Abstract
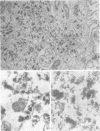
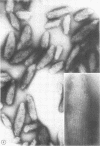
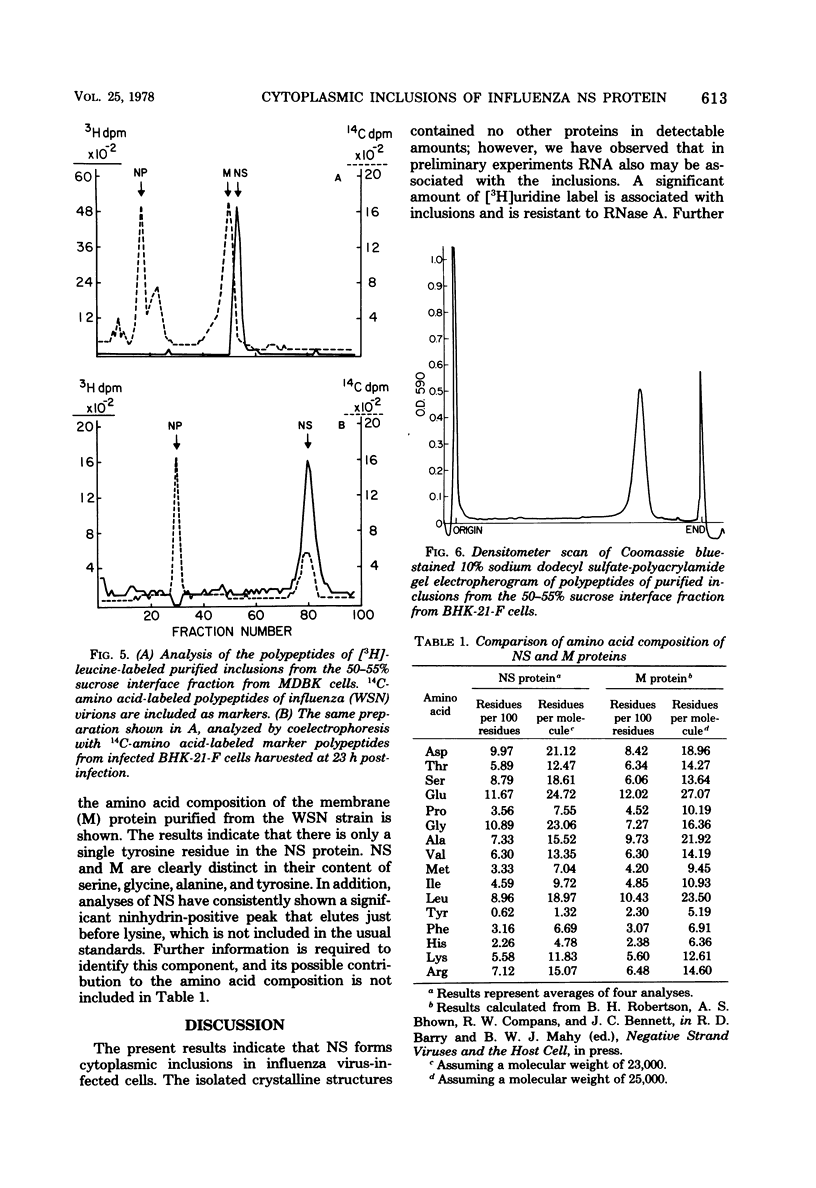
Influenza A viruses induce the accumulation of electron-dense inclusions in the cytoplasm of infected cells during the latter stages of the replication cycle. Cell fractionation studies showed that these inclusions could be recovered in subcellular fractions containing ribosomes and polysomes. Isolation of these inclusions was accomplished by procedures involving RNase treatment of these fractions followed by repurification, or by fluorocarbon extraction and gradient centrifugation. Electron microscopy indicated that the isolated inclusions exhibited a major periodicity of approximately 8 nm with minor periodicities of approximately 4 nm. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed that the influenza virus coded nonstructural protein was the only protein component present in isolated inclusions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Bell T. M., Narang H. K., Field E. J. Influenzal encephalitis in mice. A histopathological and electron microscopical study. Arch Gesamte Virusforsch. 1971;34(2):157–167. doi: 10.1007/BF01241717. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Klenk H. D., Choppin P. W. The proteins of the parainfluenza virus SV5. 1. Separation of virion polypeptides by polyacrylamide gel electrophoresis. Virology. 1969 Nov;39(3):460–466. doi: 10.1016/0042-6822(69)90094-4. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Ciampor F. Electron microscopy of tissue culture cells infected with myxoviruses. I. Nucleo-cytoplasmic changes in A0-WSN influenza virus-infected chick embryo cells. Acta Virol. 1972 Jun;16(1):9–16. [PubMed] [Google Scholar]
- Compans R. W., Dimmock N. J. An electron microscopic study of single-cycle infection of chick embryo fibroblasts by influenza virus. Virology. 1969 Nov;39(3):499–515. doi: 10.1016/0042-6822(69)90098-1. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Distinct carbohydrate components of influenza virus glycoproteins in smooth and rough cytoplasmic membranes. Virology. 1973 Oct;55(2):541–545. doi: 10.1016/0042-6822(73)90199-2. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Holmes K. V., Dales S., Choppin P. W. An electron microscopic study of moderate and virulent virus-cell interactions of the parainfluenza virus SV5. Virology. 1966 Nov;30(3):411–426. doi: 10.1016/0042-6822(66)90119-x. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- HARFORD C. G., HAMLIN A., PARKER E. Electron microscopy of early cytoplasmic changes due to influenza virus. J Exp Med. 1955 Jun 1;101(6):577–590. doi: 10.1084/jem.101.6.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Choppin P. W. On the role of the response of the cell membrane in determining virus virulence. Contrasting effects of the parainfluenza virus SV5 in two cell types. J Exp Med. 1966 Sep 1;124(3):501–520. doi: 10.1084/jem.124.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J. V., Kempf J. E., Kroeger A. V. Cytoplasmic inclusions observed by electron microscopy late in influenza virus infection of chicken embryo fibroblasts. Virology. 1968 Dec;36(4):681–683. doi: 10.1016/0042-6822(68)90200-6. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Etkind P. R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology. 1973 Nov;56(1):334–348. doi: 10.1016/0042-6822(73)90310-3. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert H., Compans R. W. Time course of synthesis and assembly of influenza virus proteins. J Virol. 1974 Nov;14(5):1083–1091. doi: 10.1128/jvi.14.5.1083-1091.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrongiello M. P., Dales S. Characterization of cytoplasmic inclusions formed during influenza/WSN virus infection of chick embryo fibroblast cells. Intervirology. 1977;8(5):281–293. doi: 10.1159/000148903. [DOI] [PubMed] [Google Scholar]
- Nagayama A., Dales S. Rapid purification and the immunological specificity of mammalian microtubular paracrystals possessing an ATPase activity. Proc Natl Acad Sci U S A. 1970 Jun;66(2):464–471. doi: 10.1073/pnas.66.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. Studies on the replication of influenza virus RNA. Virology. 1972 Mar;47(3):823–832. doi: 10.1016/0042-6822(72)90574-0. [DOI] [PubMed] [Google Scholar]
- Saito Y., Yoshioka I., Igarashi Y., Nakagawa S. Nuclear inclusions observed by electron microscope in Cynomolgus monkey kidney cells infected with influenza virus. Virology. 1970 Feb;40(2):408–410. doi: 10.1016/0042-6822(70)90420-4. [DOI] [PubMed] [Google Scholar]