Summary
Background
The anti-inflammatory effects of a homeopathic remedy, Traumeel S, have been observed in experimental and clinical studies; however, its antioxidant properties have not been elucidated. The aim of the present study was to evaluate the antioxidant effects of Traumeel S on peripheral blood neutrophils in patients with periodontitis.
Material/Methods
The study was performed using venous blood of 22 individuals with chronic periodontitis and 21 healthy subjects. The antioxidant effects of Traumeel S on the production of reactive oxygen species by unstimulated and stimulated with unopsonized E. coli neutrophils were investigated using luminol- and lucigenin-dependent chemiluminescence (CL).
Results
Polymorphonuclear leukocytes of periodontitis patients produced higher levels (p<0.01) of light output of lucigenin-dependent chemiluminescence and significantly reduced (p<0.01) light output of luminol-dependent chemiluminescence than analogous cells of healthy subjects. Highly diluted (10−4 of the stem solution) Traumeel S significantly (by approximately 50%) reduced superoxide-induced oxidation of lucigenin by unstimulated and stimulated with unopsonized E. coli polymorphonuclear leukocytes of periodontitis patients and had a tendency to intensify luminol-dependent chemiluminescence. Preincubation of the unstimulated and stimulated with unopsonized E. coli polymorphonuclear leukocytes of healthy subjects with Traumeel S exerts no inhibitory action on the luminol- and lucigenin-dependent chemiluminescence of the above-mentioned cells.
Conclusions
This study indicates that Traumeel S may significantly reduce production of superoxide anion by unstimulated and stimulated peripheral blood polymorphonuclear neutrophils of periodontitis patients.
Keywords: Traumeel S, luminol, lucigenin, chemiluminescence, neutrophils, superoxide anion
Background
Investigations have shown that periodontal diseases are chronic infectious disorders caused primarily by bacteria [1,2], but the presence of microorganisms alone is not the only factor responsible for periodontal destruction. The responses of the host to pathogenic bacteria are thought to be critically important [3].
Polymorphonuclear neutrophils (PMN) are the predominant leukocytes in the gingival pocket epithelium and the adjacent connective tissue, and they protect the host from potential pathogens [4]. The accumulation of these cells at the sites of inflammation is accompanied by modification of their activity/ability to release granule content and reactive oxygen species (ROS) [5]. Matthews et al [6] hypothesized that peripheral neutrophils in periodontitis also show both hyperreactivity to plaque organisms and hyperactivity in terms of the baseline, unstimulated generation and release of ROS.
The ROS are thought to be produced at both the plasma membrane and the phagolysosomal membrane, and consequently released into phagolysosomes and the extracellular environment [7], which contribute to killing of the bacteria [8]. Extracellular bacteria can also be killed, but principally by O2-dependent mechanisms [9]. The generation of ROS from stimulated neutrophils is thought to play an important role in host defense and tissue damage [10]. When neutrophils are activated on contact with soluble stimuli or by ingestion of foreign materials into phagosomes (phagocytosis), they initiate a “respiratory burst” by consuming molecular oxygen (O2), resulting in the formation of superoxide radical (O2−) via the action of plasma-membrane NADPH oxidase [11]. The function of NADPH oxidase in PMN is thus to provide ROS that kill organisms. Superoxide anion is important as the primary product for the neutrophil-induced generation of ROS, but it is quickly converted to H2O2 spontaneously or by superoxide dismutase (SOD) [11]. H2O2 is not an inherently reactive compound; however, H2O2 can be transformed into highly reactive and deleterious products: (i) the interactions of H2O2 with O2− or with trace levels of the transition metals can lead to formation of hydroxyl radicals (HO•) [12]; (ii) myeloperoxidase (MPO), a hydrogen peroxide oxidoreductase, which is specifically found in mammalian granulocytic leukocytes – including neutrophils, monocytes, basophils, and eosinophils – contributes considerably to the bactericidal capabilities of these cells via formation of hypochlorous acid (HOCl) from H2O2 and chlorine ions [13].
Although the responses of neutrophils to different stimulating conditions and pathogens are generally beneficial for host defense, they can also be deleterious to the host if these cells are inappropriately activated. In this sense, overproduction of free radicals and proteolytic enzymes used as defenses against bacteria and microorganisms can be highly toxic to the surrounding cells and tissues [14] and can cause periodontitis as well as cardiovascular and other diseases [15]. Genetic disorders can modify the host defense mechanisms or influence the homeostasis of the periodontium, thus increasing the patient’s susceptibility to periodontal disease [16].
Recently, efforts of many researchers have been focused on the free radical scavenging activity of plants [17–22], which are important components in many Chinese traditional medicines as well as in homeopathic medicine.
The cardinal principle on which the theory of homeopathic medicine is based is that of ‘similarity’, according to which a homeopathic remedy in a healthy subject will produce certain sets of symptoms, while the same remedy will cure similar sets of symptoms in unhealthy (sick) subjects [23]. Hahnemann’s theory withstands the test of time, and has been supported by scientific findings in an array of fields [24,25]. Homeopaths treat people based on genetic and personal health history, body type, and current physical, emotional, and mental symptoms. Treatments are “individualized”, or tailored to each person. Homeopathic remedies are derived from natural substances that come from plants, minerals, or animals.
A homeopathic complex medication, Traumeel S, has been sold over the counter in German, Austrian and Swiss pharmacies for over 50 years. It contains extracts from the following plants and minerals, all of them highly diluted (10−1–10−9 of the stem solution): Arnica montana, Calendula officinalis, Achillea millefolium, Matricaria chamomilla, Symphytum officinale, Atropa belladonna, Aconitum napellus, Bellis perennis, Hypericum perforatum, Echinacea angustifolia, Echinacea purpurea, Hamamelis virginiana, Mercurius solubilis Hahnemanni, and Hepar sulfuris. It has been found to be beneficial to humans suffering from a wide spectrum of pathological conditions, including trauma, inflammation, and degenerative processes [20,21]. Traumeel S has a wide range of indications, but its mode of action has been insufficiently studied [26].
The aim of the present study was to evaluate the effects of Traumeel S on oxidative function of peripheral blood neutrophils in vitro.
The yields of superoxide ions and other ROS can be measured by means of assessment of the lucigenin- and luminol-dependent chemiluminescence (CL) [27,28].
It has been proven [29] that the intensity of leukocyte chemiluminescence depends on the nature and concentration of substances stimulating these cells. Isolated PMN can be primed and activated in vitro by several classes of natural and synthetic compounds. Opsonized microorganisms, lipopolysaccharide (LPS), phorbol myristate acetate (PMA), and other chemical substances were most commonly used. In medical literature, we have found little data concerning the CL studies of peripheral blood leukocytes taken from severe periodontitis patients stimulated by unopsonized microorganisms. However, studies have shown that neutrophil leukocytes stimulated by unopsonized bacteria can produce large amounts of ROS [30]. It is possible that neutrophil leukocytes in the gingival crevice have contact with unopsonized microorganisms; hence, the response of PMN to unopsonized microorganisms can play a decisive role in initiation and development of periodontitis.
The luminol technique was used to detect total ROS generation (intra- and extracellular ROS levels) and lucigenin for measuring O2−-induced oxidation of lucigenin.
Material and Methods
Reagents
Luminol, lucigenin, Hank’s balanced salts solution and dimethyl sulfoxide (DMSO) were obtained from Sigma chemical Co. (St. Louis, Missouri, USA). Plastic vials and other disposable pieces of plastic ware were obtained from Carl Rot GmbH & Co KG (Karlsruhe, Germany). Traumeel S (water solution for injections) was obtained from the Biologische Heilmittel Heel GmbH (Baden-Baden, Germany).
Escherichia coli ATCC 25922 (E. coli) samples were grown in the Microbiological Laboratory, the Hospital of Kaunas University of Medicine. Specimens of E. coli culture for the investigations were used within 24 hours. The final E. coli concentration was 8×107 cells/mL.
Luminol and lucigenin were dissolved in dimethyl sulfoxide at 50 mM. Fresh working solutions of luminol and lucigenin in Hank’s balanced salt solution were prepared every day.
Fresh working solution of Traumeel S (1×10−2) in Hank’s balanced salt solution was also prepared every day. The final concentration of Traumeel S in the medium was set at 10−4 based on recommendations provided in literature [21,26].
Selection of patients
Patients (n=22) for the study were selected from a large number of individuals treated at the Department of Odontology, Kaunas University of Medicine. They were examined clinically and radiographically, and were diagnosed as having advanced periodontitis. For periodontal status evaluation, we used Russell’s [31] periodontal index (PI). The periodontitis patients had to meet the following inclusion criteria: minimum 18 remaining teeth; periodontal pocket depth ≥6mm on at least 2–3 teeth in quadrant; vertical and horizontal bone resorption visible on X-ray; missing teeth removed due to complications of periodontitis, as indicated in the medical history. Only the patients with very marked signs of periodontitis (PI >6) were included in our study (Table 1).
Table 1.
Demographic and clinical characteristics of the studied groups.
| Studied groups | Age (years) | Sex | PI index (points) | |
|---|---|---|---|---|
| Male | Female | |||
| Control group n=21 | 31.6±4.2 | 9 | 12 | 0.02±0.01 |
| Periodontitis group n=22 | 35.8±4.5 | 9 | 13 | 6.91±0.32 |
The control group consisted of 21 subjects with healthy periodontal tissues (no periodontal pockets and no bleeding on probing; sulcus gingivalis 1–2 mm); 2–3 teeth may be missing, but not due to complications of periodontitis.
Both groups were medically healthy, were non-smokers, not pregnant, and had used no medication (including anti-inflammatory drugs or vitamins) at least 2 months before the study. The age of the studied subjects ranged from 18 to 50 years.
All experiments were conducted in accordance with the rules and regulations approved by Kaunas Regional Bioethics Committee (approval Nr. BE-2-21). All subjects involved in this study signed the informed consent form approved by the Kaunas Regional Bioethics Committee.
Neutrophil preparation
Fifteen milliliters of venous blood were collected from the subjects by venipuncture, and anticoagulated with heparin (20 u./mL). We attempted to create conditions for neutrophil leukocytes similar to those in the gingival crevice. For this purpose, PMN were isolated from blood by applying the method proposed by Hirschhorn and Weissmann [32]. Leukocyte-rich plasma was obtained by spontaneous erythrocyte sedimentation from venous blood kept at 4°C for 90 min, and the supernatant was then gently removed. Cells were counted and morphologically evaluated using a hematological blood analyzer ADVIA 2120 (Siemens Healthcare Diagnostics, Dublin, Ireland).
Neutrophil leukocytes make a major contribution to the total CL response of the whole blood or isolated cell suspensions [33]; therefore, the intensity of CL induced by resting and activated neutrophil leukocytes can be calculated from the CL intensity of the total leukocyte fraction [34].
Lucigenin-dependent chemiluminescence assay
Lucigenin is extensively used to measure the production of reactive oxygen species by chemiluminescence. To detect O2−, lucigenin must first be reduced by 1 electron to produce the lucigenin cation radical [35], and the same biological system may reduce lucigenin and produce the O2−. The lucigenin cation radical then reacts with the biologically derived O2− to yield an unstable dioxetane intermediate. The lucigenin dioxetane decomposes to produce 2 molecules of N-methylacridone, 1 of which is in an electronically excited state, which upon relaxation to the ground state emits a photon [35]. Through sensitive measurement of the photon emission, the biological production of O2− can be monitored. Lucigenin is capable of penetrating into the cells and localizing in mitochondria, triggering a significant chemiluminescent response through interaction with the intermitochondrial superoxide [36]. Therefore, lucigenin-dependent CL kinetics is determined by 2 different processes: superoxide production through the respiratory burst activation, and superoxide production in mitochondria.
Thus, lucigenin-dependent CL still appears to be a valid probe for detecting O2− production by enzymatic and cellular sources [37]. In this method, the response to xanthine-xanthine oxidase presented a positive correlation with light measurement and did not show augmentation of chemiluminescence when myeloperoxidase (MPO) was added to the assay medium [27]. Also, neutrophil chemiluminescence induced by fMet-Leu-Phe (fMLP) is dose-dependently inhibited by the scavengers of superoxide anions, but not by azide, catalase, manitol, or taurine [38]. Superoxide anion release can also be assessed using a variety of methods, including the electron spin resonance technique [39], and the reduction of cytochrome C [40]. The electron spin resonance technique could not be applied due to a lack of the necessary equipment, while the reduction of cytochrome C is less sensitive than the lucigenin-dependent CL [28].
Procedure
For measuring the vial reaction, 2 mixtures each containing 1 mL of plasma with leukocytes (1×106 neutrophils) and lucigenin (50 μM) were prepared. One mixture was supplemented with 0.01 mL of Traumeel S (1×10−2), and the other – with 0.01 mL of buffer. The literature [26] indicates that Traumeel S is effective when diluted from 10−1 to 10−10. To investigate the activity of the working solution of Traumeel S, we conducted several tests, applying various levels of Traumeel S dilution. We selected the dilution of 10−4 because it had a marked effect on PMN superoxide anion production.
The samples were allowed to equilibrate to the desired temperature for 5 min, and chemiluminescence response of unstimulated PMN at 0 time point was measured. Immediately after the measurement, the system was activated by addition of unopsonized E. coli (8×107 cells/mL). The chemiluminescence response was monitored at regular 10 min intervals for up to 120 min post-stimulation at 37°C in a liquid-scintillation system Delta-300 (Tracor Analytic, Elk Grove, Illinois, USA) at the Department of Biochemistry, Kaunas University of Medicine. Activated PMN emit photons that can be detected during luminol- and lucigenin-dependent CL assay [41]. Light emission in cpm (counts per minute) was recorded at the beginning of the experiment to assess the ROS release from unstimulated leukocytes, and then for 120 min after stimulation.
The results shown were taken from 1 representative experiment out of 3 identical tests if the difference between 3 test results was slight (measured in single points), or from the mean value of identical experiments when the difference between the test results was marked (measured in tens of points).
Evaluation of luminol-based assays for the measurement of ROS production
Luminol is a chemical light amplifier. Since luminol can easily permeate the cellular membrane, luminol-dependent chemiluminescence reflects the total MPO-driven NADPH-oxidase activity [42], and the intensity of luminol-dependent CL depends on the concentration of all participants of the process – superoxide, hydroxyl radicals, peroxinitrite, H2O2 (in the presence of the peroxidase), and other compounds reacting with hypochlorite and antioxidants – as well as on peroxidase activity in the investigated biological systems [42].
Neutrophils were treated as described above in the presence of luminol (50 μM final concentration). Chemiluminescence was measured as described above.
Statistical methods
Experimental results are presented as mean values (±SD).
Data analysis was performed by applying statistical software PASW Statistics 18 and PASS 11. Differences between the groups were established by applying non-parametric one-way analysis of variance (the exact Kruskal-Wallis test). The power of the test was evaluated via modeling (PASS 11 procedure “One-Way analysis of Variance [Simulations]”). Multiple pair-wise comparisons (post-hoc) were conducted using Dunn’s test, whose power was also evaluated by modeling (PASS 11 procedure “Pair-Wise Multiple Comparisons [Simulations]”).
Sample sizes were determined by selecting type I error α=0.05 and type II error β=0.2. The application of these techniques showed that the required sample size should be not less than 16 measurements.
Results
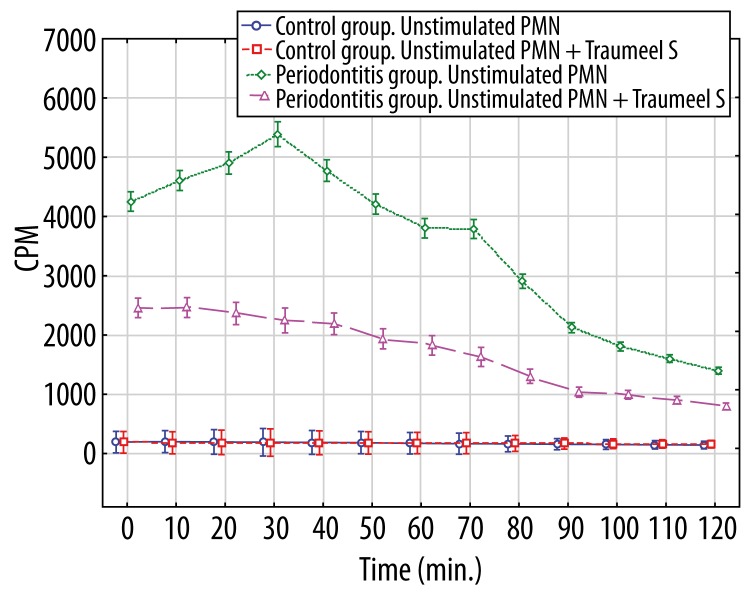
The measurement of neutrophil-burst was performed in 2 venous blood assays of periodontitis patients and controls. In the first assay, the neutrophil-burst was measured by chemiluminescence through monitoring the O2−-induced oxidation of lucigenin. Unstimulated neutrophils taken from patients produced higher levels (p<0.01 at all time points) of light output (Figure 1), compared to those from controls. Pre-incubation of unstimulated neutrophils of patients with Traumeel S in the patient group significantly (p<0.01 at all time points) inhibited light output from the baseline lucigenin-dependent chemiluminescence, while this had no effect (p>0.05 at all time points) on analogous neutrophils of the control group (Figure 1).
Figure 1.
Lucigenin-dependent chemiluminescence of 1×106 unstimulated polymorphonuclear leukocytes and unstimulated polymorphonuclear leukocytes affected by Traumeel S. * p>0.05 – between Control group unstimulated PMN and Control group unstimulated PMN+Traumeel S at all time points. p<0.01 – between Periodontitis group unstimulated PMN and Periodontitis group unstimulated PMN+Traumeel S at all time points; – between Periodontitis group unstimulated PMN and both Control groups at all time points; – between Periodontitis group unstimulated PMN+Traumeel S and both Control groups at all time points.
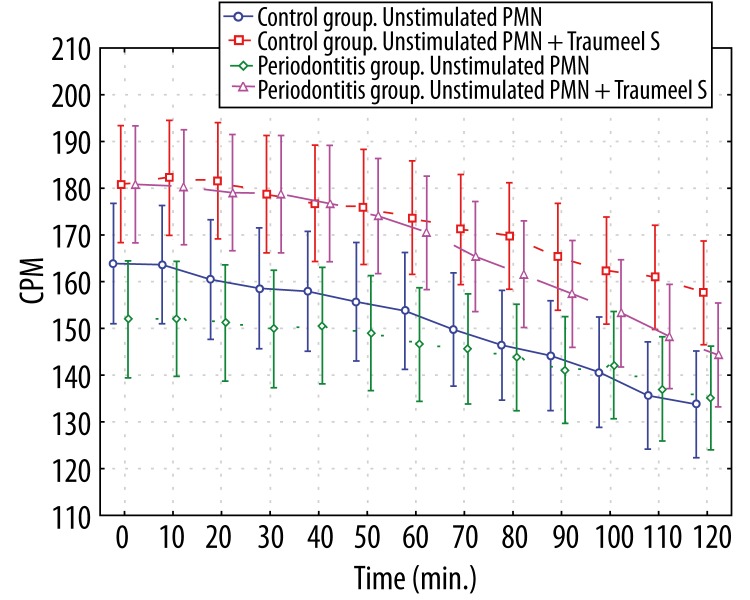
In the second assay, the neutrophil burst was measured by chemiluminescence through monitoring the ROS-induced oxidation of luminol. Luminol-dependent CL of unstimulated neutrophils did not differ significantly between patients and controls (p>0.05 at all time points, Figure 2).
Figure 2.
Luminol-dependent chemiluminescence of 1×106 unstimulated polymorphonuclear leukocytes and unstimulated polymorphonuclear leukocytes affected by Traumeel S. * p>0.05 – between all groups at all time points.
Pre-incubation of unstimulated neutrophils of patients and controls with medication Traumeel S had no effect on luminol-dependent chemiluminescence.
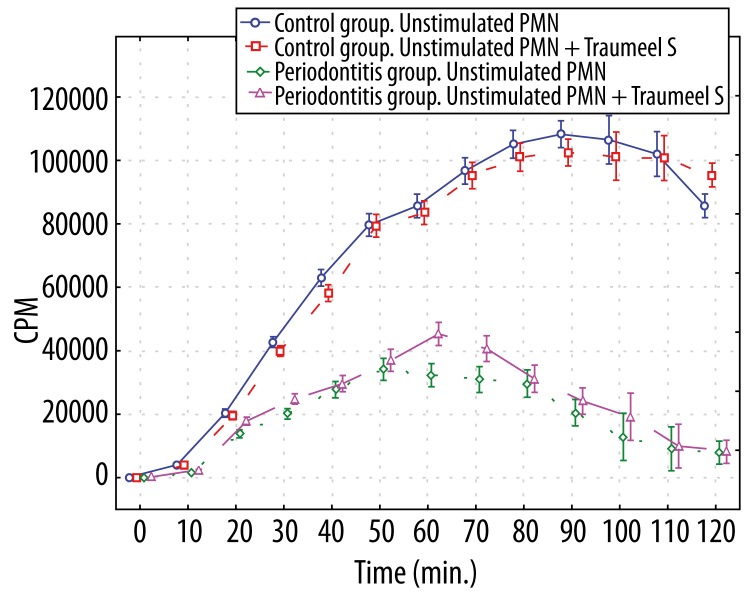
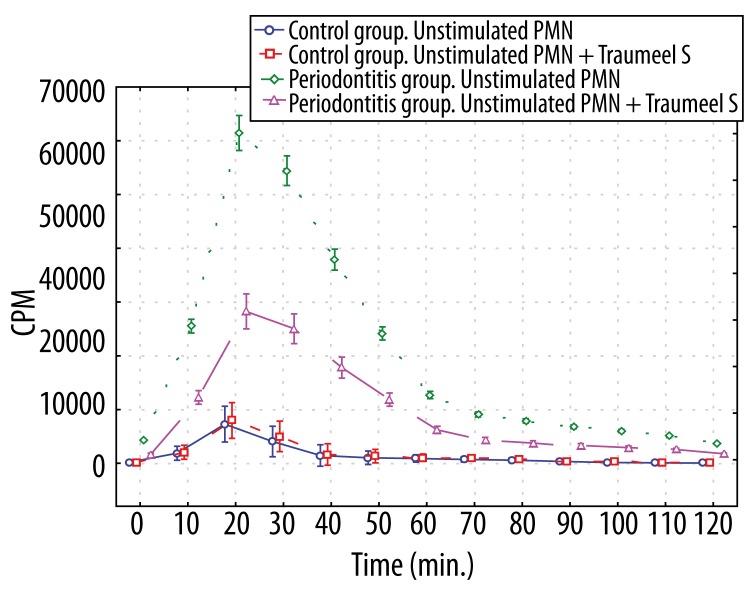
Stimulation of both patient and control neutrophils with unopsonized E. coli induced significantly increased luminol- and lucigenin-dependent chemiluminescence (p<0.01 at most time points). The level of luminol-dependent chemiluminescence of the control group neutrophils induced by unopsonized E. coli was higher (p<0.01 (30–120 min.)), compared to those in periodontitis patients (Figure 3), whereas lucigenin-dependent CL of stimulated periodontitis patient neutrophils produced significantly higher (p<0.01 at all time points) light output than those in controls (Figure 4).
Figure 3.
Luminol-dependent chemiluminescence of 1×106 polymorphonuclear leukocytes stimulated by unopsonized E. coli and polymorphonuclear leukocytes stimulated by unopsonized E. coli and affected by Traumeel S. *p>0.05 – between all groups 0–20 min; – between Periodontitis group stimulated PMN and Periodontitis group stimulated PMN+Traumeel S at all time points; – between Control group stimulated PMN and Control group stimulated PMN+Traumeel S at all time points. p<0.01 – between both Control groups and both Periodontitis groups (30–120 min).
Figure 4.
Lucigenin-dependent chemiluminescence of 1×106 polymorphonuclear leukocytes stimulated by unopsonized E. coli and polymorphonuclear leukocytes stimulated by unopsonized E. coli and affected by Traumeel S. * p>0.05 – between Control group stimulated PMN and Control group stimulated PMN+Traumeel S at all time points; p<0.01 – between Periodontitis group stimulated PMN and Periodontitis group stimulated PMN+Traumeel S (10–90 min); – between Periodontitis group stimulated PMN and both Control groups at all time points; – between Periodontitis group stimulated PMN+Traumeel S and both Control groups at all time points (10–90 min); p<0.05 – between Periodontitis group stimulated PMN and Periodontitis group stimulated PMN+Traumeel S (0, 100–120 min); – between Periodontitis group stimulated PMN+Traumeel S and both Control groups at all time points (0, 100–120 min).
Pre-incubation of the control group activated neutrophils with Traumeel S in the control group did not exert any inhibitory action (p>0.05) on luminol- and lucigenin-dependent chemiluminescence, whereas the preincubation with Traumeel S of analogous neutrophils from the periodontitis patients significantly (p<0.01 [10–90 min.], p<0.05 [0, 100–120 min.]) inhibited O2−-induced oxidation of lucigenin (Figure 4). The total light output of luminol-dependent CL recorded during the whole experiment was not significantly (p>0.05) greater than the analogous output without the action of Traumeel S.
Discussion
Histologic examination of gingivitis or periodontitis lesions revealed that the polymorphonuclear leukocytes appear to play a key role in the maintenance of periodontal health [4]. These cells line the junctional epithelium in large numbers, and appear to attempt to wall-off the underlying tissues from the bacterial biofilm. Their appearance is the result of the presence or generation of chemostatic factors in the gingival sulcus and underlying tissues [43]. Plaque bacteria are replete with chemostatic factors. They are further capable of generating chemostatic factors from plasma via activation of the complement, coagulation, fibrinolytic and kinin systems, or from surrounding cells following interactions with cellular receptors [44]. The accumulation of these cells at the sites of inflammation is accompanied by modification of their activity/ability to release granule content and reactive oxygen species [5]. Matthews et al [6] hypothesized that peripheral neutrophils in periodontitis also show both hyperreactivity to plaque organisms and hyperactivity in terms of the baseline, unstimulated generation and release of ROS.
The data obtained during the investigation of the oxidative function of the venous blood PMN taken from the periodontitis patients confirmed the hypothetic statement reported by the aforementioned authors. According to our lucigenin-dependent CL data, the PMN (both stimulated and non-stimulated) in patients with periodontitis generated significantly more superoxide anions (p<0.01) than the analogous cells of healthy subjects. The results of studies investigating the kinetics of superoxide production with unstimulated and stimulated PMN [6,45] are comparable with those obtained in the present study. Superoxide anion is the initial product that is generated by the reduction of oxygen via the activity of NADPH-oxidase. This process initiates a respiratory burst characterized by a marked increase in cellular oxygen consumption [11]. Thus, superoxide generation is a crucial step for neutrophil cytotoxic activity. However, it is necessary to emphasize that according to our data, the activated PMN of the periodontitis patients had significantly reduced (p<0.01) luminol-dependent CL.
The results of studies in the literature concerning this problem differ. Some authors report significantly increased [6] luminol-dependent CL of the venous blood activated PMN taken from periodontitis patients, whereas other authors [5] – especially those who investigated the oxidative function of the venous blood activated PMN in diabetes mellitus via luminol-dependent CL [46] – found it to be significantly decreased (p<0.01). The differences in the study results may be due the usage of different substances for the activation of the PMN, different methods applied for isolation of these cells, or other factors.
On the other hand, the active forms of oxygen (O2−, H2O2), abundantly produced by the activated PMN in periodontitis patients, can inactivate myeloperoxidase [47]. The data of the above-mentioned authors on increased luminol-dependent CL produced by the activated venous blood PMN taken from the periodontitis patients should be critically evaluated.
It is clear that NADPH oxidase-dependent generation of superoxide anions initiates a cascade resulting in the production of an array of ROS that mediate the bactericidal activity of PMN [11].
Although the ability to generate ROS is essential for optimal bactericidal activity of PMN, ROS do not discriminate between pathogens and host tissue, and induce injury to both [48]. Increasing evidence suggests that ROS comprise some of the most injurious substances released from cells and that they exert their deleterious effect through a variety of mechanisms, including lipid peroxidation and the modification of DNA, which can include the induction of strand breaks [49,50]. In addition, there is evidence that the oxidizing environment created by the generation of ROS can enhance PMN-derived protease activity, the latter of which is also injurious to host tissue [49]. The increasing awareness of the extent to which tissue injury is induced by activated PMN continues to generate interest in the development of therapeutics that can limit extracellular generation of ROS without impairing microbicidal activity of PMN against phagocytosed bacteria. Therefore, many researchers have tried to obtain bioactive substances that have cytoprotective ability against cellular oxidative damage, as well as ability to enhance antioxidant enzyme activities [51]. One of the most popular alternative medications in Germany is Traumeel S, where it is also used by conventional physicians (primarily orthopedists). Traumeel S, at dilutions of 10−1–10−7 from mother solutions, inhibits the secretion of the pro-inflammatory cytokines IL-1β and TNF-α, as well as the chemokine IL-8 from mobile human leukocytes and resident epithelial cells in vitro[26]. The results support the characterization of Traumeel S as an anti-inflammatory medication [26]. The data of our studies concerning the oxidative function of venous blood PMN taken from periodontitis patients under the influence of Traumeel S have shown that this homeopathic medication possesses antioxidant properties. Traumeel S at dilution of 10−4 prepared from the mother solutions very significantly decreased (p<0.01) lucigenin-dependent CL of PMN, directly reflecting the production of superoxide anions by the above-mentioned cells [38]. It is known [49] that increased intracellular superoxide concentrations are pro-inflammatory in neutrophils. On the one hand, our investigation data showed the antioxidant action of Traumeel S, whereas, on the other hand, the fact that Traumeel S reduced the amount of O2−, confirmed the reports of some authors [21,26] on the anti-inflammatory properties of this homeopathic medication.
In phagocytes, superoxide is mainly generated by the reaction of oxygen and NADPH through the NADPH oxidase complex [11]. In human PMN, the NADPH oxidase exhibits 3 different phenotypes – resting, primed, and activated – with their characteristic arrangement of membrane and cytosolic oxidase subunits. PMN of periodontitis patients are primed and generate higher amounts of ROS [52]. The production of O2− is dependent on translocation of the oxidase subunits [53], and multiple factors regulate its translocation and activity [11]. Attenuation of NADPH oxidase-dependent superoxide production may occur via blockage of PMN receptors or via inhibition of the membrane components of NADPH oxidase [54]. Superoxide can be also generated through the mitochondrial electron transport chain, xantine-xantine oxidase, and cytochrome P450 [55]. Changes in the mitochondrial generation of superoxide can be assessed by the addition of mitochondrial uncouplers that are depolarizing agents and inhibitors of the respiratory chain [56]. The mechanisms of action of Traumeel S on the generation of superoxide remain unknown.
It is also unclear whether only 1 of its components is biologically active or whether the effects are due to the action of several components. The marked effects seen in this study were achieved using a solution of Traumeel S containing ingredients at very low concentrations. Some of the ingredients of Traumeel S are regarded by homeopaths as remedies with anti-inflammatory properties (Belladonna, Aconitum, Hepar, and Chamomilla) or mucoprotective properties (Calendula and Hamamelis). Arnica is one of the main remedies used in homeopathic treatment of trauma. Arnica, Calendula, Hamamelis and Millefolium are believed to have antihemorrhagic properties. Echinacea angustifolia and Echinacea purpurea are thought to be immunostimulatory. Hypericum has been used in patients with neural injury [21]. This suggests that several components may play a role in the mechanism of action of Traumeel S. Indeed, the observation that such a strong response is associated with such small quantities of the different remedies in Traumeel S suggests that a synergistic effect may be involved [26].
The current results should challenge researchers to study the indicated anti-activating, anti-inflammatory and anti-oxidative potential of Traumeel S, both in vitro and in vivo, in animal models of inflammation and antioxidant status. If such studies corroborate the findings presented in the current work, this would be strong support for the use of Traumeel S and possibly related medications as conventional therapeutic modalities administered to patients suffering from chronic or acute inflammatory disorders.
Additional clinical studies on how long the antioxidant effect of Traumeel S lasts in vivo would help to devise schemes for the treatment and prevention of oxidative stress.
Conclusions
Under the present experimental conditions, the homeopathic medication Traumeel S was shown to attenuate NADPH oxidase-dependent superoxide anion production in unstimulated and stimulated polymorphonuclear leukocytes of periodontitis patients, and showed no effect on superoxide anion production in analogous leukocytes of the control group subjects.
However, these findings need to be tested with long-term studies to characterize a dose-dependent response to this medication.
Footnotes
Source of support: Departmental sources
References
- 1.Kinane DF, Lappin DF. Clinical, pathological and immunological aspects of periodontal disease. Acta Odontol Scand. 2001;59(3):154–60. doi: 10.1080/000163501750266747. [DOI] [PubMed] [Google Scholar]
- 2.Albandar JM. Global risk factors and risk indicators for periodontal diseases. Periodontol 2000. 2002;29:177–206. doi: 10.1034/j.1600-0757.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- 3.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74(1):66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 4.Miyasaki KT. The neutrophil: mechanisms of controlling periodontal bacteria. J Periodontol. 1991;62(12):761–74. doi: 10.1902/jop.1991.62.12.761. [DOI] [PubMed] [Google Scholar]
- 5.Biasi D, Bambara LM, Carletto A, et al. Neutrophil migration, oxidative metabolism and adhesion in early onset periodontitis. J Clin Periodontol. 1999;26(9):563–68. doi: 10.1034/j.1600-051x.1999.260901.x. [DOI] [PubMed] [Google Scholar]
- 6.Matthews JB, Wright HJ, Roberts A, et al. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol. 2007;147(2):255–64. doi: 10.1111/j.1365-2249.2006.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi A, Shimizu A, Hashimoto T, et al. Effect of neutrophil activating substances on intracellular generation of phagocyte chemiluminescence by means of luminol-bound microspheres. Int J Tissue React. 1988;10(3):169–75. [PubMed] [Google Scholar]
- 8.Klebanoff SJ. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980;93(3):480–89. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- 9.Weiss J, Kao L, Victor M, Elsbach P. Oxygen-independent intracellular and oxygen-dependent extracellular killing of Escherichia coli S15 by human polymorphonuclear leukocytes. J Clin Invest. 1985;76(1):206–12. doi: 10.1172/JCI111947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang KD, Hill HR. Neutrophil function disorders: pathophysiology, prevention, and therapy. J Pediatr. 1991;119(3):343–54. doi: 10.1016/s0022-3476(05)82044-x. [DOI] [PubMed] [Google Scholar]
- 11.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16(1):42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridge JM. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 13.Xia Y, Zweier JL. Measurement of myeloperoxidase in leukocyte-containing tissues. Anal Biochem. 1997;245(1):93–96. doi: 10.1006/abio.1996.9940. [DOI] [PubMed] [Google Scholar]
- 14.Pelletier M, Roberge CJ, Gauthier M, et al. Activation of human neutrophils in vitro and dieldrin-induced neutrophilic inflammation in vivo. J Leukoc Biol. 2001;70(3):367–73. [PubMed] [Google Scholar]
- 15.Misra MK, Sarwat M, Bhakuni P, et al. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15(10):RA209–19. [PubMed] [Google Scholar]
- 16.Pizzo G, Lo Re D, Piscopo MR, et al. Genetic disorders and periodontal health: A literature review. Med Sci Monit. 2009;15(8):RA167–78. [PubMed] [Google Scholar]
- 17.Gabrieli CN, Kefalas PG, Kokkalou EL. Antioxidant activity of flavonoids from Sideritis raeseri. J Ethnopharmacol. 2005;96(3):423–28. doi: 10.1016/j.jep.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 18.Tsao R, Yang R, Xie S, et al. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J Agric Food Chem. 2005;53(12):4989–95. doi: 10.1021/jf048289h. [DOI] [PubMed] [Google Scholar]
- 19.Oh PS, Lim KT. Plant originated glycoprotein has anti-oxidative and anti-inflammatory effects on dextran sulfate sodium-induced colitis in mouse. J Biomed Sci. 2006;13(4):549–60. doi: 10.1007/s11373-006-9083-9. [DOI] [PubMed] [Google Scholar]
- 20.Zenner S, Weiser M. Oral Treatment of Traumatic, Inflammatory, and Degenerative Conditions with a Homeopathic Remedy. Biomedical Therapy. 1997;15(1):22–26. [Google Scholar]
- 21.Oberbaum M, Yaniv I, Ben-Gal Y, et al. A randomized, controlled clinical trial of the homeopathic medication Traumeel S in the treatment of chemotherapy-induced stomatitis in children undergoing stem cell transplantation. Cancer. 2001;92(3):684–90. doi: 10.1002/1097-0142(20010801)92:3<684::aid-cncr1371>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Broncel M, Kozirog M, Duchnowicz P, et al. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med Sci Monit. 2010;16(1):CR28–34. [PubMed] [Google Scholar]
- 23.Bellavite P, Ortolani R, Pontarollo F, et al. Immunology and Homeopathy. 5. The Rationale of the ‘Simile’. Evid Based Complement Alternat Med. 2007;4(2):149–63. doi: 10.1093/ecam/nel117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellavite P, Ortolani R, Pontarollo F, et al. Immunology and homeopathy. 4. Clinical studies – part 1. Evid Based Complement Alternat Med. 2006;3:293–301. doi: 10.1093/ecam/nel045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellavite P, Ortolani R, Pontarollo F, et al. Immunology and homeopathy. 4. Clinical studies – part 2. Evid Based Complement Alternat Med. 2006;3:397–409. doi: 10.1093/ecam/nel046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porozov S, Cahalon L, Weiser M, et al. Inhibition of IL-1beta and TNF-alpha secretion from resting and activated human immunocytes by the homeopathic medication Traumeel S. Clin Dev Immunol. 2004;11(2):143–49. doi: 10.1080/10446670410001722203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens P, Hong D. The role of myeloperoxidase and superoxide anion in the luminol- and lucigenin-dependent chemiluminescence of human polymorphonuclear leukocytes. Fed Proc. 1982;45:273. [Google Scholar]
- 28.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232(1–2):3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 29.Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immunol. 2001;99(1):7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 30.Ohkusu K, Isobe K, Hidaka H, Nakashima I. Elucidation of the protein kinase C-dependent apoptosis pathway in distinct subsets of T lymphocytes in MRL-lpr/lpr mice. Eur J Immunol. 1995;25(11):3180–86. doi: 10.1002/eji.1830251129. [DOI] [PubMed] [Google Scholar]
- 31.Russell AL. Epidemiology of periodontal disease. Int Dent J. 1967;17(2):282–96. [PubMed] [Google Scholar]
- 32.Hirschhorn R, Weissmann G. Isolation and properties of human leukocyte lysosomes in vitro. Proc Soc Exp Biol Med. 1965;119:36–39. doi: 10.3181/00379727-119-30091. [DOI] [PubMed] [Google Scholar]
- 33.Lindena J, Burkhardt H. Separation and chemiluminescence properties of human, canine and rat polymorphonuclear cells. J Immunol Methods. 1988;115(1):141–47. doi: 10.1016/0022-1759(88)90321-3. [DOI] [PubMed] [Google Scholar]
- 34.Korkina LG, Samochatova EV, Maschan AA, et al. Release of active oxygen radicals by leukocytes of Fanconi anemia patients. J Leukoc Biol. 1992;52(3):357–62. doi: 10.1002/jlb.52.3.357. [DOI] [PubMed] [Google Scholar]
- 35.Faulkner K, Fridovich I. Luminol and lucigenin as detectors for O2–. Free Radic Biol Med. 1993;15(4):447–51. doi: 10.1016/0891-5849(93)90044-u. [DOI] [PubMed] [Google Scholar]
- 36.Chen H, Cangello D, Benson S, et al. Age-related increase in mitochondrial superoxide generation in the testosterone-producing cells of Brown Norway rat testes: relationship to reduced steroidogenic function? Exp Gerontol. 2001;36(8):1361–73. doi: 10.1016/s0531-5565(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Zhu H, Kuppusamy P, et al. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J Biol Chem. 1998;273(4):2015–23. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 38.Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods. 1987;97(2):209–13. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- 39.Rosen GM, Freeman BA. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci USA. 1984;81(23):7269–73. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993;91(6):2546–51. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheson BD, Christensen RL, Sperling R, et al. The origin of the chemiluminescence of phagocytosing granulocytes. J Clin Invest. 1976;58(4):789–96. doi: 10.1172/JCI108530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Helldén L, Ericson T, Lindhe J. Neutrophil chemotactic substances in different fractions of soluble dental plaque material. Scand J Dent Res. 1973;81(4):276–84. doi: 10.1111/j.1600-0722.1973.tb00593.x. [DOI] [PubMed] [Google Scholar]
- 44.Schenkein HA, Berry CR. Activation of complement by Treponema denticola. J Dent Res. 1991;70(2):107–10. doi: 10.1177/00220345910700020201. [DOI] [PubMed] [Google Scholar]
- 45.Matthews JB, Wright HJ, Roberts A, et al. Neutrophil hyper-responsiveness in periodontitis. J Dent Res. 2007;86(8):718–22. doi: 10.1177/154405910708600806. [DOI] [PubMed] [Google Scholar]
- 46.Sadzeviciene R, Zekonis J, Zekonis G, Paipaliene P. Oxidative function of neutrophils in periodontitis patients with type 1 diabetes mellitus. Medicina (Kaunas) 2006;42(6):479–83. [PubMed] [Google Scholar]
- 47.Edwards SW, Nurcombe HL, Hart CA. Oxidative inactivation of myeloperoxidase released from human neutrophils. Biochem J. 1987;245(3):925–28. doi: 10.1042/bj2450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 50.Hampton MB, Winterbourn CC. Modification of neutrophil oxidant production with diphenyleneiodonium and its effect on bacterial killing. Free Radic Biol Med. 1995;18(4):633–39. doi: 10.1016/0891-5849(94)00181-i. [DOI] [PubMed] [Google Scholar]
- 51.Nosál’ová V, Bobek P, Cerná S, et al. Effects of pleuran (beta-glucan isolated from Pleurotus ostreatus) on experimental colitis in rats. Physiol Res. 2001;50(6):575–81. [PubMed] [Google Scholar]
- 52.Iwata T, Kantarci A, Yagi M, et al. Ceruloplasmin induces polymorphonuclear leukocyte priming in localized aggressive periodontitis. J Periodontol. 2009;80(8):1300–6. doi: 10.1902/jop.2009.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheppard FR, Kelher MR, Moore EE, et al. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. Leukoc Biol. 2005;78(5):1025–42. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 54.Harrison CB, Drummond GR, Sobey CG, Selemidis S. Evidence that nitric oxide inhibits vascular inflammation and superoxide production via a p47phox-dependent mechanism in mice. Clin Exp Pharmacol Physiol. 2010;37(4):429–34. doi: 10.1111/j.1440-1681.2009.05317.x. [DOI] [PubMed] [Google Scholar]
- 55.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70(2):200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 56.Kovacic P, Pozos RS, Somanathan R, et al. Mechanism of mitochondrial uncouplers, inhibitors, and toxins: focus on electron transfer, free radicals, and structure-activity relationships. Curr Med Chem. 2005;12(22):2601–23. doi: 10.2174/092986705774370646. [DOI] [PubMed] [Google Scholar]