Abstract
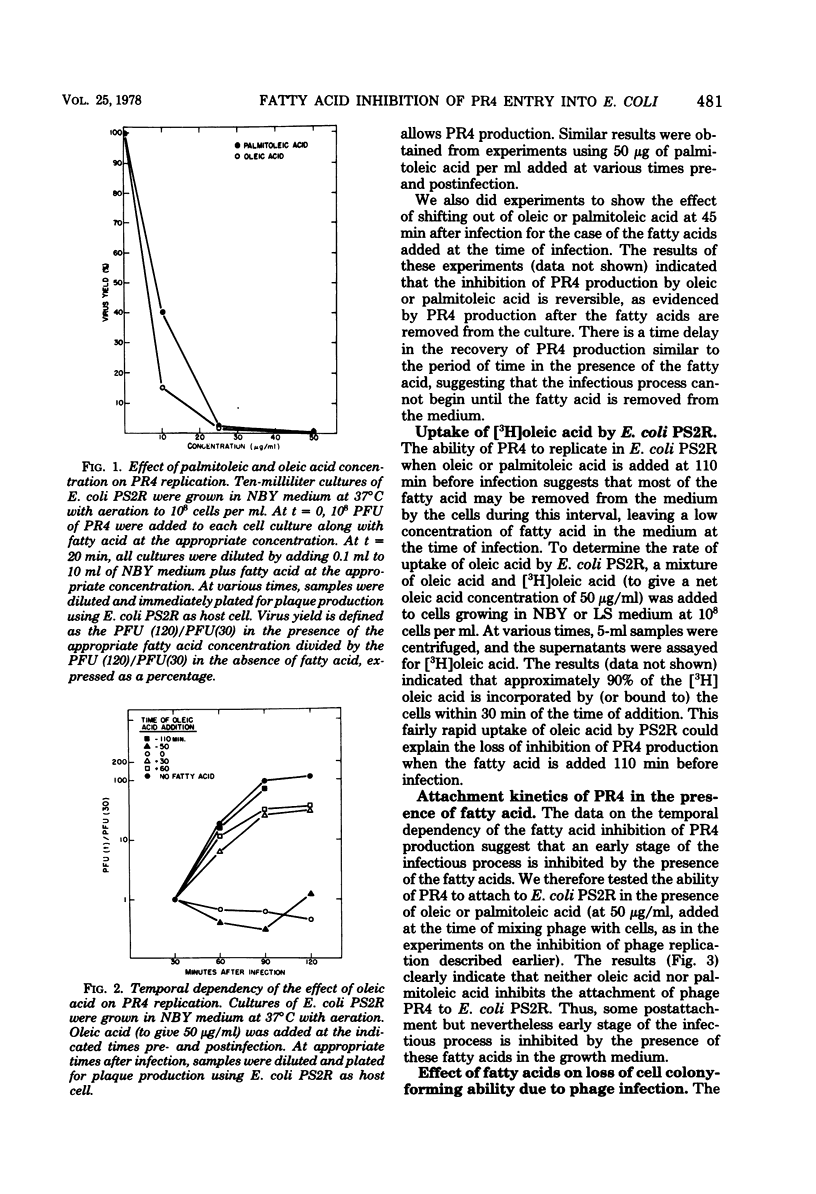
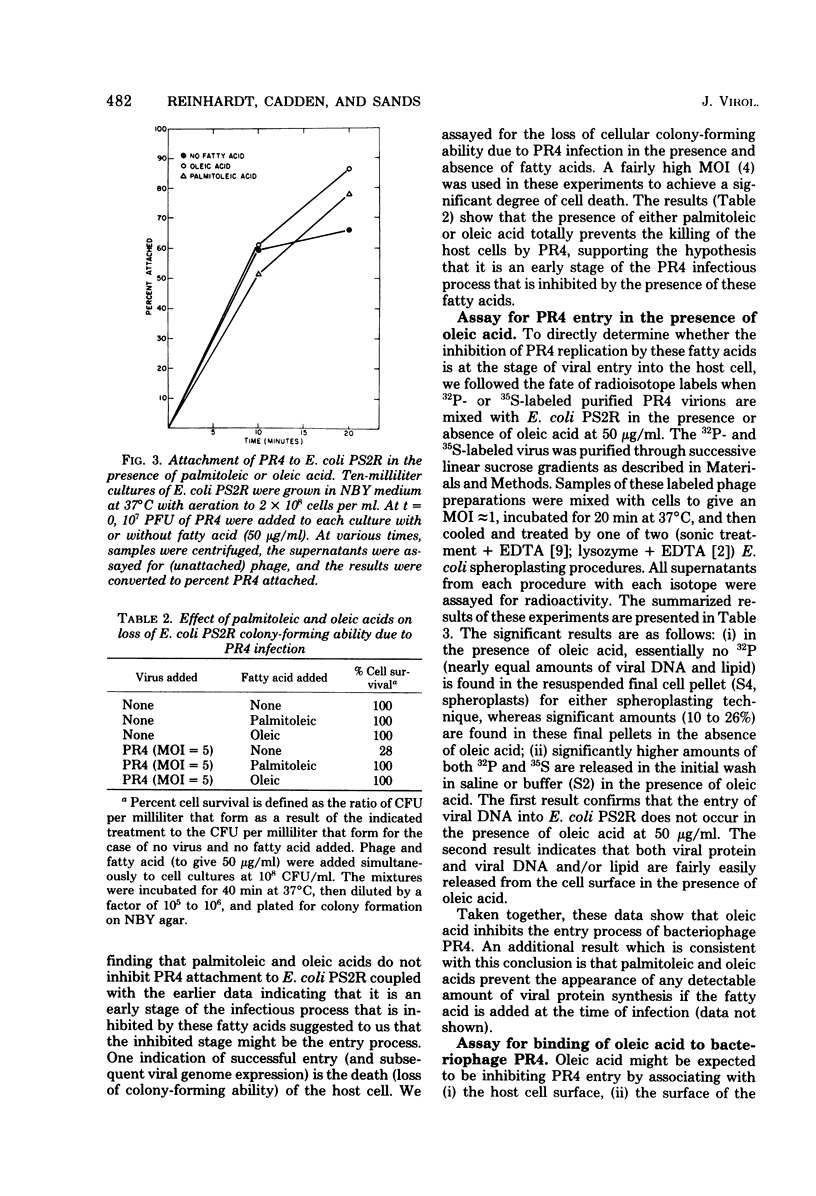
Various unsaturated fatty acids (notably palmitoleic acid and oleic acid) interfered with plaque production by the lipid-containing bacteriophage PR4 on lawns of Escherichia coli. Addition of fatty acid to give 50 μg/ml (∼0.2 mM) at the time of infection prevented phage replication. If, however, the fatty acid was added after infection, normal amounts of phage were produced. If the fatty acid was added (to 50 μg/ml) to the host cell culture a long enough time before infection such that the fatty acid concentration in the growth medium at the time of infection was reduced to ≲5 μg/ml (due to fatty acid incorporation by the host cells), normal phage replication occurred also. Neither palmitoleic acid nor oleic acid prevented PR4 attachment to E. coli. Several types of experiments indicated that it is the entry process of the virus that is inhibited by these fatty acids. Specifically, if the fatty acid was added at the time of infection, the host cells were not killed by the virus and no detectable amounts of viral protein were synthesized. In addition, experiments using purified radioisotope-labeled virions showed directly that entry is inhibited. Mutants of PR4 that did replicate in the presence of oleic acid arose spontaneously at a frequency of 10−6. Three of these mutants that have been further characterized have protein and phospholipid compositions indistinguishable from those of wild-type PR4.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamford D. H., Palva E. T., Lounatmaa K. Ultrastructure and life cycle of the lipid-containing bacteriophage phi 6. J Gen Virol. 1976 Aug;32(2):249–259. doi: 10.1099/0022-1317-32-2-249. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Adsorption of the R-specific bacteriophage PR4 to pili determined by a drug resistance plasmid of the W compatibility group. J Gen Microbiol. 1976 Jul;95(1):181–185. doi: 10.1099/00221287-95-1-181. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Rutherford E. L. Basic characterization of a lipid-containing bacteriophage specific for plasmids of the P, N, and W compatibility groups. Can J Microbiol. 1975 Feb;21(2):152–163. doi: 10.1139/m75-023. [DOI] [PubMed] [Google Scholar]
- Cadden S. P., Sands J. A. Structural proteins of a lipid-containing bacteriophage which replicates in Escherichia coli: phage PR4. Can J Microbiol. 1977 Aug;23(8):1084–1087. doi: 10.1139/m77-163. [DOI] [PubMed] [Google Scholar]
- Cupp J., Klymkowski M., Sands J., Keith A., Snipes W. Effect of lipid alkyl chain perturbations on the assembly of bacteriophage PM2. Biochim Biophys Acta. 1975 May 6;389(2):345–357. doi: 10.1016/0005-2736(75)90327-2. [DOI] [PubMed] [Google Scholar]
- Cupp J., Wanda P., Keith A., Snipes W. Inactivation of the lipid-containing bacteriophage PM2 by butylate hydroxytoluene. Antimicrob Agents Chemother. 1975 Dec;8(6):698–706. doi: 10.1128/aac.8.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Sands J. A., Auperin D. Effects of temperature and host cell genetic characteristics on the replication of the lipid-containing bacteriophage PR4 in Escherichia coli. J Virol. 1977 May;22(2):315–320. doi: 10.1128/jvi.22.2.315-320.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. A., Cupp J., Keith A., Snipes W. Temperature sensitivity of the assembly process of the enveloped bacteriophage phi6. Biochim Biophys Acta. 1974 Dec 10;373(2):277–285. doi: 10.1016/0005-2736(74)90151-5. [DOI] [PubMed] [Google Scholar]
- Sands J. A., Lowlicht R. A., Cadden S. C., Haneman J. Assembly of the enveloped bacteriophage phi 6 in environments which perturb the host cell membranes. Can J Microbiol. 1975 Aug;21(8):1287–1290. doi: 10.1139/m75-194. [DOI] [PubMed] [Google Scholar]
- Sands J. A. Origin of the phospholipids of a lipid-containing virus that replicates in Escherichia coli: bacteriophage PR4. J Virol. 1976 Aug;19(2):296–301. doi: 10.1128/jvi.19.2.296-301.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. XIX. Reconstitution of bacteriophage PM2 in vitro. J Mol Biol. 1975 Sep 5;97(1):21–34. doi: 10.1016/s0022-2836(75)80019-2. [DOI] [PubMed] [Google Scholar]
- Snipes W., Douthwright J., Sands J. Control of phospholipid synthesis and viral assembly by bacteriophage PM2. Biochim Biophys Acta. 1974 Sep 23;363(3):340–350. doi: 10.1016/0005-2736(74)90073-x. [DOI] [PubMed] [Google Scholar]
- Snipes W., Person S., Keller G., Taylor W., Keith A. Inactivation of lipid-containing viruses by long-chain alcohols. Antimicrob Agents Chemother. 1977 Jan;11(1):98–104. doi: 10.1128/aac.11.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanda P., Cupp J., Snipes W., Deith A., Rucinsky T., Polish L., Sands J. Inactivation of the enveloped bacteriophage phi6 by butylated hydroxytoluene and butylated hydroxyanisole. Antimicrob Agents Chemother. 1976 Jul;10(1):96–101. doi: 10.1128/aac.10.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]


