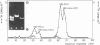Abstract
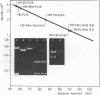
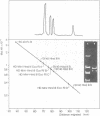
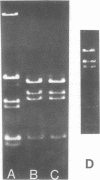
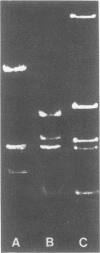
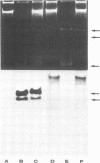
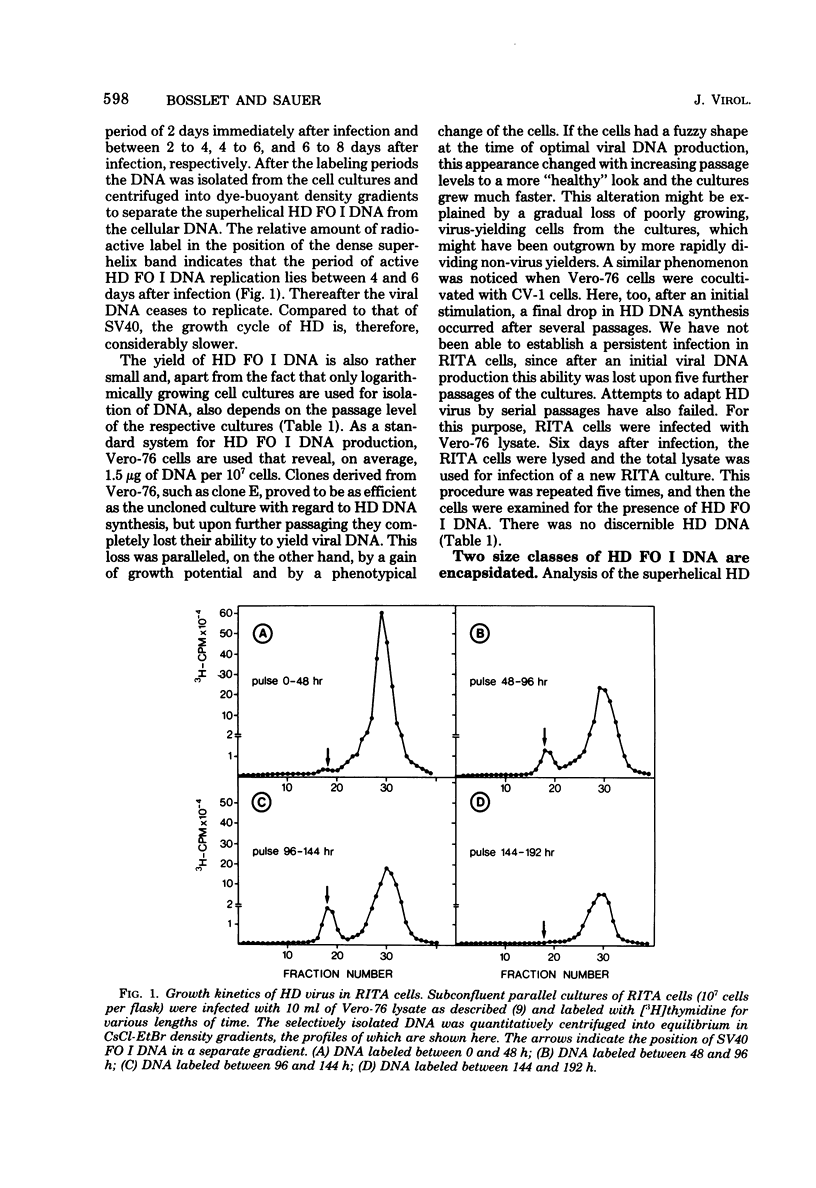
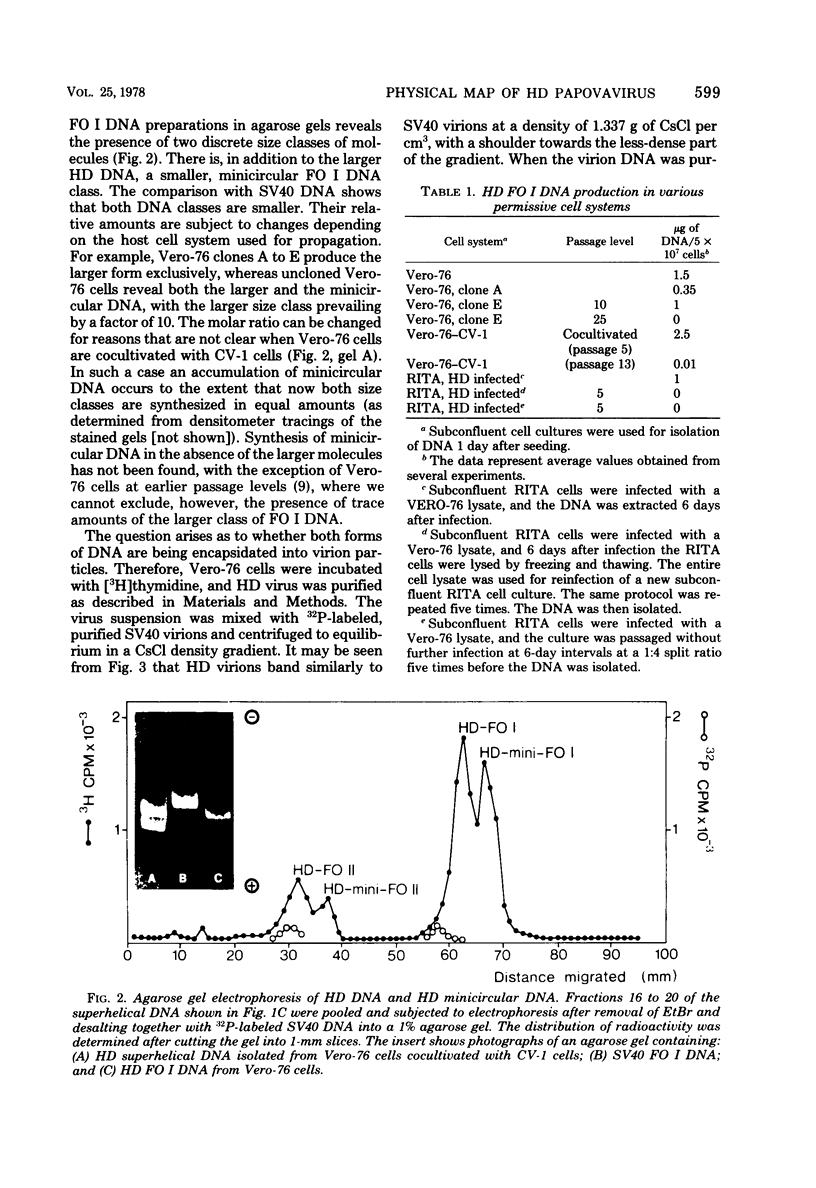
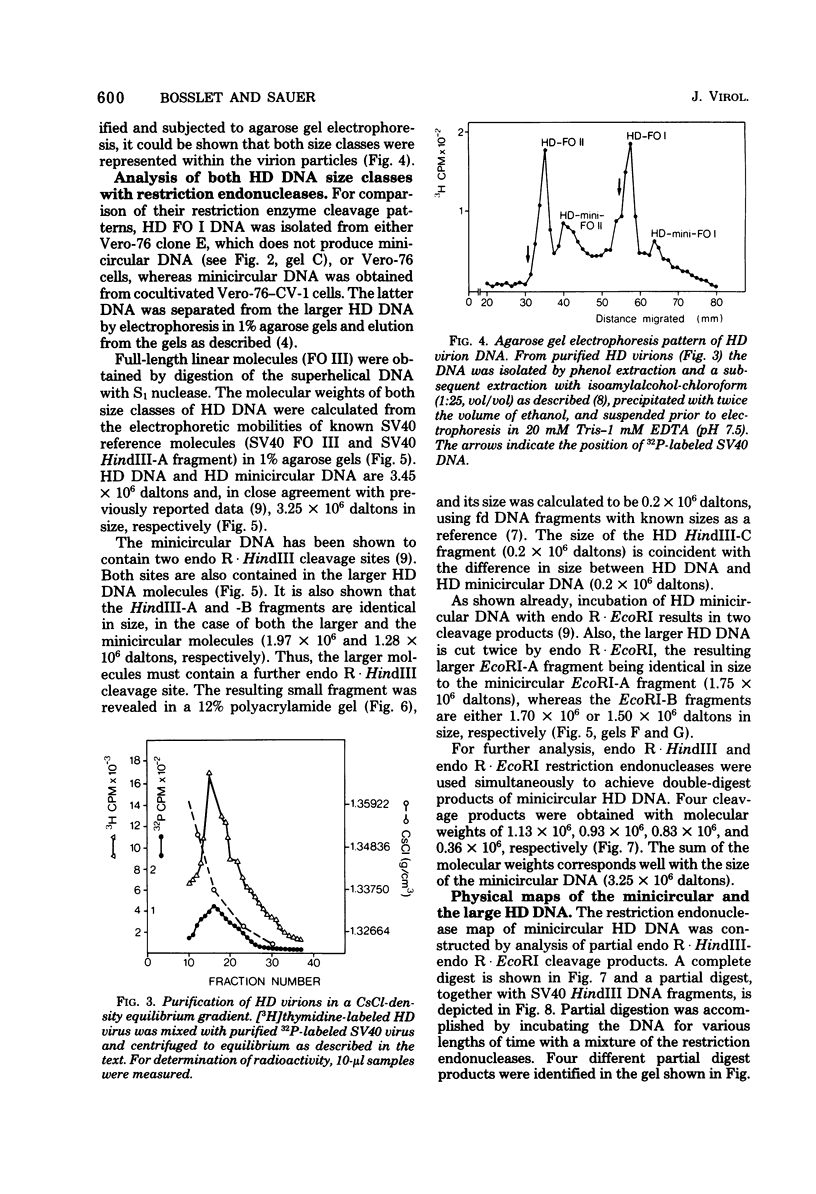
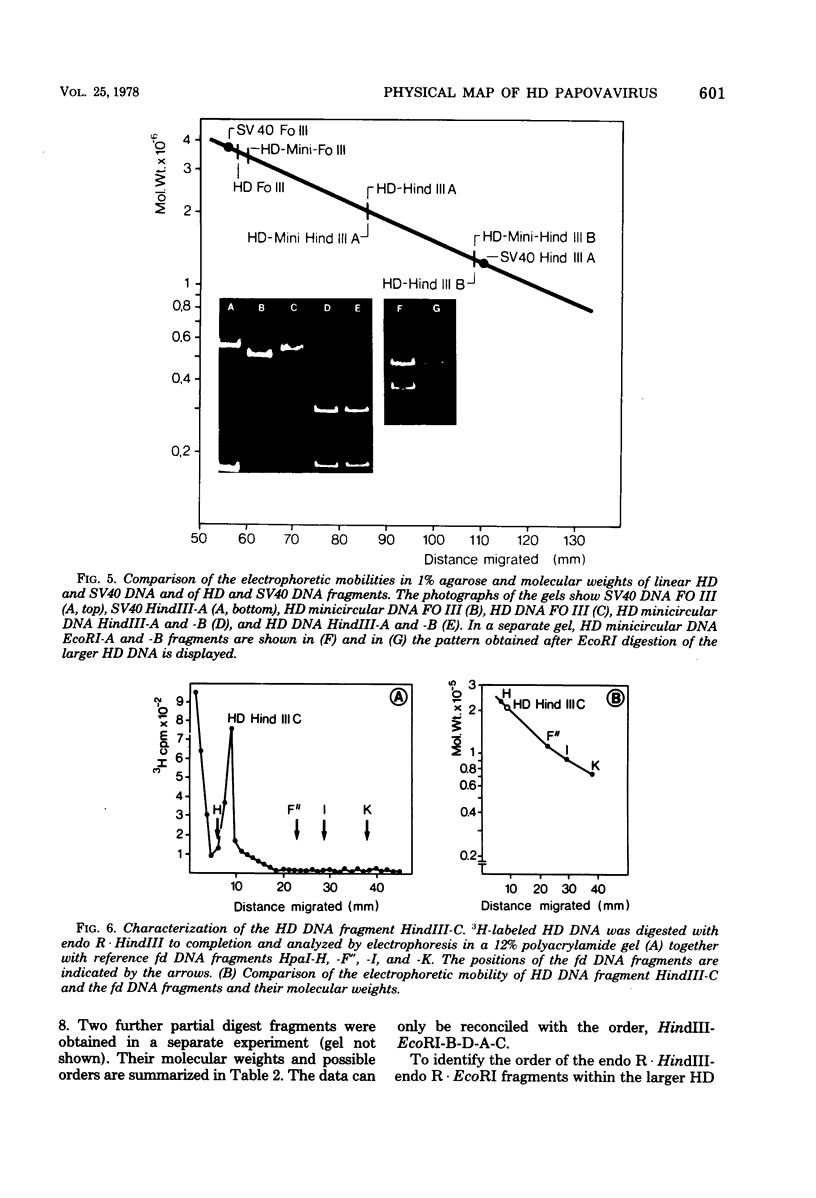
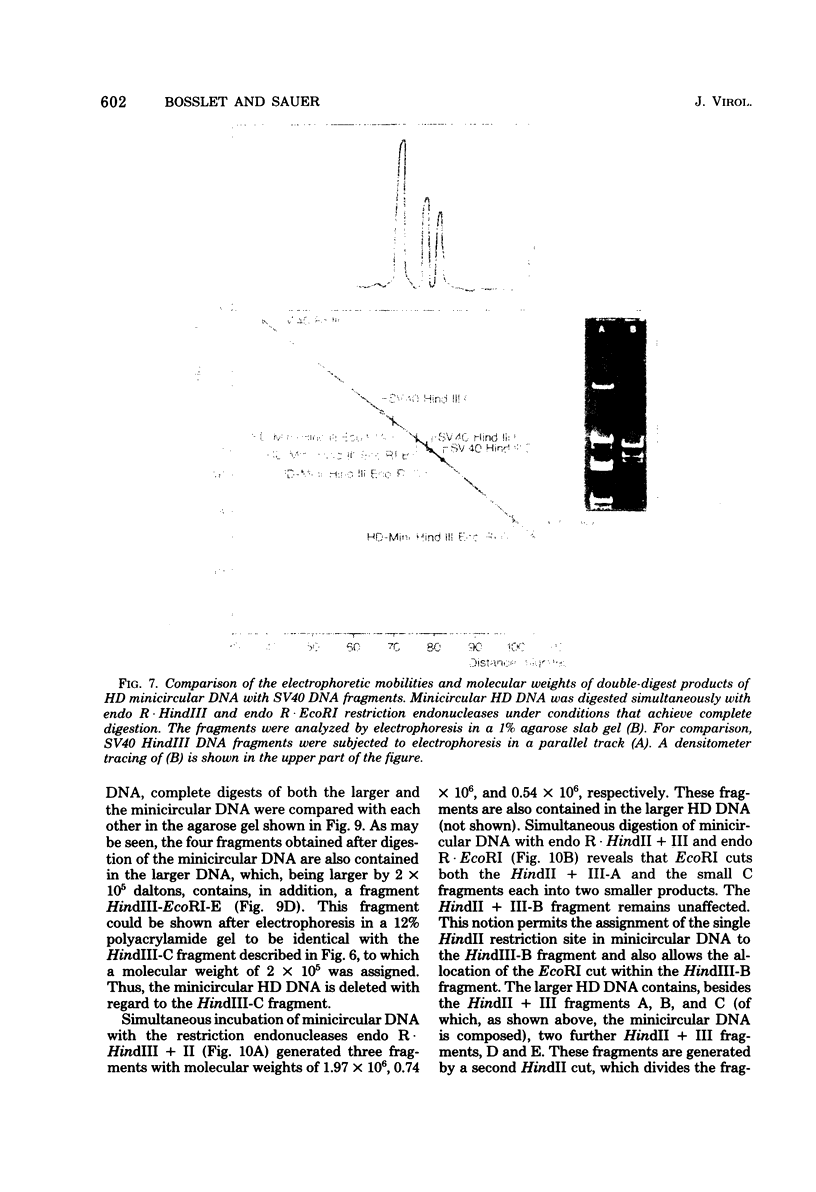
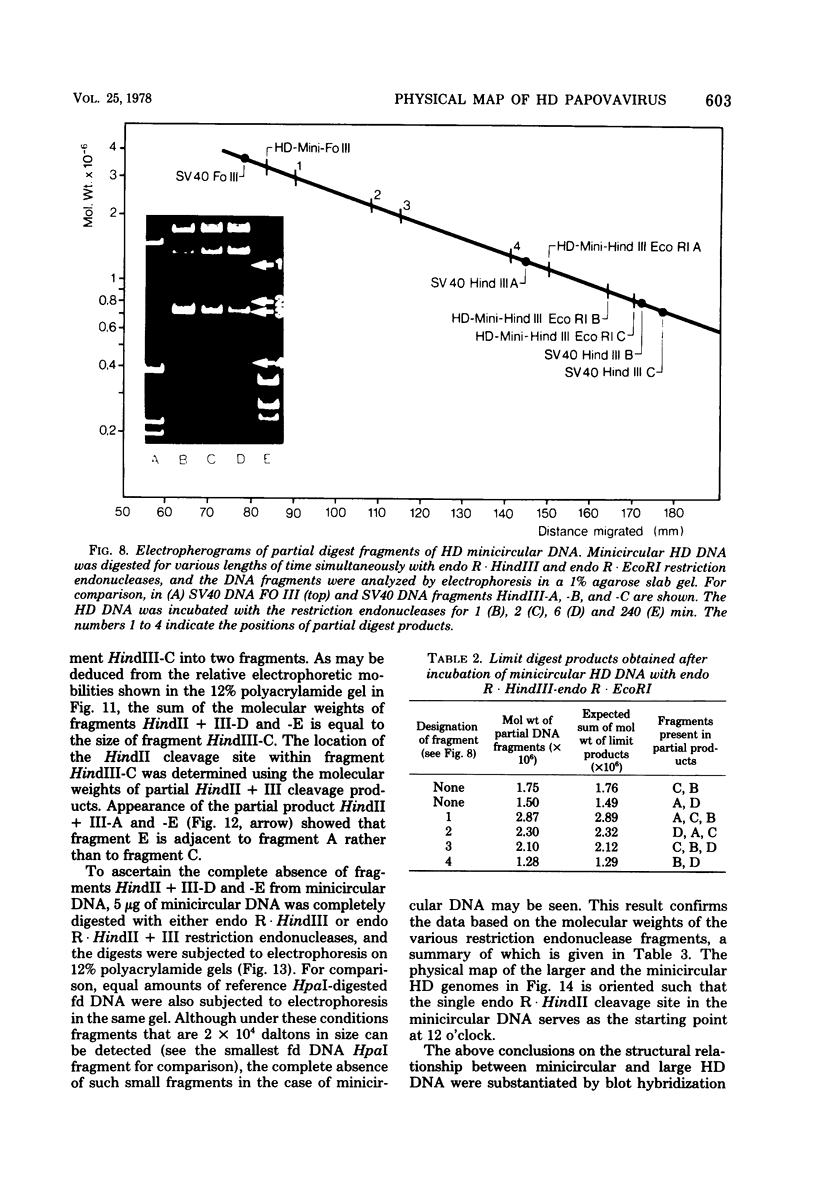
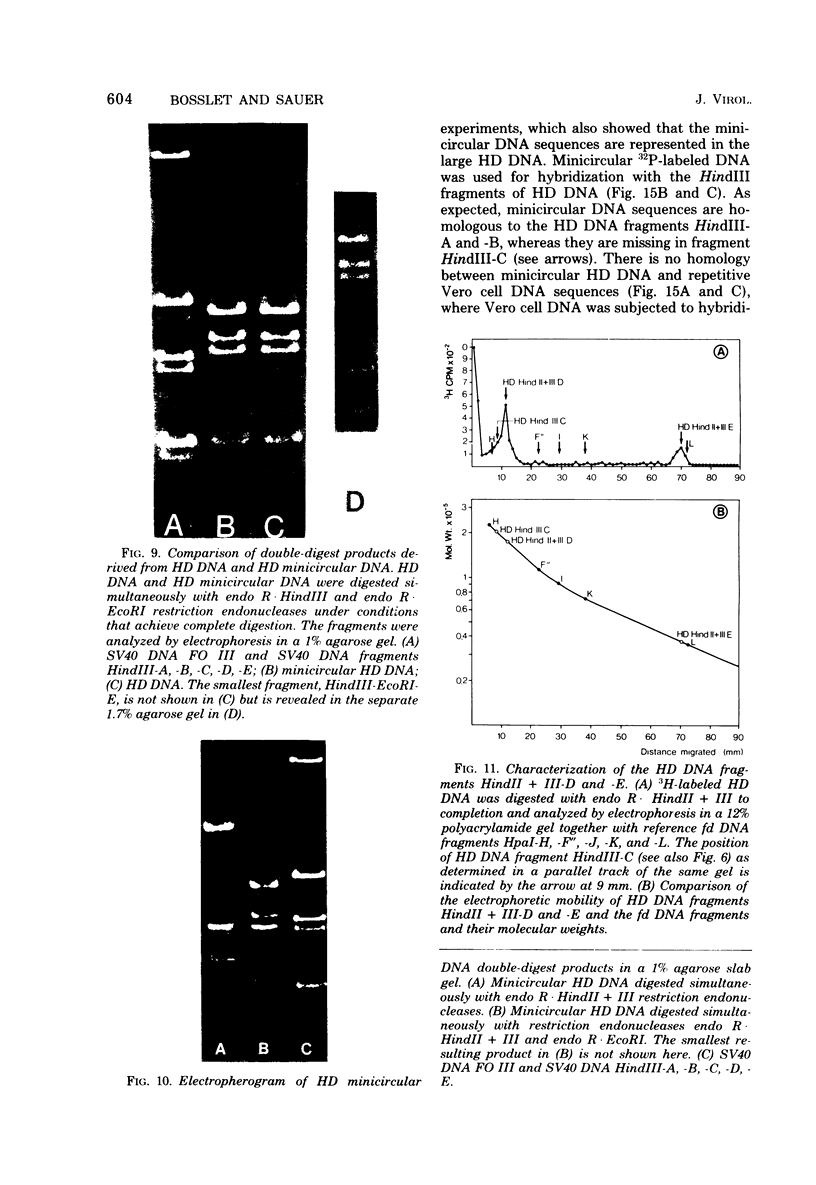
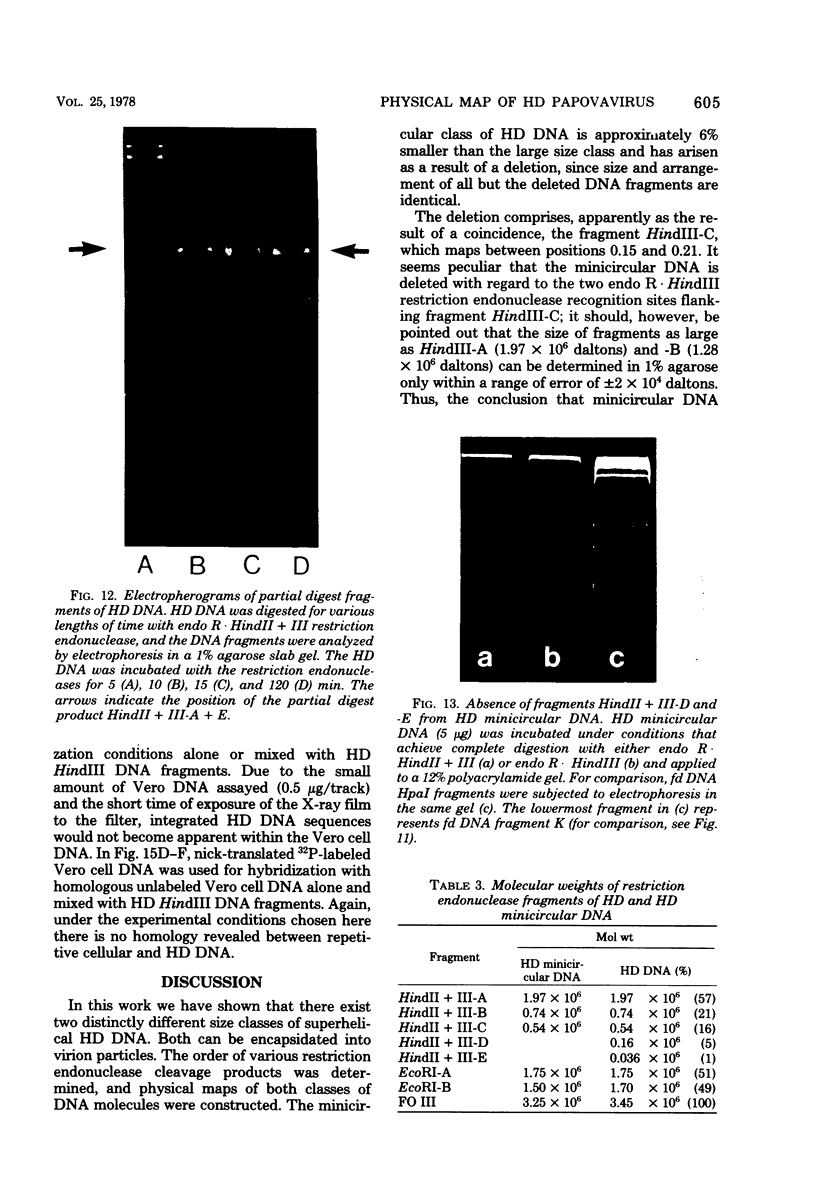
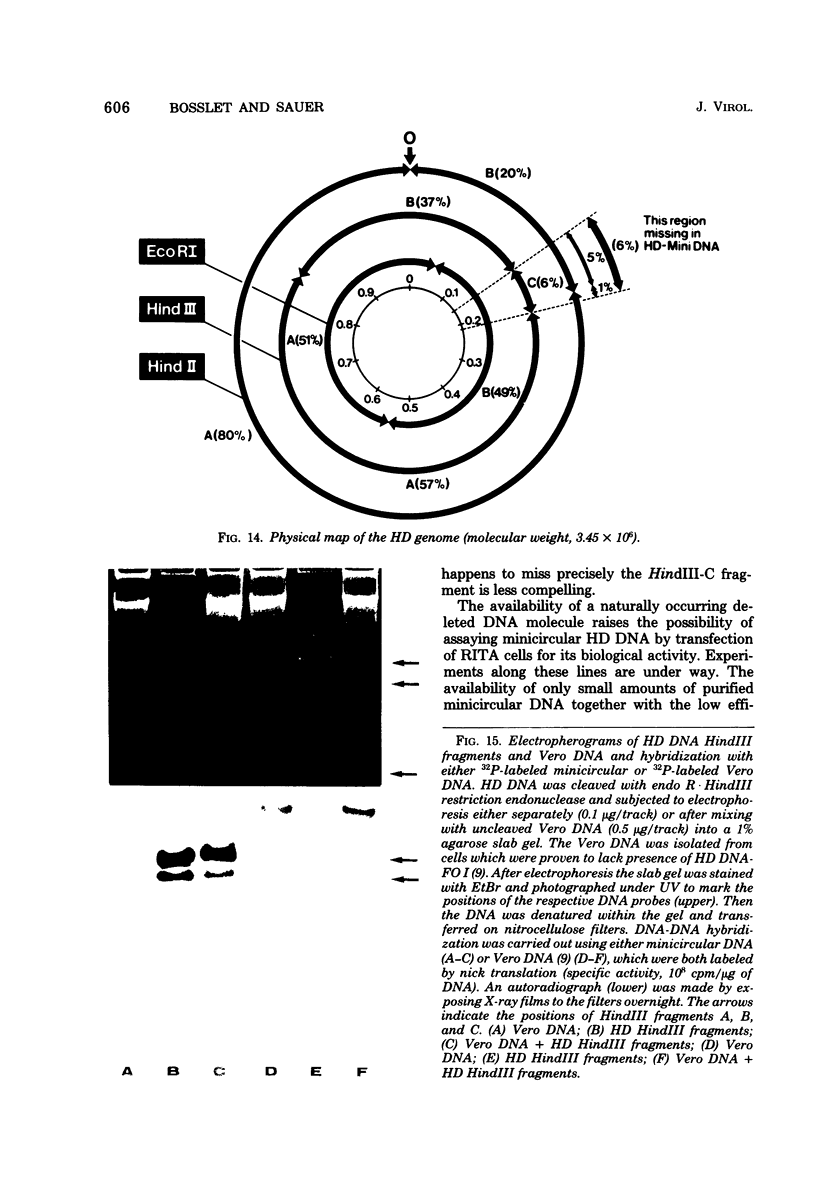
The superhelical DNA of the HD papovavirus is heterogeneous and consists of two discrete size classes with molecular weights of 3.45 X 10(6) and 3.25 X 10(6). Both size classes of DNA are encapsidated into HD virion particles. Their relative intracellular amounts differ, depending on the cell system. Vero-76 carrier cultures in which HD virus was detected contain both size classes of DNA, with the larger molecules prevailing by a factor of 10. Five clonal lines derived from Vero-76 cell cultures contain exclusively the larger DNA. On the other hand, after cocultivation of Vero-76 with CV-1 cells for several passages, minicircular DNA is accumulated such that both size classes are synthesized in equal amounts. Any of the originally viral DNA-producing cell lines may, upon subcultivation, cease yielding virus. The RITA cell line of Cercopithecus aethiops origin is the only cell line among numerous ones tested which upon infection permits the establishment of a one-step growth cycle. However, between 6 and 8 days after infection, viral DNA synthesis is discontinued, and a persistent viral infection cannot be established. Physical maps of the genomes were constructed, and it could be shown that the smaller, minicircular DNA had originated from the larger DNA as the result of a deletion. The sequences missing in the minicircular DNA are confined to the relative map position 0.15 to 0.21.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Gruss P., Sauer G. Infectious linear DNA sequences replicating in simian virus 40-infected cells. J Virol. 1977 Feb;21(2):565–578. doi: 10.1128/jvi.21.2.565-578.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss P., Sauer G. Repetitive primate DNA containing the recognition sequences for two restriction endonucleases which generate cohesive ends. FEBS Lett. 1975 Dec 1;60(1):85–88. doi: 10.1016/0014-5793(75)80424-8. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Schaller H. Mapping and characterization of promoters in bacteriophages fd, f1 and m13. J Mol Biol. 1975 Feb 25;92(2):261–277. doi: 10.1016/0022-2836(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Chowdhury K., Gruss P., Sauer G. Random cleavage of superhelical SV 40 DNA S1 nuclease. Biochim Biophys Acta. 1976 Mar 4;425(2):157–167. doi: 10.1016/0005-2787(76)90021-6. [DOI] [PubMed] [Google Scholar]
- Waldeck W., Sauer G. New oncogenic papova virus from primate cells. Nature. 1977 Sep 8;269(5624):171–173. doi: 10.1038/269171a0. [DOI] [PubMed] [Google Scholar]