Summary
Background
The lack of an experimental animal model that can reliably mimic all stages of osteonecrosis of the femoral head has hindered progress toward the successful prevention and treatment of the disease.
Material/Methods
A goat model of osteonecrosis of the femoral head (ONFH) was established and observed from the early to the intermediate-to-late stage of mechanical failure. Absolute alcohol was injected slowly into the center of bilateral femoral heads in 12 adult Small Tail Han goats. Postoperatively, the femoral heads were harvested and examined using macrostructural and histological analyses and radiographic and MRI examinations at weeks 4, 8, 12, and 25.
Results
Macrostructural and radiographic examinations revealed that the contour of both femoral heads was deformed slightly at 12 weeks, but a contour deformation with joint space narrowing was observed at 25 weeks. Histologically, a strong concordance with the natural history of ONFH in humans was found. The present model demonstrated bone trabeculae, marrow necrosis, a reconstruction deficiency and destruction of the microcirculation.
Conclusions
Among quadrupedal models, the goat model of ONFH, which is induced by a single injection of absolute alcohol, may be suitable and valuable for the evaluation of various therapeutics and side effects in the treatment of ONFH.
Keywords: osteonecrosis of the femoral head, alcohol, animal model, goat, mechanical failure
Background
Osteonecrosis of the femoral head (ONFH) is diagnosed with increasing frequency in young adults and has a significant socioeconomic impact [1]. Despite recent diagnostic advances, effective treatments have been elusive, and the majority of ONFH cases ultimately result in a collapse of the femoral head. Most surgical therapies aim to prevent collapse of the subchondral bone, but the reported clinical results are inconsistent. Surgical methods and curative effects are difficult to define because the pathogenesis of ONFH is unknown [2,3]. The lack of an experimental animal model that can reliably mimic all stages of the human disease [4] has hindered progress toward the successful prevention and treatment of ONFH.
In the present study, absolute alcohol was injected into the center of bilateral femoral heads, which could histopathologically mimic the disorder from early to intermediate-to-late stages of mechanical failure. The purpose of this study was to provide an animal model to test potential surgical interventions and new therapeutic approaches.
Material and Methods
The local Animal Care and Use Committee approved the following experimental protocol. Sixteen adult female Small Tail Han goats (age, 24–36 months; weight, 50–65 kg) were used. Twelve goats were fasted for 12 h before surgery. The goats were premedicated with ketamine (10 mg/kg) intramuscularly 10 min before induction of anesthesia with pentobarbital sodium (30 mg/kg) intravenously. The goats were placed in the right lateral position. The left hip region was shorn, and the skin was cleaned. Next, the skin was disinfected, and the left limb was prepared using standard aseptic techniques. An 8 cm posterolateral arc incision was made in the region below the left greater trochanter while carefully protecting the surrounding soft tissue. Under X-ray guidance, a 5 to 5.5 cm tunnel was drilled with a 3 mm diameter Steinman pin into the center of the femoral head. The Steinman pin was withdrawn and replaced with a hollow tube. Ten milliliters of absolute alcohol (>99.7%, v/v) was injected slowly (0.5 ml/min) through the tube, and after 30 min, the hollow tube was removed. The foramen was sealed with bone wax (Ethicon). Finally, the surgical field was irrigated with warm saline solution, and the incision was closed in layers. The right hip was injected using a technique similar to that described for the left hip. Postoperatively, all animals were injected intramuscularly with penicillin as prophylaxis. During the postoperative period, the goats were allowed to roam freely in an indoor-outdoor enclosure. The other 4 Small Tail Han goats were used as normal controls (1 per time point). Three experimental goats and 1 control goat were killed at postoperative weeks 4, 8, 12, and 25.
Macroscopic examination
The surface of the cartilage and the bone-tunnel were observed after preparation of the samples following euthanization.
X-ray examination
The general contours of the femoral head and hip articulation were observed by anteroposterior pelvic imaging.
MRI examination
Bilateral acetabula and femoral head images of the experimental goats were obtained at week 25 using MR scanning with 5 mm cross and coronal sections (including T1W, T2W and STIR).
Histological analysis
The femoral heads were sectioned into 5 mm thick slices parallel to the femoral shaft. The tissue was fixed in 10% formalin solution for 1 week. The slices were then decalcified in 10% EDTA solution for 2 weeks and cut into 4 uniform parts. The samples were embedded in paraffin, and 5 μm thick sections were generated using a microtome. The sections were stained with hematoxylin-eosin (HE) and Masson trichrome and examined under a light microscope. The histological images were converted to grayscale images using a computer to calculate the percentage of empty lacunae and the expression rate of collagen fibers.
Statistical analysis
All numerical data are presented as the mean ± standard deviation (SD). The difference between the 2 groups was calculated with the Student t-test. Differences of multiple groups were calculated with one-way ANOVA (SAS Ver. 8.1). Values were considered significant when p<0.05.
Results
Macroscopic examination
At 4 and 8 weeks postoperatively, the articular surfaces and the femoral heads were intact. Osteonecrosis was observed around the drill track and demonstrated a decrease with an increase in distance to the tip of the drill track. At 12 weeks, 3 experimental goats were examined, and 5 articular surfaces demonstrated a local loss of luster. The defect of the articular cartilage was observed at 25 weeks (Figure 1).
Figure 1.
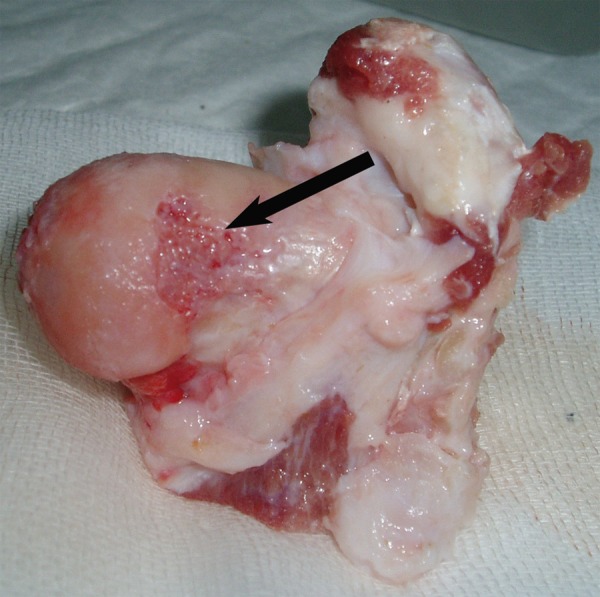
The contour of the femoral head was deformed at week 25. The joint surface was not smooth and cartilage was damaged.
X-ray examination
At week 4, 12 experimental goats were examined, and mild cystic degeneration was detected in 20 femoral heads. At 8 weeks, the bone mineral density of the 17 femoral heads had increased locally in the 9 experimental goats. At 12 weeks, 6 experimental goats were examined, and an inhomogeneous trabecular bone density was observed in all 12 femoral heads; however, slight contour changes were found in 8 heads. At 25 weeks, the last 3 experimental goats were examined, and a local collapse and narrowing of the hip joint space was observed in 4 femoral heads (Figure 2A, B).
Figure 2.
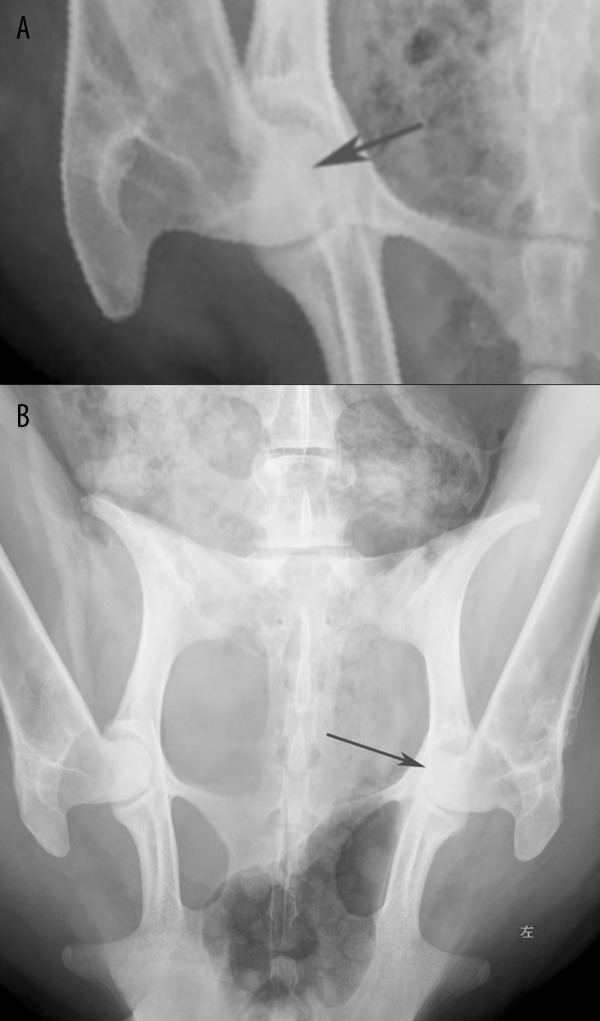
(A) X-ray examination showed slight cystic degeneration in the femoral head at week 4. However, the joint space was normal for both hips. (B) X-ray examination showed the femoral heads were not so round and narrowing of hip joint at week 25, which was more obvious at left as indicated.
MRI examination
The last 3 experimental goats were examined at week 25. In the 4 femoral heads, high signals were obtained for a narrow hip joint space, local contour collapse, and intra-articular effusion using T2-weighted and STIR imaging, and low signals were acquired using T1-weighted imaging (Figure 3).
Figure 3.
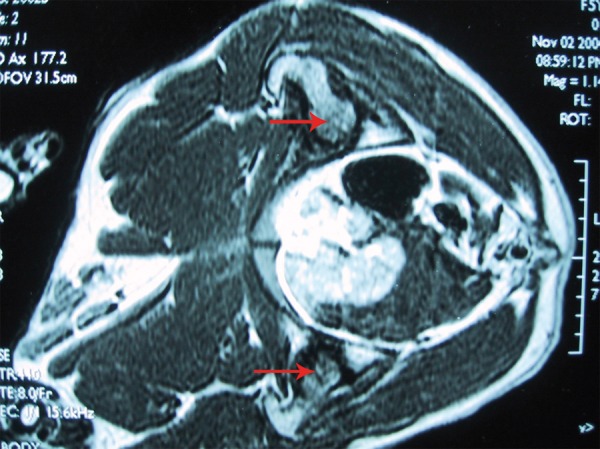
MRI showing bilateral femoral head osteonecrosis. The signal in the femoral head was characteristics of ongoing of necrosis and repairing.
Histological analysis
Four weeks
Many of the observed osteocytes were necrotic (>45% empty lacunae). Hematopoietic cells were absent, and a large number of erythrocytes had died in the marrow cavities. The nuclei of the swollen adipocytes had been lost. Sparse fibrous tissue and a modest amount of bone debris were observed in the marrow cavities, together with irregular trabecular resorption.
Eight weeks
In highly damaged necrotic areas (>60% empty lacunae), most of the marrow cavities were filled with fibrous tissue, which was characterized by a high level of cellularity with numerous macrophages. The necrotic trabecular bones had fractured. However, some new bone formation was detected and was surrounded peripherally by a large number of osteoprogenitor cells.
Twelve weeks
The percentage of empty lacunae had decreased slightly (>50%). In necrotic areas, the fibrous tissues had increased and thickened, and osteoclast proliferation and activation were observed. A number of new bones had formed, and the trabecular bones had become broader. However, the chondrocytes were dispersed and severely decreased in number.
Twenty-five weeks
The percentage of empty lacunae had decreased significantly (>35%). Massive amounts of trabecular bone had been absorbed. A large amount of bone debris and osteoclasts were detected in most of the marrow cavities, which were filled with dense fibrous tissues that exhibited a high level of cellularity with numerous fibrocytes. In the necrotic trabecular areas, active osteoblasts, bone debris and newly woven bone were present. Tenuous trabecular bone and microfractures were observed in the subchondral bone. The number of chondrocytes had decreased severely, and these cells were almost absent in the collapsed area (Figure 4A–E).
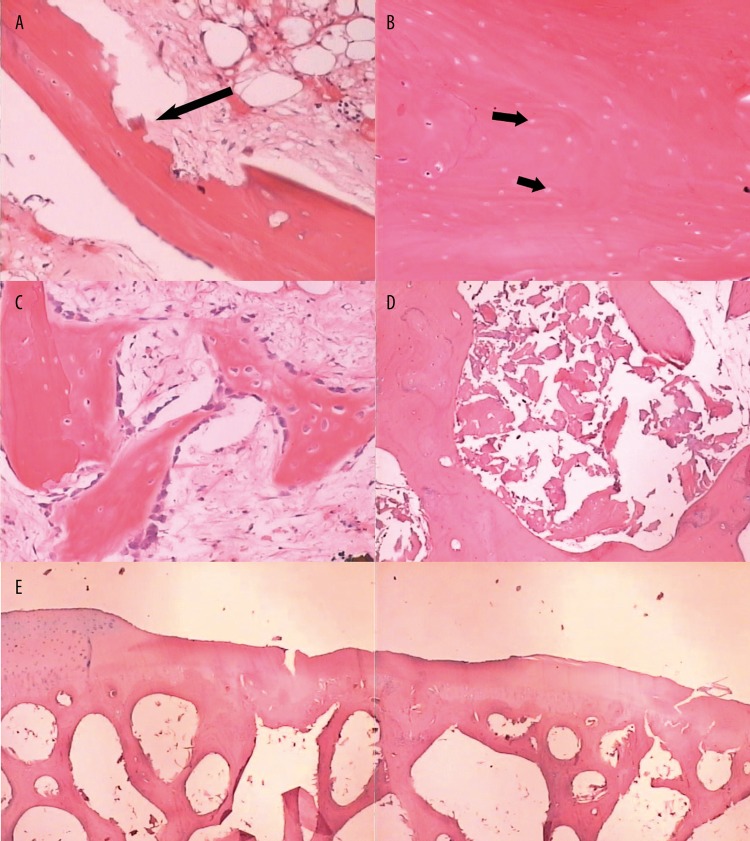
Figure 4.
(A) Histological specimen showed damaged trabecular resorption at week 4 (HE, 40×). (B) A number of the osteocyte-filled lacunae were replaced by empty lacunae at week 8 (HE, 100×). (C) Fibrous tissue with some new bone formed at week 12 (HE, 100×). (D) Massive bone fragmentation was showed in the marrow cavities at week 25 (HE, 100×). (E) Deformation of joint contour and irregularity of the subchondral bone and loss of chondrocytes was showed at week 25 (HE, 20×).
Percentage of empty lacunae and expression of fibrosis
Twelve sections each of the femoral heads were obtained from the experimental and control groups. In these sections, 500 lacunae were measured randomly under low magnification (100 ×), and the number of empty lacunae was determined. The total number of empty lacunae in the control groups was used to increase the sample size, because the time difference between the 4 control groups was not statistically significant (p=0.3281) (Table 1).
Table 1.
Percentage of empty lacunae in the experimental groups and the control group (x̄±s, CI).
| Group | Percentage of empty lacunae | Confidence Interval |
|---|---|---|
| Control group | 2.53±0.795 | 2.30~2.76 |
| 4 w | 47.23±3.492*,** | 45.02~49.45 |
| 8 w | 68.45±6.060*,** | 64.60~72.30 |
| 12 w | 54.94±5.648*,** | 51.35~58.53 |
| 25 w | 36.37±7.622*,** | 31.52~41.21 |
Difference between the experimental group and the total control group was statistically significant (p<0.0001);
difference between each experimental group was statistically significant (p<0.05).
The sections were prepared as described previously to analyze the fibrous tissue quantitatively using an image analyzing system (Axioplan 2 imaging microscope). The images were input into the computer (1200×, 1300×1030 pixels). Five random fields of view were assessed, and the mean value was determined. The numeric values for the 4 control groups were summed to increase the sample size since the time difference between the 4 control groups was not statistically significant (p=0.8485) (Table 2).
Table 2.
Percentage of fibrous tissue in the experimental groups and the control group (χ̄±s, CI).
| Group | Percentage of fibrosis | Confidence Interval |
|---|---|---|
| Control group | 4.93±2.410 | 4.23~5.63 |
| 4w | 39.61±9.474*,** | 33.59~45.63 |
| 8w | 46.35±6.979*,** | 41.93~50.77 |
| 12w | 49.64±6.909*,** | 45.26~54.02 |
| 25w | 57.05±7.588*,** | 52.23~61.87 |
Difference between the experimental group and the control group was statistically significant (p<0.0001);
difference between each experimental group was statistically significant (p<0.05), excluding the difference between the 8 week and the 12 week groups (p=0.2576).
Discussion
Osteonecrosis of the femoral head was classified clinically into 2 major types of the condition according to the etiology – traumatic and non-traumatic. The factors that determine the pathological mechanism of femoral head osteonecrosis include pathogenesis, biomechanics and the balanced relationship between bone resorption and reconstruction. Previous studies utilizing animal models have focused on quadrupeds, including dogs [5–7], rabbits [8,9], rats [10,11], sheep [12], swine [13–15], horses [16], and goats [17]. Investigations of bipeds have included chickens [18] and emus [19,20]. The techniques used for induction are extensive and have included steroids injection [14,18,21], hyperbaric exposure [12], endotoxins injection [22,23], deep freeze insults [24], vessel ligation [11], hypersensitivity reactions [17], hip luxation [25], and various combinations of these insults [24,25]. Nonetheless, a classic animal model of femoral head osteonecrosis is not available. The histological changes comprising the conspicuous features of osteonecrosis can be mimicked with relative ease. However, until recently, most quadrupeds have generally failed to progress to late stage femoral head collapse, and aggressive systemic insults have led to cachexia or death [26]. Goats were selected for the present study because their hip and vascular anatomy is similar to humans. Absolute alcohol induces cellular dehydration, protein coagulation, vasoconstriction, endothelial cell degeneration and thrombosis. Ethanol injections have been employed therapeutically (e.g., injected into bone metastases, autologous bone neoplasms or arteriovenous malformations) [27,28]. Due to the complete enzymatic degradation of ethanol, its toxic effects are limited to areas of high concentration and last only a short period. Li et al. [29] have employed ethanol injection to induce ONFH in a three-foot bearing canine model, and found subsequent resorption of necrotic trabeculae might weaken the structural properties of the femoral head via Micro-CT scanning. Reasonably, weight bearing plays an important role to result in the collapse of ONFH. Lameness, instability of the hip joint and subcapital fractures were not detected postoperatively. The experimental period was extended to 25 weeks to successfully mimic the progression of human disease from the early- to the end-stage of mechanical collapse.
Jones and Hungerford [30] have demonstrated that various etiological factors damage the microcirculation, which is a common pathological mechanism of femoral head osteonecrosis. Ethanol inactivates osteocytes directly and has toxic effects on adipocyte membranes that result in massive adipocyte swelling and a decline in the flow of the venous sinus blood. These processes directly destroy the microcirculation in the femoral head. Histopathology of the normal human femoral head has demonstrated that the percentage of osteocyte-filled lacunae correlates with age. Wong [31] has discovered that normal humans possess 92% viable osteocytes at 11–20 years of age, and the lost 8% might represent a sectioning artifact. Thereafter, osteocyte viability progressively decreases to 90% by age 30 and to 73–75% by age 40 or 50. Koo [32] considers the presence of more than 50% empty lacunae to indicate stage III (Arlet & Ficat) of ONFH. In the present study, the percentage of empty lacunae in the total control group was 2.53%, which was correlated to the natural preparation process or to normal phenomenon. However, the percentage of empty lacunae increased to 68.45% at postoperative week 8, which demonstrated that bone resorption played a predominant role. The percentage of empty lacunae and resorption and repair of bone debris occurred simultaneously at 12 and 25 weeks. Resorption might play a dominant role via the transformation of osteoprogenitor cells into osteocytes and the broadening of trabeculae in necrotic areas. Significant differences in the percentage of empty lacunae were determined between each experimental group. The percentage of empty lacunae increased at an early stage and then decreased via repair during the end stage of ONFH. These findings showed that the present animal model could successfully mimic the pathological changes observed in ONFH. In the present study, the percentage of empty lacunae was higher than that reported by Mannggold [33], most likely because the bilateral femoral heads were assessed, which resulted in an enhanced effect of weight bearing. The quantitative analysis of fibrous tissues revealed that repair of bone occurred after the induction of necrosis. Subsequently, the fibrous tissues increased in number and broadened, and they were transformed into woven bone in the necrotic area. The fibrous tissues were more mature at 25 weeks compared to 4 weeks, and this difference was statistically significant. This result indicates that the bone repair reaction was extensive. Finally, the subchondral trabeculae were fractured due to bone resorption and mechanical weight bearing, and the resorption process predominated over the repair process. In the present study, only some stages among the pathologic changes observed in ONFH were assessed; however, the pathological mechanism responsible for this condition remains unknown and requires further investigation.
Conclusions
Novel therapeutics for ONFH, including cytotherapy and physical therapy, has awaited satisfactory animal models for preclinical investigations [34,35]. The results of the current study are consistent with the ONFH changes observed in humans, and demonstrate the process of necrotic evolution. In this study, an animal model of ONFH based on histology is characterized, and this model might be suitable for the evaluation of novel therapeutic techniques in the treatment of ONFH.
Footnotes
Statement of conflict of interest
The authors declare that there are no conflicts of interest.
Source of support: This work was supported financially by the Shanghai Science Committee Foundation (Grant No.04JC14071)
References
- 1.Etienne G, Mont MA, Ragland PS. The diagnosis and treatment of nontraumatic osteonecrosis of the femoral head. Instr Course Lect. 2004;53:67–85. [PubMed] [Google Scholar]
- 2.Lieberman JR, Berry DJ, Mont MA, et al. Osteonecrosis of the hip: management in the 21st century. Instr Course Lect. 2003;52:337–55. [PubMed] [Google Scholar]
- 3.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–74. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Boss JH, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: The lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathal. 2003;40:345–54. doi: 10.1354/vp.40-4-345. [DOI] [PubMed] [Google Scholar]
- 5.da Luz PL, Forrester JS, Wyatt HL, et al. Myocardial reperfusion in acute experimental ischemia. Beneficial effects of prior treatment with steroids. Circulation. 1976;53:847–52. doi: 10.1161/01.cir.53.5.847. [DOI] [PubMed] [Google Scholar]
- 6.Jaecques SVN, Helsen JA, Mulier M, Mattheeuws D. Geometric analysis of the proximal medullary cavity of the femur in the German Shepherd dog. Vet Comp Orthop Traumatol. 1998;11:34–41. [Google Scholar]
- 7.Sophie P, Erik A, Didier M, et al. Geometric analysis of the proximal femur in a diverse sample of dogs. Res Vet Sci. 2006;80:243–52. doi: 10.1016/j.rvsc.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Vegter J, Lubsen CC. Fractional necrosis of the femoral head epiphysis after transient increase in joint pressure. An experimental study in juvenile rabbits. J Bone Joint Surg Br. 1987;69:530–35. doi: 10.1302/0301-620X.69B4.3611153. [DOI] [PubMed] [Google Scholar]
- 9.Kawai K, Tamaki A, Hirohata K. Steroid-induced accumulation of lipid in the osteocytes of the rabbit femoral head: a histochemical and electron microscopic study. J Bone Joint Surg Am. 1985;67:755–63. [PubMed] [Google Scholar]
- 10.Naito S, Ito M, Sekine I, et al. Femoral head necrosis in stroke-prone spontaneously hypertensive rats (SHRSPs) Bone. 1993;14:745–53. doi: 10.1016/8756-3282(93)90206-p. [DOI] [PubMed] [Google Scholar]
- 11.Vadasz Z, Misselevich I, Norman D, et al. Localization of vascular endothelial growth factor during the early reparative phase of the rats’ vessels deprivation-induced osteonecrosis of the femoral heads. Exp Mol Pathol. 2004;77:145–48. doi: 10.1016/j.yexmp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Lehner CE, Wilson MA, Dueland RT. Sheep model of dysbaric osteonecrosis in diverse and caisson workers. In: Urbaniak JR, Jones JP Jr, editors. Osteonecrosis: Etiology, Diagnosis, and Treatment. Chapter 20. Rosemont I1: AAOS; 1997. [Google Scholar]
- 13.Shapiro F, Connolly S, Zurakowski D, et al. Femoral head deformation and repair following induction of ischemic necrosis: A histologic and magnetic resonance imaging study in the piglet. J Bone Joint Surg Am. 2009;91:2903–14. doi: 10.2106/JBJS.H.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf D, Karen PW, Mathias HB, et al. Femoral head blood flow reduction and hypercoagulability under 24 h megadose steroid treatment in pigs. J Orthop Res. 2004;22:501–8. doi: 10.1016/j.orthres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Swiontkowski MF, Tepic S, Bahn BA, et al. The effect of fracture on femoral head blood flow. Osteonecrosis and revascularization studied in miniature swine. Acta Orthop Scand. 1993;64:196–202. doi: 10.3109/17453679308994570. [DOI] [PubMed] [Google Scholar]
- 16.Nasseri D, Hungerford D, White K, Jones L. Equine model of ischemic necrosis of bone. Transaction Orthop Res Soc. 1982:234. [Google Scholar]
- 17.Newton AS, Crawford CJ, Powers DL, Allen BL., Jr The immune goat as an animal model for Legg-Calve-Perthes disease. J Invest Surg. 1994;7:417–30. doi: 10.3109/08941939409016508. [DOI] [PubMed] [Google Scholar]
- 18.Cui Q, Wang GJ, Su CC, Balian G The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res. 1997;344:8–19. [PubMed] [Google Scholar]
- 19.Reed KL, Brown TD, Conzemius MG. Focal cryogen insults for inducing segmental osteonecrosis: computational and experimental assessments of thermal fields. J Biomech. 2003;36:1317–26. doi: 10.1016/s0021-9290(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 20.Conzemius MG, Brown TD, Zhang Y, Robinson RA. A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure. J Orthop Res. 2002;20:303–9. doi: 10.1016/S0736-0266(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissue: Corticosteroid-induced osteonecrosis on rabbits. Arthritis Rheum. 1997;40:2055–64. doi: 10.1002/art.1780401119. [DOI] [PubMed] [Google Scholar]
- 22.Sugioka Y, Hotokebuchi T, Tsutsui H. Transtrochanteric anterior rotational osteotomy for idiopathic and steroid-induced necrosis of the femoral head. Indications and long-term results. Clin Orthop Relat Res. 1992;277:111–20. [PubMed] [Google Scholar]
- 23.Kawamoto S, Shirai N, Strandberg JD, et al. Nontraumatic osteonecrosis: MR perfusion imaging evaluation in an experimental model. Acad Radiol. 2000;7:83–93. doi: 10.1016/s1076-6332(00)80455-9. [DOI] [PubMed] [Google Scholar]
- 24.Malizos KN, Darryl L, Quarles AV, et al. An experimental canine model of osteonecrosis: characterization of the repair process. J Orthop Res. 1993;11:350–57. doi: 10.1002/jor.1100110306. [DOI] [PubMed] [Google Scholar]
- 25.Nishino M, Matsumoto T, Nakamura K, Tomita K. Pathological and hemodynamic study in a new model of femoral head necrosis following traumatic dislocation. Arch Orthop Trauma Surg. 1997;116:259–62. doi: 10.1007/BF00390048. [DOI] [PubMed] [Google Scholar]
- 26.Blair WF, Brown TD, Greene ER. Pulsed ultrasound Doppler velocimetry in the assessment of microvascular hemodynamics. J Orthop Res. 1988;6:300–9. doi: 10.1002/jor.1100060219. [DOI] [PubMed] [Google Scholar]
- 27.Flandroy P, Pruvo JP. Treatment of mandibular arteriovenous malformations by direct transosseous puncture: report of two cases. Cardiovasc Intervent Radiol. 1994;17:222–25. doi: 10.1007/BF00571540. [DOI] [PubMed] [Google Scholar]
- 28.Stajcić Z. Effects of glycerol on the rat infraorbital nerve: an experimental study. Br J Oral Maxillofac Surg. 1991;29:90–93. doi: 10.1016/0266-4356(91)90088-m. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Zhang C, Zeng B. Changes in bone micro-architecture and bone mineral density following experimental osteonecrosis of the femoral head induced by local injection of ethanol in canine. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2008;22:281–89. [in Chinese] [PubMed] [Google Scholar]
- 30.Jones LC, Hungerford DS. The pathogenesis of osteonecrosis. Instr Course Lect. 2007;56:179–96. [PubMed] [Google Scholar]
- 31.Wong SY, Kariks J, Evans RA, et al. The effect of age on bone composition and viability in the femoral head. J Bone Joint Surg Am. 1985;67:274–83. [PubMed] [Google Scholar]
- 32.Koo KH, Kim R, Kim YS, et al. Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol. 2002;21:299–303. doi: 10.1007/s100670200078. [DOI] [PubMed] [Google Scholar]
- 33.Manggold J, Sergi C, Becker K, et al. A new animal model of femoral head necrosis induced by intraosseous injection of ethanol. Lab Anim. 2002;36:173–80. doi: 10.1258/0023677021912460. [DOI] [PubMed] [Google Scholar]
- 34.Yamasaki T, Yasunaga Y, Terayama H, et al. Transplantation of bone marrow mononuclear cells enables simultaneous treatment with osteotomy for osteonecrosis of the bilateral femoral head. Med Sci Monit. 2008;14(4):CS23–30. [PubMed] [Google Scholar]
- 35.Gao YS, Zhang CQ. Cytotherapy of osteonecrosis of the femoral head: a mini review. Int Orthop. 2010;34:779–82. doi: 10.1007/s00264-010-1009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]