, slow down, and start interacting with a variety of cell types beyond DCs. In Table 1 and in this section, we provide an overview of the types of cell-cell contacts that occur in stages subsequent to T-cell priming, where they typically occur, and what is known about their function.
TABLE 1.
Currently defined secondary circuits driving the emergent behavior of an immune response.
| Secondary Circuit | Molecular Basis | Site of Interaction | Reference |
|---|---|---|---|
| T-B | LFA-1/ICAM-1 SAP/CD84 “Help” CD40/40L/ Cytokines |
LN: T-B Border | (18, 75, 76) |
| T-T | LFA-1/ICAM-1 IL2, Other cytokines? |
LN: T zone, Others? | (15, 84, 96) |
| CTL-APC or Th-APC (‘re-priming’) | LFA-1/ICAM-1/pMHC/B7 | Peripheral Sites? | (97, 98) |
| T-NK | OX40/OX40L 2B4/CD48 |
Undefined | (102, 119) |
| NK-NK | SLAMF… | Undefined | (105) |
| T-Basophil | Cytokine exchange | Lung, airway proximal | (109) |
| T-Mast cell | Unknown | Peripheral Sites? | (111). |
T-B
Interaction between CD4+ T cells and B cells is the secondary immunological circuit that has been the most studied. Interactions between activated T-cell lines and activated B-cell lines have in fact served as models for IS formation for many years (41, 49, 73, 74). However, it is now recognized that most initial TCR priming happens between DCs and naive T cells, often via initially motile synapse interactions.
T-B interactions typically take place starting around 24 h after initial T-cell priming. The interaction is facilitated by a coordinated downregulation of CXCR5 and upregulation of CCR7 in B cells such that these tend to move toward the T-cell zone (18). CD4+ T cells after priming can also upregulate CXCR5, becoming T-follicular helper (Tfh) cells, allowing them to migrate toward the B-cell zones. At the border between T and B-cell zones, T and B-cell contacts are mediated by the integrins LFA-1/ICAM-1 (75) but also critically rely on interactions mediated by the adapter SAP [signaling lymphocytic activating molecule (SLAM)-associated protein] and associated SLAM family members, notably CD84 (75, 76).
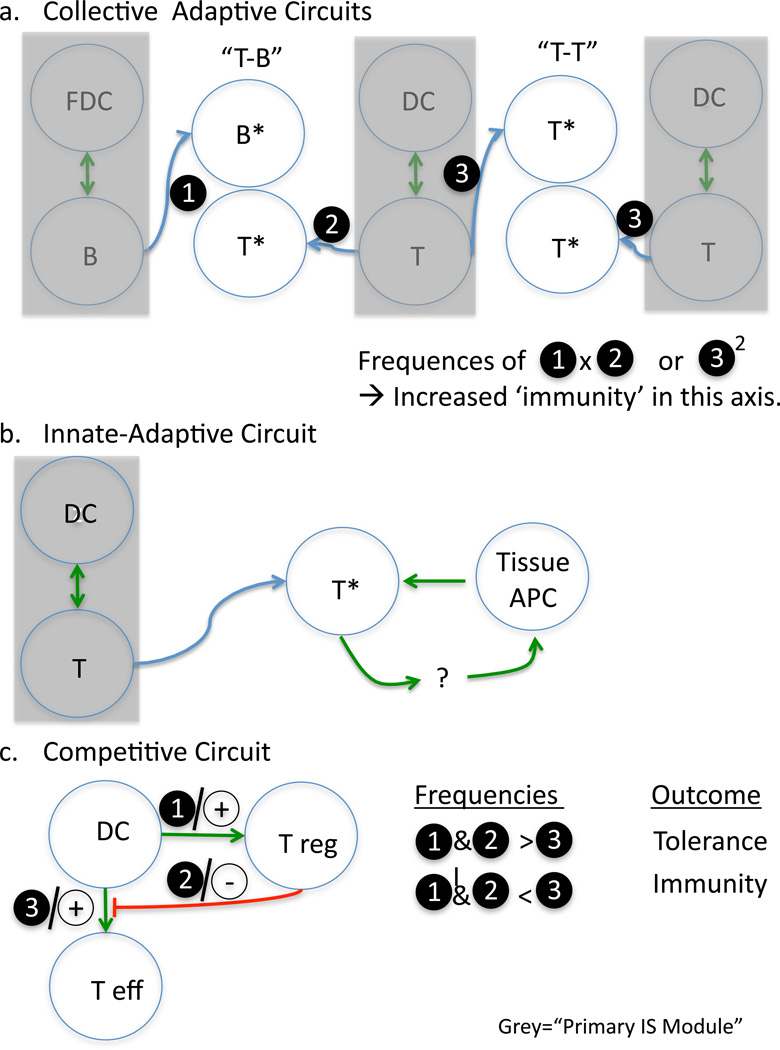
T-cell interaction with B cells is crucial for B-cell functions (77). T-cell help is typically provided by primed CD4+ T cells that upregulate CD40L, allowing them to engage CD40 on B cells at the T-B synapse (25). The molecule SAP is also a critical regulator, allowing T-cell help (78). While SAP-dependent ‘help’ for B cells has been proposed to be independent of cytokine release by T cells for uptake by B cells (79), cytokines such as interleukin-4 (IL-4) are nevertheless a critical resource that T cells can deliver in these secondary synapses. Interestingly, while T-B couples migrate during their interaction, it would appear that B cells are the most active in this process and apparently ‘lead’ T cells whose morphology appears largely round (18).This may suggest that particularly stable IS, as studied in vitro between T cells and B cells, may largely represent details of these interactions rather than those of T-DC interactions in the primary circuit. In Fig. 6A, we refer to T-B interactions in among a class of ‘collective’ circuits in which primed cells of the adaptive immune response may use coincidence detection and synaptic clustering to ‘collectively’ boost the overall response. As noted below, there are other examples of this in the immune system.
Fig. 6. Variations in ‘secondary circuits’.
Here we present cartoon version of circuits representing: (A) Collective responses in which simultaneously primed cells of different (T-B) or similar (T-T) members of the adaptive immune response form a circuit that results in the amplification or collective differentiation of the response. (B) Tissue repriming responses as an example of reciprocal stimulation of adaptive cells by innate and innate by adaptive. (C) Competitive circuits in which the flux through one circuit likely negates interactions in a second circuit.
T-T
Following recognition of their cognate antigen presented by a DC in LNs, T cells slow down and form long stable interactions with DCs (13, 14, 67, 80). During this ‘arrest phase’, sometimes called ‘Phase II’(13), several T cells are often found interacting with the same APC, forming clusters (81). Chemokines CCL3 and CCL4, produced by mature DCs and activated T cells, have been shown to attract other T cells to the sites of activation (82, 83), leading to concentration of cells in a particular region and perhaps augmenting this clustering effect. Although T-T interactions appear extremely stable in vitro, homotypic interactions between CD4+ T cells are short-lived in vivo (15). They rely on the integrins LFA-1 and ICAM-1 (84). Studies also suggested ADAM-15 (85) and the phosphatase PTP-PEST (86) as regulators of T-T interactions.
Homotypic T-cell interactions have been observed by many groups (15, 71, 87, 88) and have been considered a hallmark of T-cell activation, especially in vitro. However, the consequence of such interactions is still obscure. In vitro, T cells form synapses where increased localized IL-2 signaling complexes are found (15, 89), suggesting that T cells communicate and regulate each other through cytokine signaling. It has been proposed that CD4+ T cells regulate their expansion, activation, and differentiation (90, 91) through T-T interactions. Therefore, already differentiated T-helper 1 (Th1) or Th2 cells might participate in the skewing of newly activated CD4+ T cells through cytokine sharing at T-T synapses.
CD4+ and CD8+ T cells have been shown in some experiments to capture antigen pMHC complexes and mediate antigen-specific signaling to other CD8+ cells (92). In humans, antigen-specific CD4+ T-T interactions may regulate cell expansion following CD4+ T-cell upregulation of MHC class II expression (93). Cytotoxic CD8+ T cells can share TCRs in a contact-dependent manner, which facilitates virus control (94) and suggests that T-T interactions may help generate diversity. Finally, T cells interactions could also control the immune response, avoiding any inadequate response, as T cells can potentially kill each other through activation-induced cell death (95). The participation of regulatory T cells (Tregs) in clusters may also be another way to influence the number and activation phenotype of conventional T cells (96, see below).
T-APC (‘repriming’)
CTLs maintain cytotoxicity when engaging professional APCs bearing B7 molecules (97, 98), but CTL activity is rapidly lost in the absence of such repriming or in the absence of high concentrations of common γ chain cytokines such as IL-2 (98, 99). Thus, it is very likely that CTL-APC interactions at tissue sites serve to ‘re-prime’ these CTLs. This repriming module, though poorly characterized at present, again proceeds after the initial IS priming and relies on an appropriately activated DC or equivalent APC to be present near the target site. The details of these synapses in vivo as well as the circuitry whereby APCs are maintained in an upregulated state remain to be better identified.
In a similar manner, it is likely that CD4+ T cells also benefit from a repriming synapse in the lymph nodes and/or in the tissues. The T-B interaction module, just discussed, may represent the latter. The latter also likely involves cell types that present pMHC in non-lymphoid tissues. We recently used calcium imaging of CD4+ T cells in the lung in a model of asthma and found CD4+ T cells engaged in signaling during interaction with lung-resident DCs there (100). This observation implies a restimulation of sorts in this context, although the requirement for this interaction and the full consequences of it for the T cell remain largely unknown.
A repriming circuit may also result in reciprocal activation or modulation of the innate cells via the interaction with lymphocytes. Much of this remains to be studied in tissue sites but it has been observed, for example, that DCs in the pancreas are matured in response to T-cell infiltrates there, leading to the speculation that this occurs by a direct T-DC interaction (101).
T-NK
Although the bulk of this review concerns the adaptive arm of immunity, there is significant evidence that activated T cells and NK cells can form a secondary activating circuit, although it is not well characterized at the cell-biology level. One aspect of this, observed in vitro by Lanier and colleagues (102), is the selective upregulation of OX40 ligand on IL-2, IL-12, or IL-15-activated human NK cells following stimulation through NKG2D, the low affinity receptor for IgG (CD16) or killer cell Ig-like receptor 2DS2. CD16-activated NK cells can then costimulate TCR-induced proliferation and IFNγ production by autologous CD4+ T cells. The process is dependent upon expression of OX40 ligand and B7 by the activated NK cells (102). Activated NK cells can also enhance T-cell activation and proliferation in response to CD3 cross-linking and specific antigen through interactions between 2B4 (CD244) on NK cells and CD48 on T cells (103). Cantor and colleagues (104) demonstrated that interaction between the class Ib MHC molecule Qa-1-Qdm on activated T cells and NKG2A on NK protected activated CD4+ T cells from lysis by a subset of NKG2A+ NK cells and was essential for T-cell expansion and development of immunologic memory. The site of these interactions is presumably in the secondary lymphoid tissues, but at present, little study has been made of their interactions in situ.
NK-NK
Similar to T-T interactions, in vitro evidence has suggested critical activated NK cells crosstalk with one another, subsequent to their initial synapse and in a phase which apparently boosts killing and cytokine production (105). Like T-B interactions, these interactions are mediated, at least in part, via interactions of SLAM-family receptors 2B4/CD48 and CD2/CD58. The dynamics of NK-NK circuits in vivo and indeed a definition of the synapse between these cells remain to be established.
T-Basophil
While some data implied that basophils were APCs for T cells and therefore T-Basophil interactions would be primary modules, particularly in asthma (106, 107), more recent data suggest that they are not the primary APCs for T cells (108). Instead, data from Locksley and colleagues (109, 110) suggest basophils as secondary contacts in clusters in the secondary tissue (lung), perhaps facilitating further T-cell differentiation and/or basophil function.
T-mast cells?
Close localization between mast cells and T cells has been observed during a variety of T-cell-mediated inflammatory processes (111) and in inflamed allergic tissues (112). In vitro experiments suggest that contact between T cells and mast cells, mediated by LFA-1 and ICAM-1, are important for their reciprocal regulation. Contact between mast cells and activated T cells induces mast cells to release mediators, like histamine and TNFα (112, 113), but also cytokines and chemokines (114). Furthermore, a fraction of mast cells generated in vitro express mature MHC class II when primed with IFNγ and IL-4, allowing them to form an immunological synapse with Th cells in vitro (115). Although in vivo evidence is still missing, these data suggests that mast cells may serve as secondary APCs for T cells in situ.
Treg-T (or Treg-APC-Teff) and broadly regulatory modules
In considering these modules thus far, we have largely been referring to the ‘positive’ aspects of collective and repriming situations. Circuits may also drive the immune system into relatively inactive states or prevent differentiation of the effector response, and by far the most evident example of this ‘secondary circuit’ is the regulation of APC function by Treg cells (17, 116, 117), resulting in reduced priming or repriming of T cells. This circuit is of interest because it is likely to be both timing and frequency dependent for the outcome, since Treg and effector T cells (Teff) have competing outcomes and can each influence the DC in opposite ways. The ‘frequency versus outcome’ relationship described in Fig. 6C is at least supported by experiments; fewer Tregs are less effective but also are less effective if not administered sufficiently early (16). There are also likely to be ‘direct’ (T-T) type inhibitory circuits: both for one Th lineage to inhibit another but also likely for direct synaptic inhibition of Teff by Treg cells (118).
Perspectives: timing, milieu, and modeling the circuitry
How does immune circuitry integrate signals throughout the system, and what are the primary determinants of the emergent ‘response’ of the system to a complex stimulus such as infection or vaccination? While we are beginning to appreciate that timing of secondary circuits as well as the ‘mass action’ (or frequency) of flux through a given circuit are deterministic, many details remain to be resolved. Chief among these are to understand whether specific circuits are ‘more powerful’ than others and to determine whether timing of flux through a given circuit is critical to determine the overall outcome. Armed with this kind of information, vaccinations as well as interventions in immune diseases may prove more tractable—with interventions ‘designed’ to provide multiple and optimal triggering of the correct circuits at the correct time, via stimuli that enhance specific interactions while minimizing others.
Acknowledgements
This work was supported by grants from the American Asthma Foundation and NIH R01AI52116, U01CA141451, P01HL024136 (MFK). We thank all members of the current Krummel lab for fruitful discussions.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Cantor H, Gershon RK. Immunological circuits: cellular composition. Fed Proc. 1979;38:2058–2064. [PubMed] [Google Scholar]
- 2.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 3.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- 5.Davis MM, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 6.Lyons DS, et al. A TCR binds to antagonist ligands with lower affinities and faster dissociation rates than to agonists. Immunity. 1996;5:53–61. doi: 10.1016/s1074-7613(00)80309-x. [DOI] [PubMed] [Google Scholar]
- 7.Matsui K, Boniface JJ, Reay PA, Schild H, Fazekas de St.Groth B, Davis MM. Low affinity interactions of peptide-MHC complexes with T cell receptors. Science. 1991;254:1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- 8.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 9.Evavold BD, Allen PM. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 10.Felix NJ, et al. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8:388–397. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 11.Colf LA, et al. How a Single T Cell Receptor Recognizes Both Self and Foreign MHC. Cell. 2007;129:135–146. doi: 10.1016/j.cell.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 12.Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- 13.Mempel TR, Henrickson SE, von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 14.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 15.Sabatos CA, et al. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29:238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Q, Krummel MF. Imaging the function of regulatory T cells in vivo. Curr Opin Immunol. 2006 doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Tadokoro CE, et al. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–511. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada T, et al. Antigen-Engaged B Cells Undergo Chemotaxis toward the T Zone and Form Motile Conjugates with Helper T Cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 20.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 21.Norcross MA. A synaptic basis for T-lymphocyte activation. Ann Immunol (Paris) 1984;135D:113–134. doi: 10.1016/s0769-2625(84)81105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grakoui A, et al. The immunological synapse: A molecular machine that controls T cell activation. Science. 1999;285:221–226. [PubMed] [Google Scholar]
- 23.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A. 1999;96:15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 25.Boisvert J, Edmondson S, Krummel MF. Immunological synapse formation licenses CD40-CD40L accumulations at T-APC contact sites. J Immunol. 2004;173:3647–3652. doi: 10.4049/jimmunol.173.6.3647. [DOI] [PubMed] [Google Scholar]
- 26.Henrickson SE, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skokos D, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007 doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 28.Sims TN, et al. Opposing Effects of PKCtheta and WASp on Symmetry Breaking and Relocation of the Immunological Synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 29.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006 doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 30.Brossard C, et al. Multifocal structure of the T cell - dendritic cell synapse. Eur J Immunol. 2005;35:1741–1753. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 31.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 32.van der Merwe PA, McNamee PN, Davies EA, Barclay AN, Davis SJ. Topology of the CD2-CD48 cell-adhesion molecule complex: implications for antigen recognition by T cells. Curr Biol. 1995;5:74–84. doi: 10.1016/s0960-9822(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 33.Davis SJ, Ikemizu S, Evans EJ, Fugger L, Bakker TR, van der Merwe PA. The nature of molecular recognition by T cells. Nat Immunol. 2003;4:217–224. doi: 10.1038/ni0303-217. [DOI] [PubMed] [Google Scholar]
- 34.Shaw AS, Dustin ML. Making the T cell receptor go the distance: A topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- 35.Choudhuri K, Wiseman D, Brown MH, Gould K, van der Merwe PA. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 36.Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adapter moesin. Immunity. 2001;15:691–701. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- 37.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR Signaling from Geometrically Repatterned Immunological Synapses. Science. 2005;310:1191–1193. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 39.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:117–127. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 40.Beemiller P, Jacobelli J, Krummel MF. Integration of the movement of signaling microclusters with cellular motility in immunological synapses. Nat Immunol. 2012 doi: 10.1038/ni.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krummel MF, Sjaastad MD, Wülfing C, Davis MM. Differential Assembly of CD3ζ and CD4 During T cell Activation. Science. 2000;289:1349–1352. doi: 10.1126/science.289.5483.1349. [DOI] [PubMed] [Google Scholar]
- 42.Oddos S, et al. High-speed high-resolution imaging of intercellular immune synapses using optical tweezers. Biophys J. 2008;95:L66–L68. doi: 10.1529/biophysj.108.143198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnadieu E, Bismuth G, Trautmann A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr Biol. 1994;4:584–595. doi: 10.1016/s0960-9822(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 44.Negulescu PA, Krasieva TB, Khan A, Kerschbaum HH, Cahalan MD. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity. 1996;4:421–430. doi: 10.1016/s1074-7613(00)80409-4. [DOI] [PubMed] [Google Scholar]
- 45.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei SH, Safrina O, Yu Y, Garrod KR, Cahalan MD, Parker I. Ca2+ signals in CD4+ T cells during early contacts with antigen-bearing dendritic cells in lymph node. J Immunol. 2007;179:1586–1594. doi: 10.4049/jimmunol.179.3.1586. [DOI] [PubMed] [Google Scholar]
- 47.Gunzer M, et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 48.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci U S A. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 50.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee KH, et al. The Immunological Synapse Balances T Cell Receptor Signaling and Degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 54.Friedman RS, Beemiller P, Sorensen CM, Jacobelli J, Krummel MF. Real-time analysis of T cell receptors in naive cells in vitro and in vivo reveals flexibility in synapse and signaling dynamics. J Exp Med. 2010 doi: 10.1084/jem.20091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beal AM, et al. Kinetics of early T cell receptor signaling regulate the pathway of lytic granule delivery to the secretory domain. Immunity. 2009;31:632–642. doi: 10.1016/j.immuni.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001151. e1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown AC, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001152. e1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riedl J, et al. Lifeact mice for studying F-actin dynamics. Nat Methods. 2010;7:168–169. doi: 10.1038/nmeth0310-168. [DOI] [PubMed] [Google Scholar]
- 59.Riedl J, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci USA. 2009;106:12729–12734. doi: 10.1073/pnas.0902621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rozdial MM, Pleiman CM, Cambier JC, Finkel TH. pp56lck mediates TCR z chain binding to the microfilament cytoskeleton. J Immunol. 1998;161:5491–5499. [PubMed] [Google Scholar]
- 63.Dustin ML. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr Opin Cell Biol. 2007;19:529–533. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moreau HD, et al. Dynamic in situ cytometry uncovers T cell receptor signaling during immunological synapses and kinapses in vivo. Immunity. 2012;37:351–363. doi: 10.1016/j.immuni.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 65.Critchfield JM, et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science. 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 66.Friedman RS, Beemiller P, Sorensen CM, Jacobelli J, Krummel MF. Real-time analysis of T cell receptors in naive cells in vitro and in vivo reveals flexibility in synapse and signaling dynamics. J Exp Med. 2010;207:2733–2749. doi: 10.1084/jem.20091201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 69.Shakhar G, et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katzman SD, et al. Duration of antigen receptor signaling determines T-cell tolerance or activation. Proc Natl Acad Sci USA. 2010;107:18085–18090. doi: 10.1073/pnas.1010560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia Z, Pradelli E, Celli S, Beuneu H, Simon A, Bousso P. Competition for antigen determines the stability of T cell-dendritic cell interactions during clonal expansion. Proc Natl Acad Sci USA. 2007;104:4553–4558. doi: 10.1073/pnas.0610019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Germain RN, Robey EA, Cahalan MD. A decade of imaging cellular motility and interaction dynamics in the immune system. Science. 2012;336:1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kupfer A, Singer SJ, Janeway CA, Jr, Swain SL. Coclustering of CD4 (L3T4) molecule with the T cell receptor is induced by specific direct interaction of helper T cells and antigen-presenting cells. Proceeding of the National Accademy of Sciences, USA. 1987;84:5888–5892. doi: 10.1073/pnas.84.16.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monks CRF, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase CΘ during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 75.Cannons JL, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000;176:171–180. doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 78.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003;421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 79.Cannons JL, et al. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- 81.Bousso P, Robey E. Dynamics of CD8(+) T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 82.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 83.Hugues S, et al. Dynamic imaging of chemokine-dependent CD8+ T cell help for CD8+ T cell responses. Nat Immunol. 2007;8:921–930. doi: 10.1038/ni1495. [DOI] [PubMed] [Google Scholar]
- 84.Rothlein R, Springer TA. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J Exp Med. 1986;163:1132–1149. doi: 10.1084/jem.163.5.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Charrier L, et al. ADAM-15/metargidin mediates homotypic aggregation of human T lymphocytes and heterotypic interactions of T lymphocytes with intestinal epithelial cells. The J Biol Chem. 2007;282:16948–16958. doi: 10.1074/jbc.M700158200. [DOI] [PubMed] [Google Scholar]
- 86.Davidson D, Shi X, Zhong MC, Rhee I, Veillette A. The phosphatase PTP-PEST promotes secondary T cell responses by dephosphorylating the protein tyrosine kinase Pyk2. Immunity. 2010;33:167–180. doi: 10.1016/j.immuni.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 87.Bajenoff M, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hommel M, Kyewski B. Dynamic changes during the immune response in T cell-antigen-presenting cell clusters isolated from lymph nodes. J Exp Med. 2003;197:269–280. doi: 10.1084/jem.20021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doh J, Krummel MF. Immunological synapses within context: patterns of cell-cell communication and their application in T-T interactions. Curr Top Microbiol Immunol. 2010;340:25–50. doi: 10.1007/978-3-642-03858-7_2. [DOI] [PubMed] [Google Scholar]
- 90.Thummler K, Leipe J, Ramming A, Schulze-Koops H, Skapenko A. Immune regulation by peripheral suppressor T cells induced upon homotypic T cell/T cell interactions. J Leuk Biol. 2010;88:1041–1050. doi: 10.1189/jlb.0310122. [DOI] [PubMed] [Google Scholar]
- 91.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. Journal of immunology. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang J-F, et al. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 93.Helft J, et al. Antigen-specific T-T interactions regulate CD4 T-cell expansion. Blood. 2008;112:1249–1258. doi: 10.1182/blood-2007-09-114389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaudhri G, et al. T cell receptor sharing by cytotoxic T lymphocytes facilitates efficient virus control. Proc Natl Acad Sci USA. 2009;106:14984–14989. doi: 10.1073/pnas.0906554106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 96.Doh J, Kim M, Krummel MF. Cell-laden microwells for the study of multicellularity in lymphocyte fate decisions. Biomaterials. 2010;31:3422–3428. doi: 10.1016/j.biomaterials.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 97.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krummel MF, Heath WR, Allison J. Differential coupling of second signals for cytotoxicity and proliferation in CD8+ T cell effectors: Amplification of the lytic potential by B7. J Immunol. 1999;163:2999–3006. [PubMed] [Google Scholar]
- 99.Engelhardt JJ, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell. 2012;21:402–417. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thornton EE, et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Melli K, et al. Amplification of autoimmune response through induction of dendritic cell maturation in inflamed tissues. J Immunol. 2009;182:2590–2600. doi: 10.4049/jimmunol.0803543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 103.Assarsson E, et al. NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J Immunol. 2004;173:174–180. doi: 10.4049/jimmunol.173.1.174. [DOI] [PubMed] [Google Scholar]
- 104.Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim EO, Kim TJ, Kim N, Kim ST, Kumar V, Lee KM. Homotypic cell to cell cross-talk among human natural killer cells reveals differential and overlapping roles of 2B4 and CD2. J Biol Chem. 2010;285:41755–41764. doi: 10.1074/jbc.M110.137976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sokol CL, Chu N-Q, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. 2010;22:73–77. doi: 10.1016/j.coi.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hammad H, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sullivan BM, et al. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 111.Mekori YA, Metcalfe DD. Mast cell-T cell interactions. J Allergy Clin Immunol. 1999;104:517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 112.Gri G, et al. Mast cell: an emerging partner in immune interaction. Front Immunol. 2012;3:120. doi: 10.3389/fimmu.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Inamura N, Mekori YA, Bhattacharyya SP, Bianchine PJ, Metcalfe DD. Induction and enhancement of Fc(epsilon)RI-dependent mast cell degranulation following coculture with activated T cells: dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J Immunol. 1998;160:4026–4033. [PubMed] [Google Scholar]
- 114.Stopfer P, Mannel DN, Hehlgans T. Lymphotoxin-beta receptor activation by activated T cells induces cytokine release from mouse bone marrow-derived mast cells. J Immunol. 2004;172:7459–7465. doi: 10.4049/jimmunol.172.12.7459. [DOI] [PubMed] [Google Scholar]
- 115.Gaudenzio N, Espagnolle N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114:4979–4988. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- 116.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Serra P, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 118.Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 119.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. NK cell regulation of T cell-mediated responses. Mol Immunol. 2005;42:451–454. doi: 10.1016/j.molimm.2004.07.025. [DOI] [PubMed] [Google Scholar]