Abstract
Background
Accurate evaluation of gene expression requires normalization relative to the expression of reliable reference genes. Expression levels of “classical” reference genes can differ, however, across experimental conditions. Although quantitative real-time PCR (qRT-PCR) has been used extensively to decipher gene function in the sweetpotato whitefly Bemisia tabaci, a world-wide pest in many agricultural systems, the stability of its reference genes has rarely been validated.
Results
In this study, 15 candidate reference genes from B. tabaci were evaluated using two Excel-based algorithms geNorm and Normfinder under a diverse set of biotic and abiotic conditions. At least two reference genes were selected to normalize gene expressions in B. tabaci under experimental conditions. Specifically, for biotic conditions including host plant, acquisition of a plant virus, developmental stage, tissue (body region of the adult), and whitefly biotype, ribosomal protein L29 was the most stable reference gene. In contrast, the expression of elongation factor 1 alpha, peptidylprolyl isomerase A, NADH dehydrogenase, succinate dehydrogenase complex subunit A and heat shock protein 40 were consistently stable across various abiotic conditions including photoperiod, temperature, and insecticide susceptibility.
Conclusion
Our finding is the first step toward establishing a standardized quantitative real-time PCR procedure following the MIQE (Minimum Information for publication of Quantitative real time PCR Experiments) guideline in an agriculturally important insect pest, and provides a solid foundation for future RNA interference based functional study in B. tabaci.
Introduction
In recent years, quantitative real-time PCR (qRT-PCR) has been widely utilized for gene expression analysis because of its sensitivity, accuracy, reproducibility, and most importantly, quantitativeness [1]–[4]. There is no argument that qRT-PCR is a powerful tool for gene expression analysis, data analysis, and subsequent interpretation. However, interpretation can be challenging due to variation caused by pipetting error and different extraction techniques, transcription and amplification efficiencies among different samples [2], [5]–[7]. Therefore, controlling for internal differences and reducing errors between samples requires the use of reliable reference genes for normalization in gene expression analysis [8].
Traditionally, housekeeping genes including 18S ribosomal RNA, glyceraldehyde-3-phosphate dehydrogenase, elongation factor, ubiquitin-conjugating enzyme, alpha microtubules protein, and beta microtubule protein have been used extensively as endogenous controls for the normalization of qRT-PCR data, but the expression levels of these reference genes can differ under environmental conditions [9]. Based on previous studies, it is evident that the existence of a single universal reference gene suited for all experimental conditions is highly unlikely [5], [10]–[12]. Therefore, selection of reliable reference genes that are consistently expressed under specific experimental conditions is critical for the interpretation of qRT-PCR results. Currently, two Excel-based software tools including geNorm (http://medgen.ugent.be/~jvdesomp/genorm/) [10] and Normfinder (http://www.mdl.dk/publications normfinder.htm) [13], are widely used for evaluating the performance of reference genes. The geNorm program was used to calculate the mean pair-wise variation between an individual gene and all other tested candidate reference genes and the results were shown as expression stability (M). Normfinder is an algorithm for estimation of reference genes among a set of candidates. It ranks the candidate genes based on their expression stability.
The sweetpotato whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is one of the most destructive insect pests worldwide [14]–[15]. This whitefly damages many crops by direct feeding and by vectoring 114 plant viruses [16]. Bemisia tabaci has long been thought to comprise morphologically indistinguishable biotypes that often differ in host range, fecundity, insecticide resistance, transmission competency for begomoviruses, and the symbionts they harbor [14], [16], [17]. Recent studies suggest that most of these biotypes represent genetically distinct cryptic species [18]–[19], among which the B biotype of the Middle East-Minor Asia 1 and the Q biotype of the Mediterranean group are the most invasive and destructive [20]. Although B. tabaci was first recorded in China in the late 1940s, crop damage caused by this insect did not become serious until the introduction of the B biotype in the 1990s [21]. The Q biotype of B. tabaci was first detected in Yunnan Province, China in 2003 [22]. Since then, the Q biotype has gradually displaced the established B populations and has become the dominant B. tabaci in most of China [23].
To examine the temporal and spatial changes of gene expression in B. tabaci, β-actin and α-tubulin are the most frequently used endogenous reference genes in qRT-PCR analyses [24]–[27]. These genes were selected without the companion validation study to evaluate their suitability under specific experimental conditions. Previous studies have demonstrated that the expression of β-actin can be significantly influenced by tissue type [12]. Bustin and his colleagues proposed a MIQE guideline (Minimum Information for publication of Quantitative real time PCR Experiments) [28] to standardize qRT-PCR analysis; reference gene selection is an integral part of their recommendations. In this study, 15 housekeeping genes from a parallel B. tabaci transcriptome study [29] were selected as candidate reference genes. The overall goal of this research is to develop a standardized qRT-PCR analysis in B. tabaci following the MIQE guideline. Specifically, we evaluate the stability and performance of the above mentioned candidate reference genes under different experimental conditions including five biotic factors (host, acquisition of a plant virus, developmental stage, tissue, and whitefly biotype) and three abiotic factors (photoperiod, temperature, and insecticide exposure). The choice and number of reference genes needed under various conditions are investigated and recommended.
Materials and Methods
Ethics Statement
Bemisia tabaci B biotype strains used in this study were initially collected in the field at Beijing in 2000, and have been maintained in a greenhouse at the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences. The species in the genus Aleyrodidaeare common agricultural pests and are not included in the “List of Protected Animals in China”. No specific permits were required for the described field studies.
Candidate Reference Genes
Housekeeping genes from a previous B. tabaci transcriptomic study [29] were selected as candidate reference genes including β-actin (Actin), 18S rRNA (18S), heat shock protein (HSP20, HSP40, HSP70, HSP90), γ-tubulin, 60S ribosomal protein L29 (RPL29), succinate dehydrogenase complex subunit A (SDHA), flavoprotein, glyceraldehyde phosphate dehydrogenase (GAPDH), elongation factor 1 alpha (EF-1α), peptidylprolyl isomeraseA (PPIA), NADH dehydrogenase (NADH), Myosin light chain (Myosin L), and adenosine triphosphate enzyme (ATPase). Primer 5.0 (http://www.premierbiosoft.com/) was used to design primers for qRT-PCR analysis. The validity of these candidate reference genes were evaluated under selected biotic and abiotic conditions described in the following sections.
Biotic Conditions
Host plant
Bemisia tabaci B biotype was maintained on three different host plants including cabbage, tomato, and cucumber [30]. A total of 180 3-day-old adults were collected, snap frozen in liquid nitrogen, and stored at −80°C before qRT-PCR analysis.
Acquisition of a plant virus
Tomato plants infected with Tomato yellow leaf curl virus (TYLCV) were obtained by Agrobacterium tumefaciens-mediated inoculation using a cloned TYLCV genome (GenBank accession ID: AM282874) [31]. Plants were inoculated with the virus at the 3-true-leaf stage. Viral infection of tomato plants was confirmed by the development of characteristic leaf curl symptoms and was further validated by molecular analysis [32]. Viruliferous B. tabaci were obtained by caging non-viruliferous B. tabaci adults with TYLCV-infected tomato plant for a 72 h acquisition access period [33]. Non-viruliferous B. tabaci were obtained by caging non-viruliferous B. tabaci adults with healthy tomato plants for 72 h. A total of 180 3-day-old adult whiteflies from both virus-infected and virus-free tomato plants, respectively, were snap frozen and stored as described earlier.
Developmental stage
Three developmental stages (egg, pupa, and adult) were collected from B. tabaci B biotype maintained on healthy cabbage plants. A total of 900 eggs, 900 pupae, and 300 adults were snap frozen and stored as described earlier.
Tissue
A dissection needle and a stereo microscope (Leica, DFC425) were used to obtain three body regions (head, thorax, and abdomen) from 3-day-old B. tabaci adults (TH-S). These sections were dissected from adults reared on cabbage plants; snap frozen, and stored as described earlier.
Whitefly biotype
Bemisia tabaci B and Q biotype strains were collected from Beijing, China in 2000 and 2008, respectively, and have been maintained in a greenhouse at the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences [30].
Abiotic Conditions
Photoperiod
A total of 200 3-day-old B. tabaci adults were placed into nine screen cages, respectively, and provisioned with cabbage plants at the 5 to 7-true-leaf stage. These cages were kept in growth chambers (27±0.5°C, 60±10% RH) with photoperiods (L/D) of 24∶0, 0∶24, and 14∶10, respectively. After 96 h, B. tabaci adults were snap frozen and stored as described earlier.
Temperature
A total of 720 3-day-old B. tabaci adults (80 whiteflies×9 replications) were collected from cabbage plant and placed individually into 30 ml specimen tubes. The tubes were then placed in climatic chambers at 4.0, 25.0, and 37.5°C, respectively. After 1 h, the live adults were snap frozen and stored as described earlier.
Insecticide susceptibility
Thiamethoxam susceptible (TH-S) and resistant (TH-R) B. tabaci strains were established from the same populations described previously [34]. Before sample collection, a leaf-dip bioassay [34] was conducted to confirm that the resistance factor [LC50 (TH-R)/LC50 (TH-S)] was over 70-fold. A total of 180 adults from both TH-S and TH-R were collected, snap frozen, and stored as described earlier.
Total RNA Extraction and cDNA Synthesis
Total RNA was extracted with a Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was quantified by measuring the absorbance at 260 nm with a Nano Vue UV/Vis spectrophotometer (GE Healthcare). The purity of RNA was assessed at an absorbance ratio of OD260/280 and OD260/230, and the integrity was checked with 1% agarose gel electrophoresis. Then, 1 µg of RNA was used to synthesize the first-strand cDNA using the PrimeScript®RT reagent Kit (Takara Bio, Tokyo, Japan) with gDNA Eraser (Perfect Real Time, TaKara, Shiga, Japan) according to the manufacturer's protocol. The synthesized cDNA was stored at −20°C.
Quantitative Real-time PCR analysis
Quantitative Real-time PCR (qRT-PCR) was performed on an ABI 7500 real-time system. The cDNA of each sample representing one biological replicate was diluted to a working concentration of 17 ng/µl for the qRT-PCR analysis. The melt temperature was 60°C and product contained between 80 and 200 base pairs (Table 1). The 25 µl reaction system contained 1 µl of diluted cDNA, 11.25 µl of SYBR® Green Real-time PCR Master Mix (TIANGEN, Corp, Beijing, China), and 0.5 µl of each primer. The cycling parameters were as follows: 95°C for 3 min followed by 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 35 s. A 3-fold serial dilution of cDNA was used to construct a standard curve to determine the PCR efficiency that would be used to convert the quantification cycles (Ct-values) into the relative quantities (relative gene expressions).
Table 1. Primers used for qRT-PCR analysis.
| Gene | Accession Number | Primer sequences (5′to 3′)1 | Amplicon (bp) | Tm (°C)2 | E (%)3 | R 2 4 |
| HSP20 | EU934239 | F-AAGAAGTCAGCGTGAAAGTCGR-GTACCTCCTAGTGAAAGATCGG | 107 | 60 | 99.5 | 0.9978 |
| HSP40 | EE597535 | F-AGATGAGGCTCATGATGGTCAAR-TGAGAAGCGCATTGCATTGT | 81 | 60 | 109.4 | 0.9992 |
| HSP70 | EU934240 | F-AGCACTCCGGCGTCTACGR-CGAACCTGGCACGGGACAC | 134 | 60 | 109.6 | 0.9944 |
| HSP90 | EU934241 | F-ATCGCCAAATCTGGAACTAAAGCR-GTGTTTTGAGACGACTGTGACGGTG | 135 | 60 | 100.9 | 0.9951 |
| PPIA | JU470456 | F-ATGTTTTGGGCTTTGGTCR-CGTTGCCATCTGAATGAAATAC | 148 | 60 | 96.9 | 0.9988 |
| EF-1α | EE600682 | F-TAGCCTTGTGCCAATTTCCGR-CCTTCAGCATTACCGTCC | 110 | 60 | 103.9 | 0.998 |
| SDHA | JU470457 | F-GCGACTGATTCTTCTCCTGCR-TGGTGCCAACAGATTAGGTGC | 141 | 60 | 92.4 | 0.9986 |
| NADH | JU470455 | F-ATAGTTGGCTGTAGAACCAGAGTGR-ACACGAAGGGAAGAGCACATA | 96 | 60 | 93.5 | 0.9973 |
| γ- tubulin | JU470458 | F-CCACAATCCATGCAAATCR-CCGAAATGGCCTCTGCTA | 117 | 60 | 75.3 | 0.9832 |
| Myosin L | EE597481 | F-TTTCAGACGAGGATGTCGCAR-CGTCATAGATTTCGAACGCG | 81 | 60 | 108.0 | 1.0000 |
| RPL29 | EE596314 | F-TCGGAAAATTACCGTGAGR-GAACTTGTGATCTACTCCTCTCGTG | 144 | 60 | 101.3 | 0.9909 |
| ATPase | JU470453 | F-AGAGCGAGTGTTTGGGTGR-GACGGCGATTCGAGAAGG | 138 | 60 | 98.9 | 0.9994 |
| 18S | U20401 | F-CGGCTACCACATCCAAGGAAR-GCTGGAATTACCGCGGCT | 187 | 60 | 99.5 | 0.9987 |
| Actin | AF071908 | F-TCTTCCAGCCATCCTTCTTGR-CGGTGATTTCCTTCTGCATT | 174 | 60 | 95.0 | 0.9973 |
| GAPDH | JU470454 | F-GGACACGGAAAGCCATACCAGR-ACCACCGCTACCCAAAAGACC | 166 | 60 | 77.0 | 0.9943 |
: F, forward primer; R, reverse primer;
: Tm, Annealing temperature;
: E, Efficiency;
: R 2, Coefficient of determination.
Data Analysis
Expression of reference genes was evaluated with two web-based analysis tools: geNorm and NormFinder. geNorm was used to calculate the M stability value as the mean pairwise variation between an individual gene and all other tested candidate genes. The lower the M value, the more stable the reference genes. The value of Vn/Vn+1 indicates the pairwise variation between two sequential normalization factors and determines the optimal number of reference genes required for accurate normalization. A value below 0.15 indicates that an additional reference gene will not significantly improve normalization. Normfinder evaluates the overall variation of the candidate reference genes under the experimental conditions and estimates the variation between and within groups. For each candidate gene, Normfinder provides a stability value that is a direct and rapid measurement of expression variation. This stability value enables the user to estimate the systematic error introduced when selecting a suitable reference gene.
Results
Expression profiles of candidate reference genes
For each reference gene, a dissociation curve with a single-peak ensured that the primer sets amplified a unique PCR product ranging from 81 to 187 bp. The PCR efficiency was consistently high for candidate reference genes except γ-tubulin (75.3%) and GAPDH (77.0%) (Table 1). The raw Ct values ranged from 11.63 (18S) to 31.11 (γ-tubulin) with different host plants; from 12.02 (18S) to 31.25 (HSP70) with different photoperiods; from 11.85 (18S) to 30.37 (γ-tubulin) with different temperatures; from 11.49 (18S) to 30.86 (HSP70) for non-viruliferous and viruliferous adults; from 12.44 (18S) to 29.58 (γ-tubulin) in thiamethoxam-resistant and -susceptible adults; from 12.17 (18S) to 29.58 (γ-tubulin) for different developmental stages; from 9.15 (18S) to 33.13 (γ-tubulin) for different tissues; and from 12.56 (18S) to 30.71 (γ-tubulin) for B and Q biotype adults.
Stability of candidate reference gene expression
geNorm
The geNorm program was used to calculate the average expression stability values (M stability values) and to plot the influence of different factors using pairwise comparisons. The least stable genes have the highest M values and were successively excluded. The program also indicated the minimum number of reference genes for accurate normalization in B. tabaci by the pairwise variation value. Values (V2/3) under 0.15 shows that no additional genes are required for the normalization (Figure S1 and S2).
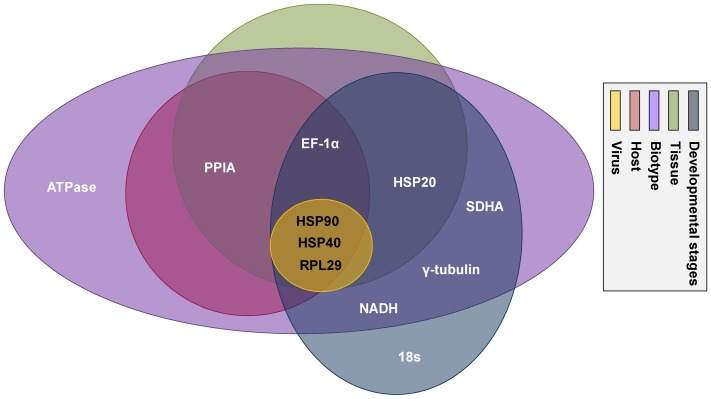
For different hosts, reference genes with M values<0.5 are ranked (from highest to lowest stability) in the order of PPIA+EF1-α > HSP90 > HSP40 > RPL29 (Figure 1). For virus status (with or without TYLCV), RPL29, HSP90, and HSP40 are the most suited reference genes. For developmental stage, the ranking of reference gene stability among those with M values < 0.5 is HSP90+NADH > 18S > γ-tubulin > RPL29 > EF1-α > HSP20 > HSP40 > SDHA. For different B. tabaci tissues, HSP20, HSP40, HSP90, PPIA, RPL29, and EF1-α are relatively stable. For whitefly biotype, reference genes with M values < 0.5 are ranked (from highest to lowest stability) in the order of HSP40+NADH > SDHA > HSP90 > EF1-α > ATPase > PPIA > γ-tubulin > RPL29 > HSP20. Based on data obtained with five biotic factors, the ideal reference genes according to geNorm are RPL29, HSP40, and HSP90.
Figure 1. Reference genes selected by geNorm under various biotic conditions.
The expression stability measure (M) is the mean of the stability values of the remaining genes. The least stable genes have the highest M values. The genes listed here are considered stable based on a cutoff M value of less than 0.5. Each circle with a distinct color represents a different set of biotic condition. Genes located within one circle are stable under a specific biotic condition, and genes shared with multiple circles are stable across those conditions.
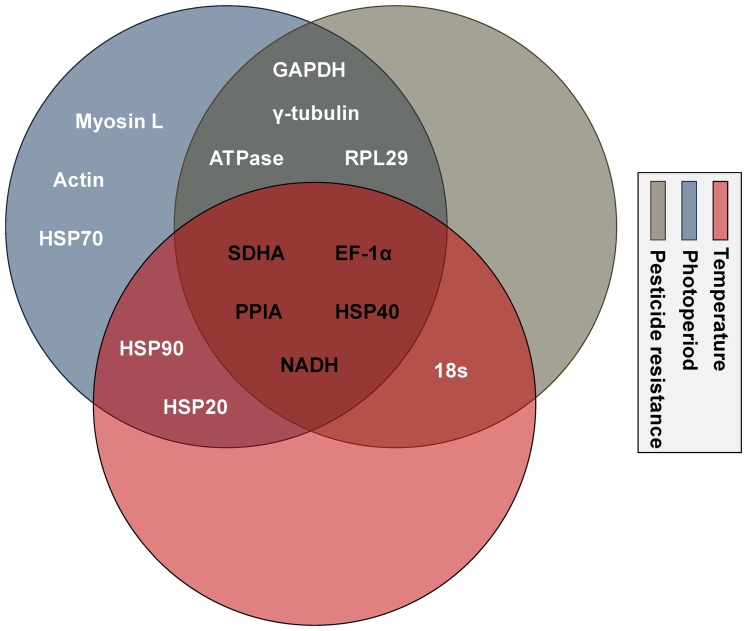
For photoperiod, the M values are <0.5 for all candidate reference genes (Figure 2). For temperature, reference genes with M values <0.5 are ranked (from highest to lowest stability) in the order of EF1-α+NADH > SDHA > RPL29 > PPIA > HSP40 > ATPase > 18S > GAPDH > γ-tubulin. For pesticide resistance, reference genes with M values<0.5 are ranked (from highest to lowest stability) in the order of PPIA+NADH>HSP20>HSP40>HSP90>18S>EF1-α>SDHA. Based on data obtained from three abiotic factors, the ideal reference genes are EF1-α, PPIA, NADH, SDHA, and HSP40.
Figure 2. Reference genes selected by geNorm under various abiotic conditions.
The expression stability measure (M) is the mean of the stability values of the remaining genes. The least stable genes have the highest M values. The genes listed here are considered stable based on a cutoff M value of less than 0.5. Each circle with a distinct color represents a different set of biotic condition. Genes located within one circle are stable under a specific abiotic condition, and genes shared with multiple circles are stable across those conditions.
Normfinder
Normfinder indicated that RPL29, GAPDH, and NADH are the most stable reference genes for host plants, tissues, biotypes, respectively (Table 2). Specifically, for developmental stages and viruliferous/non-viruliferous B. tabaci, SDHA is the most stable reference gene. A similar trend is observed under selected abiotic factors, in which HSP20, HSP40, and EF1-α are ranked as the most stable reference genes for photoperiod, temperature, and insecticide susceptibility, respectively (Table 3).
Table 2. Ranking of candidate reference genes in response to biotic factors.
| Rank | Host | TYLCV | Developmental stages | Tissue | Biotype | |||||
| Gene | SV1 | Gene | SV | Gene | SV | Gene | SV | Gene | SV | |
| 1 | RPL29 | 0.197 | SDHA | 0.207 | SDHA | 0.212 | GAPDH | 0.116 | NADH | 0.150 |
| 2 | HSP90 | 0.318 | RPL29 | 0.394 | HSP90 | 0.332 | EF-1α | 0.203 | HSP40 | 0.172 |
| 3 | SDHA | 0.351 | HSP90 | 0.394 | EF-1α | 0.359 | RPL29 | 0.295 | HSP90 | 0.178 |
| 4 | NADH | 0.418 | γ-tubulin | 0.457 | RPL29 | 0.378 | HSP70 | 0.323 | RPL29 | 0.263 |
| 5 | EF-1α | 0.457 | 18s | 0.502 | NADH | 0.393 | ATPase | 0.340 | EF-1α | 0.344 |
| 6 | PPIA | 0.491 | HSP40 | 0.592 | HSP20 | 0.525 | SDHA | 0.363 | ATPase | 0.425 |
| 7 | ATPase | 0.568 | PPIA | 0.593 | HSP40 | 0.599 | HSP20 | 0.559 | HSP20 | 0.489 |
| 8 | HSP40 | 0.604 | NADH | 0.633 | 18s | 0.605 | HSP40 | 0.675 | γ-tubulin | 0.536 |
| 9 | γ-tubulin | 0.638 | HSP20 | 0.672 | ATPase | 0.605 | HSP90 | 0.807 | PPIA | 0.585 |
| 10 | GAPDH | 0.672 | EF-1α | 0.797 | GAPDH | 0.706 | PPIA | 0.858 | Myosin L | 0.812 |
| 11 | HSP20 | 0.681 | ATPase | 0.875 | PPIA | 0.759 | Actin | 0.979 | 18s | 0.841 |
| 12 | 18s | 0.710 | HSP70 | 1.221 | Myosin L | 0.901 | NADH | 1.244 | Actin | 0.945 |
| 13 | Actin | 1.186 | Actin | 1.233 | γ-tubulin | 0.936 | 18s | 1.318 | GAPDH | 1.125 |
| 14 | Myosin L | 1.216 | Myosin L | 1.310 | Actin | 1.101 | Myosin L | 1.512 | SDHA | 1.455 |
| 15 | HSP70 | 1.240 | GAPDH | 1.587 | HSP70 | 1.252 | γ-tubulin | 2.121 | HSP70 | 1.563 |
: Stability Value was evaluated by Normfinder.
Table 3. Ranking of candidate reference genes in response to abiotic factors.
| Rank | Photoperiod | Temperature | Insecticide Susceptibility1 | |||
| Gene | SV2 | Gene | SV | Gene | SV | |
| 1 | HSP90 | 0.069 | HSP40 | 0.263 | EF-1α | 0.162 |
| 2 | HSP20 | 0.093 | HSP90 | 0.353 | ATPase | 0.269 |
| 3 | ATPase | 0.162 | EF-1α | 0.375 | SDHA | 0.279 |
| 4 | HSP40 | 0.181 | PPIA | 0.425 | HSP90 | 0.335 |
| 5 | RPL29 | 0.244 | NADH | 0.429 | PPIA | 0.364 |
| 6 | HSP70 | 0.262 | SDHA | 0.451 | HSP20 | 0.447 |
| 7 | EF-1α | 0.292 | RPL29 | 0.473 | RPL29 | 0.453 |
| 8 | SDHA | 0.293 | γ-tubulin | 0.569 | 18s | 0.502 |
| 9 | NADH | 0.306 | 18s | 0.587 | Actin | 0.514 |
| 10 | γ-tubulin | 0.341 | ATPase | 0.589 | NADH | 0.590 |
| 11 | PPIA | 0.379 | GAPDH | 0.684 | HSP40 | 0.607 |
| 12 | Actin | 0.385 | Myosin L | 0.917 | γ-tubulin | 0.680 |
| 13 | GAPDH | 0.463 | Actin | 1.306 | HSP70 | 0.767 |
| 14 | Myosin L | 0.605 | HSP20 | 1.804 | GAPDH | 1.461 |
| 15 | 18s | 0.893 | HSP70 | 3.981 | Myosin L | 1.484 |
: Thiamethoxam-resistant and -susceptible whiteflies.
: Stability Value was evaluated by Normfinder.
Discussion
Because it is highly sensitive, specific, accurate, and reproducible, qRT-PCR is, in many ways, superior to conventional methods (northern hybridization and semi-quantitative PCR), and has become an essential tool for gene expression analysis [13], [28], [35]–[39]. qRT-PCR analysis, however, is influenced greatly by the selection of reference genes [10], [40]–[41]. The endogenous reference genes should be stable across different experimental treatments; otherwise, a variable reference gene can compromise the qRT-PCR analysis by introducing artificial changes or masking true changes in target gene expression. Some commonly used reference genes can vary substantially in response to specific experimental conditions [11], [42]–[43]. In this study, we used two Excel-based algorithms geNorm and Normfinder to evaluate the stability of 15 candidate reference genes in B. tabaci in response to five biotic factors (host, virus, stage, tissue, and biotype) and three abiotic factors (photoperiod, temperature, and insecticide susceptibility).
A major conclusion of this study is that many of the candidate genes in B. tabaci should not be used as the default reference genes because their expression is highly variable under certain conditions. Our results indicate that the stability of reference gene expression must be validated for each experimental condition under investigation. The ranking of these reference genes differs somewhat for geNorm and Normfinder, because these programs have different algorithms and different sensitivities toward co-regulated reference genes. Despite the discrepancies, both programs identified a similar set of reference genes suited for the respective experimental conditions.
The ideal reference genes in response to biotic factors were RPL29, HSP40, and HSP90 according to geNorm and RPL29 based on Normfinder, respectively. Combing these results, RPL29 is a consensus reference gene that is reliable across a range of biotic conditions (Table 4), and this is consistent with the performance of the other ribosomal protein L32, a widely used single normaliser in gene expression studies [11], [43]–[47]. Despite subtle ranking differences between geNorm and Normfinder, the ideal reference genes in response to abiotic factors were determined to be EF1-α, PPIA, NADH, SDHA, and HSP40 (Table 4). EF-1α has rarely been used as a normaliser in the past but has recently been selected as a suitable reference gene in salmon [48], humans [47], [49], Orthoptera [46], [50], and Hymenoptera [43]. PPIA was considered sufficiently stable for normalization in this study, which is consistent with a previous report in human cervical tissues [47].
Table 4. Recommended reference genes for various experimental conditions.
| Experimental Conditions | Recommended Reference Genes | ||
| Biotic Factors | |||
| Host | HSP90 | RPL29 | EF-1α |
| TYLCV | HSP90 | RPL29 | |
| Developmental stages | NADH | HSP90 | RPL29 |
| Tissue | RPL29 | EF-1α | |
| Biotype | NADH | HSP90 | EF-1α |
| Abiotic Factors | |||
| Photoperiod | HSP40 | HSP90 | PPIA |
| Temperature | EF-1α | NADH | SDHA |
| Thiamethoxam susceptibility | PPIA | EF-1α | HSP20 |
Another conclusion of our study is that some genes that have been consistently used for the normalization study showed high levels of variation in response to certain treatments. Previously, 18S has been considered an ideal reference gene because the expression level of rRNA appears to vary considerably less than mRNA [51]. In this study, the raw Ct values of 18S ranged from 9.92 to 15.94 depending on insect body region and host plants, suggesting that the expression of 18S can be highly variable and consequently, it could not be used as a reference gene under certain experimental conditions. This result is consistent with some earlier studies on 18S RNA [11], [42]. Another commonly used reference gene, actin, encodes a major component of the protein scaffold that supports the cell and determines its shape. The expression of actin is moderately abundant in most cell types, and actin has been used extensively as a reference gene in B. tabaci and in many other insects including the desert locust [46], European honey bee [45], and two species of Collembola [52]. In our study actin was not stable among different tissues (body regions) and hosts; disqualifying actin as a suitable reference gene under these conditions.
In recent years, more researchers have adopted a multiple reference gene approach to analyze gene expression [38], [53]. Our results demonstrated that the expression of several reference genes from B. tabaci were consistently stable across selected experimental conditions. However, the best-suited reference genes can be different in response to diverse biotic and abiotic factors (Table 4). Our finding is the very first step toward establishing a standardized qRT-PCR procedure following the MIQE (Minimal Information required for Publication of Quantitative Real-Time PCR) guideline in an agriculturally important insect pest. More importantly, this study provides a solid foundation for future RNAi-based functional study in B. tabaci.
Supporting Information
Optimal number of reference genes required for accurate normalization of gene expression under biotic conditions. Based on geNorm analysis, average pairwise variations are calculated between the normalization factors NFn and NFn+1 to indicate whether inclusion of an extra reference gene increases the stability of the normalization factor. Values<0.15 indicate that additional genes are not required for the normalization of gene expression.
(TIFF)
Optimal number of reference genes required for accurate normalization of gene expression under abiotic conditions. Based on geNorm analysis, average pairwise variations are calculated between the normalization factors NFn and NFn+1 to indicate whether inclusion of an extra reference gene adds to the stability of the normalization factor. Values<0.15 indicate that additional genes are not required for the normalization of gene expression.
(TIFF)
Acknowledgments
The authors are grateful to the editor and anonymous reviewers for their constructive comments. We also thank Chun Liang (University of Hong Kong) and John Obrycki (University of Kentucky) for their engaging suggestions on an earlier draft.
Funding Statement
This research was supported by the National Science Fund for Distinguished Young Scholars (31025020), the 973 Program (2013CB127602), and the National Natural Science Foundation of China (No. 30900153). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ginzinger DG (2002) Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 30: 503–512. [DOI] [PubMed] [Google Scholar]
- 2. Bustin SA, Benes V, Nolan T, Pfaffl MV (2005) Quantitative real-time RT-PCR a perspective. J Mol Endocrinol 34: 597–601. [DOI] [PubMed] [Google Scholar]
- 3. Kubista M, Andrade JM, Bengtsson M, Forootan M, Jonák J, et al. (2006) The real-time polymerase chain reaction. Mol Aspects Med 27: 95–125. [DOI] [PubMed] [Google Scholar]
- 4. Van Guilder HD, Vrana KE, Freeman WM (2008) Twenty-five years of quantitative PCR for gene expression analysis. Biotech 44: 619–626. [DOI] [PubMed] [Google Scholar]
- 5. Thellin O, Zorzi W, Lakaye B, Coumans B, Hennen G, et al. (1999) Housekeeping genes as internal standards: use and limits. J Biotech 75: 291–295. [DOI] [PubMed] [Google Scholar]
- 6. Suzuki T, Higgins PJ, Crawford DR (2000) Control selection for RNA quantization. Biotech 29: 332–337. [DOI] [PubMed] [Google Scholar]
- 7. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotech Lett 26: 509–515. [DOI] [PubMed] [Google Scholar]
- 8. Tunbridge EM, Eastwood SL, Harrison PJ (2011) Changed relative to what? housekeeping genes and normalization strategies human brain gene expression studies. Biol Psychiatry 69: 173–179. [DOI] [PubMed] [Google Scholar]
- 9. Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, et al. (2004) Guideline to reference gene selection for quantitative real-time PCR. Biochem Bioph Res Co 313: 856–862. [DOI] [PubMed] [Google Scholar]
- 10. Vandesompele J, De Preter K, Pattyn F, Poppe B, Roy NV, et al. (2002) Accurate normalization of realtime quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ponton F, Chapuis MP, Pernice M, Sword GA, Simpson SJ (2011) Evaluation of potential reference genes for reverse transcription qPCR studies of physiological responses in Drosophila melanogaster . J Insect Physiol 57: 841. [DOI] [PubMed] [Google Scholar]
- 12. Zhou XG, Tarver MR, Bennett GW, Oi FM, Scharf ME (2006) Two hexamerin genes from the termite Reticulitermes flavipes: sequence, expression, and proposed functions in caste regulation. Gene 376: 47–58. [DOI] [PubMed] [Google Scholar]
- 13. Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 14. Brown JK, Frohlich DR, Rosell RC (1995) The sweetpotato or silverleaf whiteflies: biotypes of Bemisia tabaci or a species complex? Annu Rev Entomol 40: 511–534. [Google Scholar]
- 15. Inbar M, Gerling D (2008) Plant-mediated interactions between whiteflies, herbivores and natural enemies. Ann Rev Entomol 53: 431–448. [DOI] [PubMed] [Google Scholar]
- 16. Jones DR (2003) Plant viruses transmitted by whiteflies. Eu J Plant Pathol 109: 195–219. [Google Scholar]
- 17. Perring TM (2001) The Bemisia tabaci species complex. Crop Prot 20: 725–737. [Google Scholar]
- 18. Dinsdale A, Cook L, Riginos C, Buckley YM, De Barro PJ (2010) Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann Entomol Soc Am 103: 196–208. [Google Scholar]
- 19. Xu J, De Barro PJ, Liu SS (2010) Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull Entomol Res 100: 359–366. [DOI] [PubMed] [Google Scholar]
- 20. De Barro PJ, Liu SS, Boykin LM, Dinsdale A (2011) Bemisia tabaci: a statement of species status. Annu Rev Entomol 56: 1–19. [DOI] [PubMed] [Google Scholar]
- 21. Luo C, Yao Y, Wang RJ, Yan FM, Hu DX (2002) The use of mitochondrial cytochrome oxidase mtCO1 gene sequences for the identification of biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomologica Sinica 4: 759–763. [Google Scholar]
- 22. Chu D, Zhang YJ, Brown JK, Cong B, Xu BY, et al. (2006) The introduction of the exotic biotype Q of Bemisia tabacifrom the Mediterranean region into China on ornamental crops. Fla Entomol 89: 168–174. [Google Scholar]
- 23. Pan HP, Chu D, Ge DQ, Wang SL, Wu QJ, et al. (2011) Further spread of and domination by Bemisia tabaci biotype Q on field crops in China. J Econ Entomol 104: 978–985. [DOI] [PubMed] [Google Scholar]
- 24. Mahadav A, Kontsedalov S, Czosnek H, Ghanim M (2009) Thermotolerance and gene expression following heat stress in the whitefly Bemisia tabaci B and Q biotypes. Insect Biochem Mol Biol 39: 668–676. [DOI] [PubMed] [Google Scholar]
- 25. Ghanim M, Kontsedalov S (2009) Susceptibility to insecticides in the Q biotype of Bemisia tabaci is correlated with bacterial symbiont densities. Pest Manag Sci 65: 939–942. [DOI] [PubMed] [Google Scholar]
- 26. Upadhyay SK, Chandrashekar K, Thakur N, Verma PC, Borgio JF, et al. (2011) RNA interference for the control of whiteflies (Bemisia tabaci) by oral route. J Biosci 36: 153–161. [DOI] [PubMed] [Google Scholar]
- 27. Lü ZC, Wan FH (2011) Using double-stranded RNA to explore the role of heat shock protein genes in heat tolerance in Bemisia tabaci (Gennadius). J Exp Biol 214: 764–769. [DOI] [PubMed] [Google Scholar]
- 28. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 29. Xie W, Meng QS, Wu QJ, Wang SL, Yang X, et al. (2012) Pyrosequencing the Bemisia tabaci transcriptome reveals a highly diverse bacterial community and a robust system for insecticide resistance. PLoS ONE 7: e35181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie W, Wang SL, Wu QJ, Feng YT, Pan HP, et al. (2011) Induction effects of host plants on insecticide susceptibility and detoxification enzymes of Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag Sci 67: 87–93. [DOI] [PubMed] [Google Scholar]
- 31. Wu JB, Dai FM, Zhou XP (2006) First report of Tomato yellow leaf curl virus in China. Ann Appl Biol 155: 439–448. [DOI] [PubMed] [Google Scholar]
- 32. Ghanim M, Morin S, Zeidan M, Czosnek M (1998) Evidence for transovarial transmission of tomato yellow leaf curl virus by its vector, the whitefly Bemisia tabaci . Virology 240: 295–303. [DOI] [PubMed] [Google Scholar]
- 33. Jiu M, Zhou XP, Tong L, Xu J, Yang X, et al. (2007) Vector-virus mutualism accelerates population increase of an invasive whitefly. PLoS ONE 2: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feng YT, Wu QJ, Wang SL, Chang XL, Xie W, et al. (2010) Cross-resistance study and biochemical mechanisms of thiamethoxam resistance in B-biotype Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag Sci 66: 313–318. [DOI] [PubMed] [Google Scholar]
- 35. Willems E, Mateizel I, Kemp C, Cauffman G, Sermon K, et al. (2006) Selection of reference genes in mouse embryos and in differentiating human and mouse ES cells. Int J Dev Biol 50: 627–635. [DOI] [PubMed] [Google Scholar]
- 36. Hoogewijs D, Houthoofd K, Matthijssens F, Vandesompele J, Vanfleteren JR (2008) Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans . BMC Mol Biol 9: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langnaese K, John R, Schweizer H, Ebmeyer U, Keilhoff G (2008) Selection of reference genes for quantitative real-time PCR in a rat asphyxial cardiac arrest model. BMC Mol Biol 9: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tatsumi K, Ohashi K, Taminishi S, Okano T, Yoshioka A, et al. (2008) Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochem Bioph Res Co 374: 106–110. [DOI] [PubMed] [Google Scholar]
- 39. Huis R, Hawkins S, Neutelings G (2010) Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.). BMC Plant Biol 10: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29: 23–39. [DOI] [PubMed] [Google Scholar]
- 41. Gutierrez L, Mauriat M, Guenin S, Pelloux J, Lefebvre JF, et al. (2008) The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. J Plant Biotech 6: 609–618. [DOI] [PubMed] [Google Scholar]
- 42. Shen GM, Jiang HB, Wang XN, Wang JJ (2010) Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol Biol 11: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hornáková D, Matoušková P, Kindl J, Valterová I, Pichová I (2010) Selection of reference genes for real-time polymerase chain reaction analysis in tissues from Bombus terrestris and Bombus lucorum of different ages. Anal Biochem 397: 118–120. [DOI] [PubMed] [Google Scholar]
- 44. Jiang H, Liu Y, Tang P, Zhou A, Wang J (2009) Validation of endogenous reference genes for insecticide-induced and developmental expression profiling of Liposcelis bostrychophila (Psocoptera: Liposcelididae). Molecular Biol Rep 37: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 45. Scharlaken B, de Graaf DC, Goossens K, Brunain M, Peelman LJ, et al. (2008) Reference gene selection for insect expression studies using quantitative real-time PCR: The head of the honeybee, Apis mellifera, after a bacterial challenge. J Insect Sci 8: 33. [Google Scholar]
- 46. Hiel MBV, Wielendaele PV, Temmerman L, Soest SV, Vuerinckx K, et al. (2009) Identification and validation of housekeeping genes in brains of the desert locust Schistocerca gregaria under different developmental conditions. BMC Mol Biol 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen YM, Li Y, Ye F, Wang FF, Lu WG, et al. (2010) Identification of suitable reference genes for measurement of gene expression in human cervical tissues. Anal Biochem 405: 224–229. [DOI] [PubMed] [Google Scholar]
- 48. Olsvik P, Lie K, Jordal AE, Nilsen T, Hordvik I (2005) Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Mol Bio l6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Silver N, Best S, Jiang J, Thein SL (2006) Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marie-Pierre C, Donya TE, Tim D, Laurence B, Fleur P, et al. (2011) Assessment and validation of a suite of reverse transcription-quantitative PCR reference genes for analyses of density-dependent behavioral plasticity in the Australian plague locust. BMC Mol Biol 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25: 169–193. [DOI] [PubMed] [Google Scholar]
- 52. De Boer M, de Boer T, Marien J, Timmermans M, Nota B, et al. (2009) Reference genes for QRT-PCR tested under various stress conditions in Folsomia candida and Orchesella cincta (Insecta, Collembola). BMC Mol Biol 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kylee J, Veazey, Michael C (2011) Golding selection of stable reference genes for quantitative RT-PCR comparisons of mouse embryonic and extra-embryonic stem cells. PLoS ONE 6: e27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Optimal number of reference genes required for accurate normalization of gene expression under biotic conditions. Based on geNorm analysis, average pairwise variations are calculated between the normalization factors NFn and NFn+1 to indicate whether inclusion of an extra reference gene increases the stability of the normalization factor. Values<0.15 indicate that additional genes are not required for the normalization of gene expression.
(TIFF)
Optimal number of reference genes required for accurate normalization of gene expression under abiotic conditions. Based on geNorm analysis, average pairwise variations are calculated between the normalization factors NFn and NFn+1 to indicate whether inclusion of an extra reference gene adds to the stability of the normalization factor. Values<0.15 indicate that additional genes are not required for the normalization of gene expression.
(TIFF)