Abstract
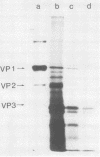
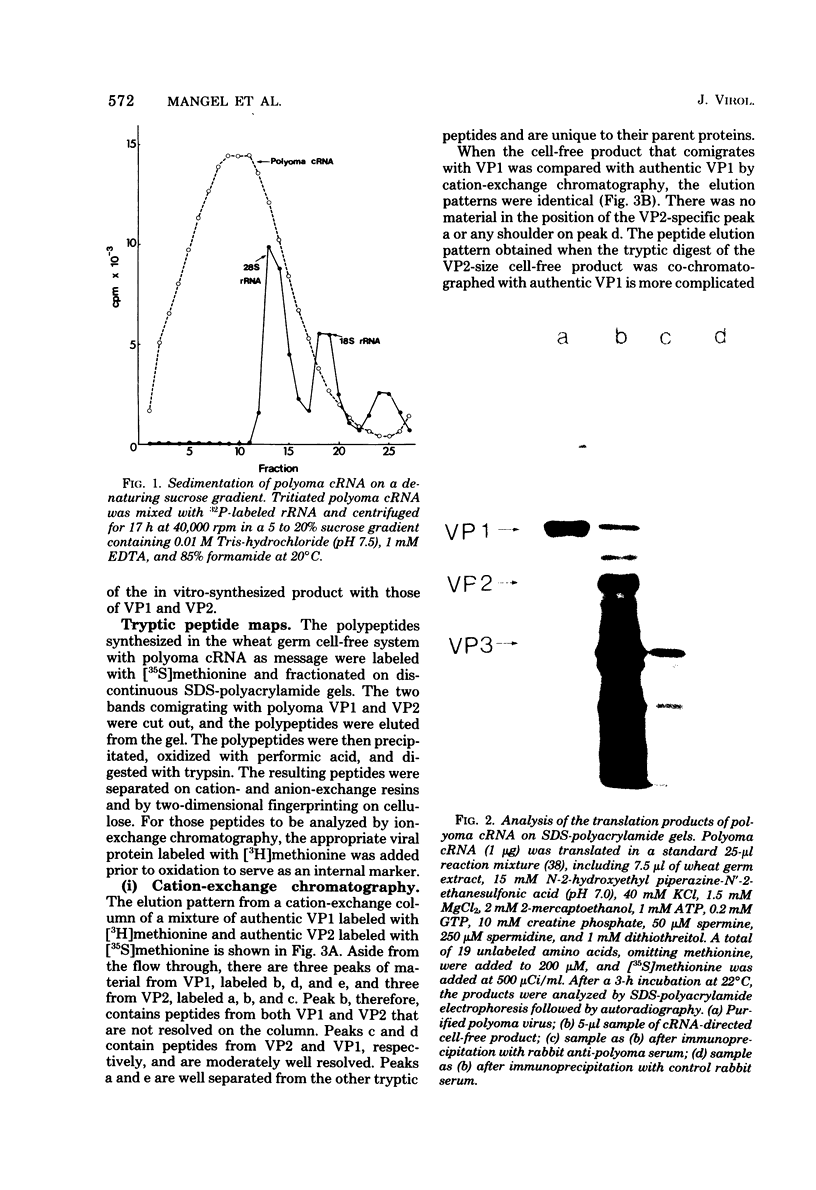
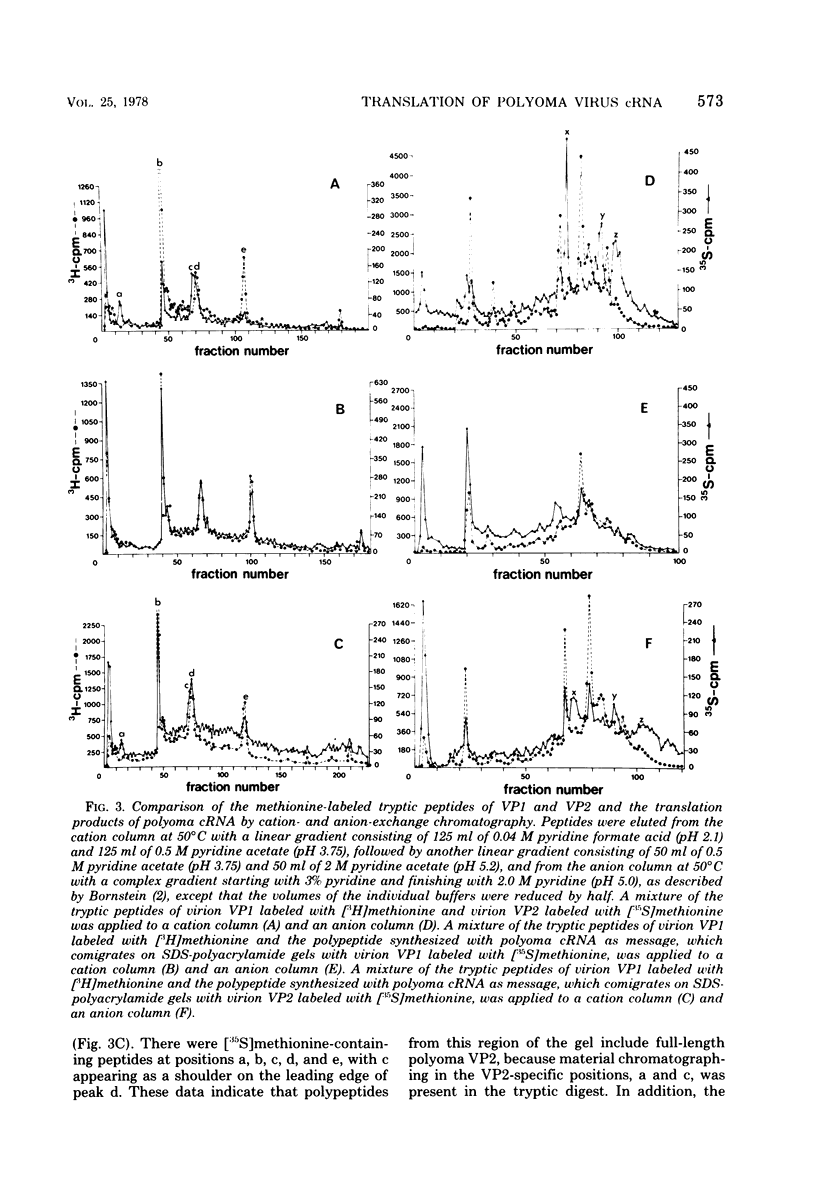
Polyoma virus complementary RNA, synthesized in vitro by using highly purified Escherichia coli RNA polymerase and nondefective form I polyoma DNA, was translated in a wheat germ cell-free system. Polypeptides were synthesized that comigrated on sodium dodecyl sulfate-polyacrylamide gels with the polyoma capsid proteins VP1 and VP2, although most of the cell-free products were of smaller molecular weights. The VP1-size protein specifically immunoprecipitated with anti-polyoma virus serum, and upon digestion by trypsin yielded [35S]methionine-labeled tryptic peptides that co-chromatographed with the [3H]methionine-labeled tryptic peptides of virion-derived VP1 on both cation-exchange and anion-exchange resins. The VP2-size in vitro product contained all the virion VP2 methionine-labeled tryptic peptides, as shown by cation- and anion-exchange chromatography and two-dimensional fingerprinting on cellulose. We conclude that full-length polyoma VP1 and VP2 are synthesized in response to complementary RNA and consequently that the viral capsid proteins VP1, VP2, and VP3 are entirely virus coded.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornstein P. Structure of alpha-1-CB8, a large cyanogen bromide produced fragment from the alpha-1 chain of rat collagen. The nature of a hydroxylamine-sensitive bond and composition of tryptic peptides. Biochemistry. 1970 Jun 9;9(12):2408–2421. doi: 10.1021/bi00814a004. [DOI] [PubMed] [Google Scholar]
- Both G. W., Banerjee A. K., Shatkin A. J. Methylation-dependent translation of viral messenger RNAs in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1189–1193. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buetti E. Characterization of late polyoma mRNA. J Virol. 1974 Aug;14(2):249–260. doi: 10.1128/jvi.14.2.249-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani D., Revel M., Groner Y. Translational discrimination of 'capped' and 'non-capped' mRNAS: inhibition of a series of chemical analogs of m7GpppX. FEBS Lett. 1976 May 1;64(2):326–331. doi: 10.1016/0014-5793(76)80321-3. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Boyer H. W., Tischer E. G., Goodman H. M. Electron microscopic mapping of the attachment sites on SV40 DNA during lytic infection. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):109–117. doi: 10.1101/sqb.1974.039.01.016. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Crawford L. V. Polyoma virus basic proteins. J Gen Virol. 1972 Feb;14(2):141–155. doi: 10.1099/0022-1317-14-2-141. [DOI] [PubMed] [Google Scholar]
- Friedmann T., David D. Structural roles of polyoma virus proteins. J Virol. 1972 Oct;10(4):776–782. doi: 10.1128/jvi.10.4.776-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann T., Eckhart W. Virion proteins of polyoma temperature-sensitive mutants: late mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):243–246. doi: 10.1101/sqb.1974.039.01.031. [DOI] [PubMed] [Google Scholar]
- Friedmann T. Genetic economy of polyoma virus: capsid proteins are cleavage products of same viral gene. Proc Natl Acad Sci U S A. 1974 Feb;71(2):257–259. doi: 10.1073/pnas.71.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Polyoma virus proteins: a description of the structural proteins of the virion based on polyacrylamide gel electrophoresis and peptide analysis. Virology. 1974 Dec;62(2):319–336. doi: 10.1016/0042-6822(74)90395-x. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Fried M., Waterfield M. D. Nonhistone virion proteins of polyoma: characterisation of the particle proteins by tryptic peptide analysis by use of ion-exchange columns. Virology. 1975 Aug;66(2):408–419. doi: 10.1016/0042-6822(75)90213-5. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Waterfield M. D., Miller L. K., Fried M. Correlation between genetic loci and structural differences in the capsid proteins of polyoma virus plaque morphology mutants. Cell. 1977 Jun;11(2):331–338. doi: 10.1016/0092-8674(77)90049-6. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A., Baglioni C. Inhibition of initiation of protein synthesis by 7-methylguanosine-5'-monophosphate. Proc Natl Acad Sci U S A. 1976 Jan;73(1):19–23. doi: 10.1073/pnas.73.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hölzel F., Sokol F. Integration of progeny simian virus 40 DNA into the host cell genome. J Mol Biol. 1974 Apr 15;84(3):423–444. doi: 10.1016/0022-2836(74)90450-1. [DOI] [PubMed] [Google Scholar]
- Kamen R., Lindstrom D. M., Shure H., Old R. W. Virus-specific RNA in cells productively infected or transformed by polyoma virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):187–198. doi: 10.1101/sqb.1974.039.01.025. [DOI] [PubMed] [Google Scholar]
- Kamen R., Shure H. Topography of polyoma virus messenger RNA molecules. Cell. 1976 Mar;7(3):361–371. doi: 10.1016/0092-8674(76)90165-3. [DOI] [PubMed] [Google Scholar]
- Mangel W. F. Initial steps in the large-scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. Arch Biochem Biophys. 1974 Jul;163(1):172–177. doi: 10.1016/0003-9861(74)90466-4. [DOI] [PubMed] [Google Scholar]
- Miller L. K., Fried M. Construction of infectious polyoma hybrid genomes in vitro. Nature. 1976 Feb 19;259(5544):598–601. doi: 10.1038/259598a0. [DOI] [PubMed] [Google Scholar]
- Prives C. L., Aviv H., Gilboa E., Revel M., Winocour E. The cell-free translation of SV40 messenger RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):309–316. doi: 10.1101/sqb.1974.039.01.041. [DOI] [PubMed] [Google Scholar]
- Roberts B. E., Gorecki M., Mulligan R. C., Danna K. J., Rozenblatt S., Rich A. Simian virus 40 DNA directs synthesis of authentic viral polypeptides in a linked transcription-translation cell-free system. Proc Natl Acad Sci U S A. 1975 May;72(5):1922–1926. doi: 10.1073/pnas.72.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]
- Rozenblatt S., Mulligan R. C., Gorecki M., Roberts B. E., Rich A. Direct biochemical mapping of eukaryotic viral DNA by means of a linked transcription-translation cell-free system. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2747–2751. doi: 10.1073/pnas.73.8.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. Adenovirus amazes at Cold Spring Harbor. Nature. 1977 Jul 14;268(5616):101–104. doi: 10.1038/268101a0. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Kamen R., Mangel W. F., Shure H., Wheeler T. Location of the sequences coding for capsid proteins VP1 and VP2 on polyoma virus DNA. Cell. 1976 Nov;9(3):481–487. doi: 10.1016/0092-8674(76)90093-3. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Weil R., Salomon E., May E., May P. A simplifying concept in tumor virology: virus-specific "pleiotropic effectors". Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):381–395. doi: 10.1101/sqb.1974.039.01.050. [DOI] [PubMed] [Google Scholar]
- Wheeler T., Bayley S. T., Harvey R., Crawford L. V., Smith A. E. Cell-free synthesis of polyoma virus capsid proteins VP1 and VP2. J Virol. 1977 Jan;21(1):215–224. doi: 10.1128/jvi.21.1.215-224.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Frenkel N., Lavi S., Osenholts M., Rozenblatt S. Host substitution in SV40 and polyoma DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):101–108. doi: 10.1101/sqb.1974.039.01.015. [DOI] [PubMed] [Google Scholar]