D. melanogaster Gαo-subunit and the RGS domain of its interacting partner CG5036 have been overproduced and purified; the crystallization and preliminary X-ray crystallographic analysis of the complex of the two proteins are reported.
Keywords: Drosophila melanogaster, Gαo-subunit, G-protein signalling
Abstract
Regulator of G-protein signalling (RGS) proteins negatively regulate heterotrimeric G-protein signalling through their conserved RGS domains. RGS domains act as GTPase-activating proteins, accelerating the GTP hydrolysis rate of the activated form of Gα-subunits. Although omnipresent in eukaryotes, RGS proteins have not been adequately analysed in non-mammalian organisms. The Drosophila melanogaster Gαo-subunit and the RGS domain of its interacting partner CG5036 have been overproduced and purified; the crystallization of the complex of the two proteins using PEG 4000 as a crystallizing agent and preliminary X-ray crystallographic analysis are reported. Diffraction data were collected to 2.0 Å resolution using a synchrotron-radiation source.
1. Introduction
Heterotrimeric G proteins play key roles in eukaryotic signal transduction, functioning as immediate intracellular interaction partners of, and signal transmitters from, transmembrane receptors of the G-protein-coupled receptor (GPCR) superfamily (Pierce et al., 2002 ▶). Upon interaction with ligand-activated GPCR, the initially heterotrimeric G protein consisting of the GDP-bound α-subunit and the βγ-heterodimer exchanges the guanine nucleotide for GTP and dissociates into Gα-GTP and Gβγ. Both of the components of the G protein are competent to engage downstream effectors. Hydrolysis of GTP on the α-subunit recreates Gα-GDP, which can bind back the βγ-subunits and close the G-protein activation cycle.
An important regulatory function in this cycle is played by the family of regulator of G-protein signalling (RGS) proteins (Ross & Wilkie, 2000 ▶). Comprising several families, these proteins exert a number of activities, the most important of which is that mediated by the core RGS domain: acceleration of GTP hydrolysis on the Gα-subunits. The crystal structures of several mammalian Gα–RGS complexes reveal that the RGS stabilizes the transition state in the GTP-to-GDP hydrolysis process, mimicked in the crystal by the Gα–GDP–AlF4 − conformation (Chen et al., 1996 ▶; Watson et al., 1996 ▶; Tesmer et al., 1997 ▶; Hunt et al., 1996 ▶; Slep et al., 2008 ▶).
Although heterotrimeric G-protein signalling has been extensively studied in mammalian systems, it is omnipresent in eukaryotes. Interestingly, compared with mammals, other taxa may contain strongly expanded G-protein and RGS families or highly unusual members of such families (Anantharaman et al., 2011 ▶). These findings highlight the importance of extending the analysis of heterotrimeric G-protein signalling to non-mammalian species. Drosophila melanogaster represents a powerful genetic model system. Our previous investigations concentrated on the role of its heterotrimeric G-protein signalling, mediated by the Gαo-subunit, in canonical Frizzled signalling (Egger-Adam & Katanaev, 2010 ▶) and planar cell polarity (Katanaev et al., 2005 ▶), regulation of asymmetric cell division (Katanaev & Tomlinson, 2006 ▶) and endocytic trafficking (Purvanov et al., 2010 ▶). In a search to identify Gαo binding partners, we conducted a yeast two-hybrid screen using constitutively activated (GTP-hydrolysis-deficient) Drosophila Gαo as the bait (Kopein & Katanaev, 2009 ▶). The details and extensive results of this screening, as well as the exhaustive characterization of the interaction of Gαo with one of the binding partners emerging in this screen (the RGS protein CG5036), will be published elsewhere. Characterized by a relatively simple domain composition, CG5036 is the only Drosophila member of the RZ subfamily of RGS proteins, represented in mammals by RGS17, RGS19 and RGS20. The current paper describes the cloning, expression and purification of the RGS domain of CG5036, as well as its crystallization in complex with Drosophila Gαo. Determination of the structure of this complex will be relevant for understanding the mechanisms of interaction of Gα subunits with RGS domains. It will also be the first example of a non-mammalian Gα–RGS complex.
2. Materials and methods
2.1. Sequence amplification and cloning
Drosophila Gαo was PCR-amplified from the plasmid pQE32-Gαo using the oligonucleotides forward, 5′-ATACAGATCCATCGAAGGTCGTAAGAATCTAAAGGAGGATGGAATCC-3′, and reverse, 5′-TGAATAAGCTTTTAGTACAGTCCACAGCCGCGCAGG-3′, deleting the 21 N-terminal residues from Gαo. Purified PCR products were subcloned into the pQE30 expression vector (Qiagen) by BamHI and HindIII.
Drosophila CG5036 cDNA LD40005 was obtained from the Berkeley Drosophila Genome Project cDNA collection. The sequence of the C-terminal half of the protein containing the RGS domain (deleting 109 N-terminal amino acids) was PCR-amplified using the following oligonucleotides: forward, 5′-CCGGATCCCTGGCCATCAAGAATGCCGATG-3′, and reverse, 5′-GGACATGTCGACCTAAGTTGGACTATCCG-3′. The purified PCR products were subcloned into pQE30 by BamHI and SalI.
Plasmids pQE30-GαoΔ21 and pQE30-CG5036Δ109 were subsequently verified by sequencing. The resulting constructs contained N-terminal hexahistidine tags.
2.2. Protein expression and purification
pQE30-GαoΔ21 was transformed into Escherichia coli strain M15 (pREP4) (Qiagen). The transformed cells were cultured at 310 K in LB medium containing 100 µg ml−1 ampicillin and 50 µg ml−1 kanamycin. When the OD600 reached 0.6, the culture was cooled to 290 K and induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG). Following overnight incubation at 290 K, the cells were harvested by centrifugation at 5000g for 10 min at 277 K. The cell pellets were resuspended in lysis buffer [buffer A: 50 mM Tris–HCl pH 7.5, 300 mM NaCl, 5 mM β-mercaptoethanol (β-ME), 10 mM MgCl2, 50 µM GDP, 20 mM imidazole, 1 mM phenylmethylsulfonyl fluoride] and disrupted using an EmulsiFlex-C3 high-pressure homogenizer (Avestin). Subsequently, cell debris was removed by centrifugation at 277 K for 30 min at 10 000g. The His-tagged protein was partially purified by Ni–NTA affinity chromatography. Fractions containing the target protein were pooled, concentrated, diluted sixfold with buffer B (50 mM Tris–HCl pH 7.5, 5 mM β-ME, 10 mM MgCl2, 50 µM GDP), loaded onto a column with Q Sepharose (GE Healthcare) and washed with buffer C (50 mM Tris–HCl pH 7.5, 10 mM MgCl2, 50 mM NaCl, 5 mM β-ME, 50 µM GDP). The protein was eluted with a 50–500 mM NaCl gradient in buffer C over 200 ml. Fractions containing GαoΔ21 were pooled, concentrated to 0.5 ml and injected onto a Superdex 75 10/30 column equilibrated in buffer D (50 mM Tris–HCl pH 7.5, 5 mM MgCl2, 100 mM NaCl, 2 mM DTT, 50 µM GDP, 10% glycerol, 30 mM NaF, 1 mM AlCl3). The purified GαoΔ21 was concentrated to 10 mg ml−1.
pQE30-CG5036Δ109 was transformed into E. coli strain M15 (pREP4) (Qiagen). The transformed cells were cultured at 310 K in LB medium containing 100 µg ml−1 ampicillin and 50 µg ml−1 kanamycin. When the OD600 reached 0.6, the culture was cooled to 300 K and induced with 0.5 mM IPTG. After 6 h induction, the cells were harvested by centrifugation at 5000g for 10 min at 277 K. The cell pellets were resuspended in lysis buffer consisting of 50 mM Tris–HCl pH 7.5, 250 mM MgCl2, 1 M LiCl, 20 mM imidazole, 2 mM DTT, 0.3 mM PMSF and disrupted using an EmulsiFlex-C3 high-pressure homogenizer (Avestin). Cell debris was removed by centrifugation at 20 000g for 30 min at 277 K. The 6×His-tagged protein was purified by Ni–NTA affinity chromatography (Qiagen). The purified protein sample was pooled, exchanged into 20 mM Tris–HCl pH 7.5, 2 mM MgCl2, 150 mM NaCl, 2 mM DTT and concentrated to 25 mg ml−1.
To obtain a 1:1 stoichiometric ratio of Gαo:CG5036, a 1.4-fold molar excess of CG5036 was added to 420 µl 180 µM Gαo in buffer D, mixed and incubated on ice for 1 h. The sample was injected onto a Superdex 75 10/30 column equilibrated in buffer D and the 1:1 Gαo–CG5036 complex was separated from excess CG5036. Fractions containing the complex were pooled and concentrated to 10 mg ml−1.
2.3. Crystallization
Screening for initial crystallization conditions was performed by the hanging-drop vapour-diffusion method using commercially available crystal screening kits from Jena Bioscience (JBScreen Nuc-Pro 1, JBScreen Nuc-Pro 2, JBScreen Nuc-Pro 3 and JBScreen Nuc-Pro 4) and Molecular Dimensions (ProPlex and MIDAS) at 295 K. Droplets consisting of 1.5 µl Gαo–CG5036 solution and 1.5 µl reservoir solution were equilibrated against 300 µl reservoir solution in 24-well plates. After 8–10 d, diamond-shaped crystals (Fig. 1 ▶) appeared in condition No. 3 of JBScreen Nuc-Pro 2 (20% PEG 4000, 100 mM HEPES sodium salt pH 7.5). The approximate dimensions of the crystals were 150 × 150 × 30 µm.
Figure 1.
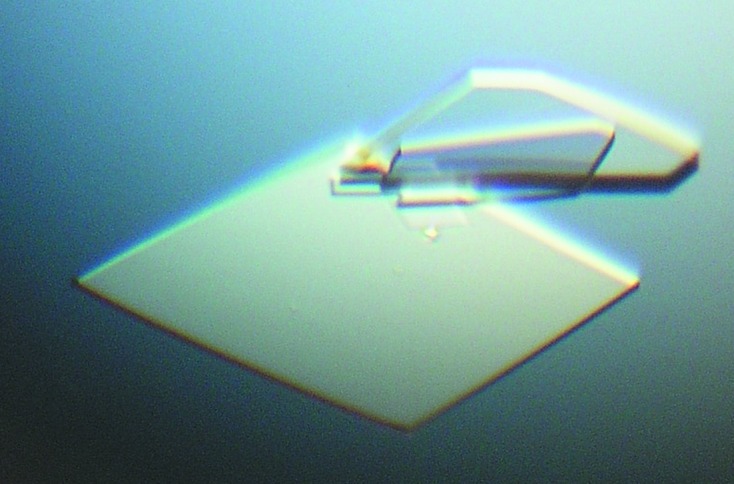
Crystals of the Gαo–CG5036 complex from Drosophila.
2.4. X-ray diffraction analysis
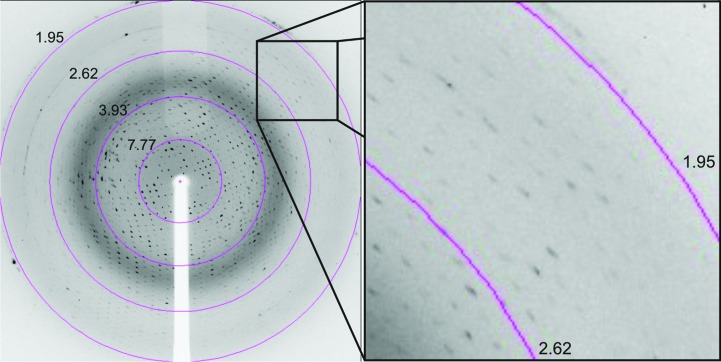
For diffraction data collection, a single crystal was flash-cooled after soaking it in a cryosolution consisting of 33% PEG 4000, 100 mM HEPES sodium salt pH 7.5 prior to data collection. X-ray diffraction data were collected on beamline BL14.1 at BESSY, Berlin, Germany. The wavelength of the radiation source was set to 0.91841 Å and a MAR Mosaic 225 detector was used to record X-ray diffraction intensities as 260 images with an oscillation range of 0.5° per image (Fig. 2 ▶). The intensities were indexed, integrated and scaled using the program XDS (Kabsch, 2010 ▶). Details of the data-collection and processing statistics are summarized in Table 1 ▶. The Matthews coefficient was calculated to be 2.03 Å3 Da−1 (Matthews, 1968 ▶), corresponding to a solvent content of 39.52%. Experimental phasing, model fitting and refinement are in progress.
Figure 2.
X-ray diffraction pattern: concentric rings indicate resolution ranges and the high-resolution diffraction pattern is magnified.
Table 1. Data-collection and processing statistics.
Values in parentheses are for the highest resolution shell.
| Space group | C2 [No. 5] |
| Unit-cell parameters (Å, °) | a = 136.61, b = 95.00, c = 89.09, α = γ = 90, β = 125.35 |
| Radiation source | BL14.1, BESSY, Berlin, Germany |
| Wavelength (Å) | 0.91841 |
| Temperature (K) | 100 |
| Detector | MAR Mosaic 225 |
| Oscillation range (°) | 0.5 |
| No. of frames | 260 |
| Resolution range (Å) | 50.0–1.95 (2.0–1.95) |
| Mosaicity (°) | 0.52 |
| Total reflections | 180995 (11567) |
| Total independent reflections | 65397 (4676) |
| R merge † | 0.086 (0.526) |
| Average I/σ(I) | 8.33 (2.21) |
| Average multiplicity | 2.7 (2.5) |
| Completeness (%) | 96.3 (94.4) |
R
merge = 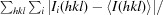
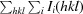 , where I
i(hkl) is the intensity of the ith measurement of reflection hkl and 〈I(hkl)〉 is the average intensity of reflection hkl.
, where I
i(hkl) is the intensity of the ith measurement of reflection hkl and 〈I(hkl)〉 is the average intensity of reflection hkl.
3. Results and discussion
GαoΔ21 (molecular mass 38 kDa) and CG5036Δ109 (molecular mass 20 kDa) were purified using the protocols described above and their purity was checked by SDS–PAGE (Fig. 3 ▶, lanes 1 and 2). The Gαo–CG5036 complex was purified by Superdex 75 10/30 HiLoad size-exclusion chromatography and the elution profile showed a peak corresponding to the complex (58 kDa). SDS–PAGE was used to confirm the presence of the complex and showed two bands with molecular weights of GαoΔ21 and CG5036Δ109 (Fig. 3 ▶, lane 3). Their purity was sufficient for protein crystallization. SDS–PAGE of dissolved crystals showed two bands corresponding to Gαo and CG5036 (Fig. 3 ▶, lane 4). Structure determination and model building are ongoing.
Figure 3.
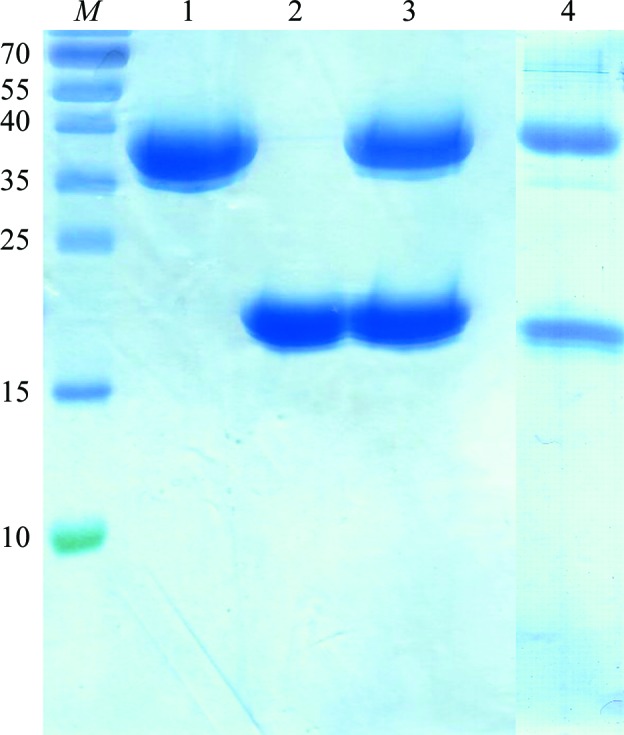
SDS–PAGE gel of purified GαoΔ21 (lane 1), CG5036Δ109 (lane 2), Gαo–CG5036 (lane 3) and dissolved crystals of Gαo–CG5036 (lane 4). Molecular-weight markers (lane M) are shown together with the sizes (in kDa) of specific bands.
Acknowledgments
This work was supported by the Russian Academy of Sciences, the Russian Foundation for Basic Research (grant No. 11-04-00859 to ST) and the Swiss National Science Foundation (grant No. 31003A_138350 to VLK).
References
- Anantharaman, V., Abhiman, S., de Souza, R. F. & Aravind, L. (2011). Gene, 475, 63–78. [DOI] [PMC free article] [PubMed]
- Chen, C.-K., Wieland, T. & Simon, M. I. (1996). Proc. Natl Acad. Sci. USA, 93, 12885–12889. [DOI] [PMC free article] [PubMed]
- Egger-Adam, D. & Katanaev, V. L. (2010). Dev. Dyn. 239, 168–183. [DOI] [PubMed]
- Hunt, T. W., Fields, T. A., Casey, P. J. & Peralta, E. G. (1996). Nature (London), 383, 175–177. [DOI] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Katanaev, V. L., Ponzielli, R., Sémériva, M. & Tomlinson, A. (2005). Cell, 120, 111–122. [DOI] [PubMed]
- Katanaev, V. L. & Tomlinson, A. (2006). Proc. Natl Acad. Sci. USA, 103, 6524–6529. [DOI] [PMC free article] [PubMed]
- Kopein, D. & Katanaev, V. L. (2009). Mol. Biol. Cell, 20, 3865–3877. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. (2002). Nature Rev. Mol. Cell Biol. 3, 639–650. [DOI] [PubMed]
- Purvanov, V., Koval, A. & Katanaev, V. L. (2010). Sci. Signal. 3, ra65. [DOI] [PubMed]
- Ross, E. M. & Wilkie, T. M. (2000). Annu. Rev. Biochem. 69, 795–827. [DOI] [PubMed]
- Slep, K. C., Kercher, M. A., Wieland, T., Chen, C.-K., Simon, M. I. & Sigler, P. B. (2008). Proc. Natl Acad. Sci. USA, 105, 6243–6248. [DOI] [PMC free article] [PubMed]
- Tesmer, J. J. G., Berman, D. M., Gilman, A. G. & Sprang, S. R. (1997). Cell, 89, 251–261. [DOI] [PubMed]
- Watson, N., Linder, M. E., Druey, K. M., Kehrl, J. H. & Blumer, K. J. (1996). Nature (London), 383, 172–175. [DOI] [PubMed]