Abstract
Objectives
Lower birth weight within the normal range predicts adult chronic diseases, but the same birth weight in different ethnic groups may reflect different patterns of tissue development. Neonatal body composition was investigated among non-Hispanic Caucasians and African Americans, taking advantage of variability in gestational duration to understand growth during late gestation.
Methods
Air displacement plethysmography assessed fat and lean body mass among 220 non-Hispanic Caucasian and 93 non-Hispanic African American neonates. The two ethnic groups were compared using linear regression.
Results
At 36 weeks gestation, the average lean mass of Caucasian neonates was 2,515 g vs. that of 2,319 g of African American neonates (difference, P = 0.02). The corresponding figures for fat mass were 231 and 278 g, respectively (difference, P = 0.24). At 41 weeks, the Caucasians were 319 g heavier in lean body mass (P < 0.001) but were also 123 g heavier in fat mass (P = 0.001). The slopes for lean mass vs. gestational week were similar, but the slope of fat mass was 5.8 times greater (P = 0.009) for Caucasian (41.0 g/week) than for African American neonates (7.0 g/week).
Conclusions
By 36 weeks of gestation, the African American fetus developed similar fat mass and less lean mass compared with the Caucasian fetus. Thereafter, changes in lean mass among the African American fetus with increasing gestational age at birth were similar to the Caucasian fetus, but fat accumulated more slowly. We hypothesize that different ethnic fetal growth strategies involving body composition may contribute to ethnic health disparities in later life.
INTRODUCTION
Lower birth weight within the normal range has been shown to predict later chronic diseases, including cardiovascular disease, type 2 diabetes, and the metabolic syndrome (Barker, 1995), diseases for which African Americans have a high risk compared with Caucasians (Beckles et al., 2011; Keenan and Shaw, 2011). These associations have been ascribed to variability in fetal growth patterns. Birth weight is a crude marker of fetal growth, however, and the same weight can reflect many different paths of growth. Further studies are required to uncover the nature of anatomical and physiological underpinnings of developmental bases for adult disease to clarify causality and promote interventional strategies.
Understanding the mechanisms underlying the associations between size at birth and subsequent health has been limited by the relative absence of data relating neonatal body size to mediators of metabolic effects and disease risk. One of these mediators is body composition and its fetal antecedents. Among south Asian neonates, for example, there is a “thin-fat” phenotype. Compared with neonates in the United Kingdom, babies born in Pune, India are long and thin, with a deficit of nonfat soft tissues but preservation of fat (Muthayya et al., 2006; Yajnik et al., 2003).
There are few investigations of African American neonatal body composition, and these offer conflicting observations. Using body composition information from dual-energy X-ray absorptiometry (DXA), no significant mean differences from Caucasian neonates were found (Koo et al., 2000). Based on anthropometry, lower birth weight among African American neonates was associated with lower average lean mass, but no difference in subcutaneous fat mass (Singh et al., 2010).
A significant challenge to the clarification of fetal growth as a source for lifelong health is the technical limitation faced by the longitudinal study of the in utero fetus. There are no reports considering ethnic differences in the timing of fetal tissue differentiation. This is an important evidentiary gap because variations in body composition at birth are associated with differences in later health outcomes. For example, coronary heart disease among men is more strongly associated with thinness at birth than with low birth weight (Weiss, 2007). Ethnic diversity in the pathological sequelae of the metabolic syndrome has been reported (Demerath et al., 2007; Lancaster et al., 2006), including variability in vascular consequences of the same risk factors (Bang et al., 2009). As a case in point, percent body fat is an independent predictor of arterial stiffness among Caucasians, but not among south Asians (Eapen et al., 2011). To address this information gap, we use air displacement plethysmography (ADP; Peapod™) to investigate potential differences in neonatal body composition between non-Hispanic Caucasians and African Americans at the time of delivery, taking advantage of variability in length of gestation to understand the accumulation of fat and lean mass in late gestation.
METHODS
Women delivering at William Beaumont Hospital, Royal Oak, Michigan, and Hutzel Women’s Hospital, Detroit, Michigan, were invited to participate in this prospective, cross-sectional investigation between August 2004 and April 2009. Exclusion criteria for study participation included delivery before the third trimester, multiple gestations, or congenital anomalies. The study sample comprised 220 non-Hispanic Caucasian and 93 non-Hispanic African American neonates defined by maternal self-assigned ethnicity. Hispanics represent only 5% of the population of Detroit and were not included. This study was approved by the Institutional Review boards of William Beaumont Hospital, Wayne State University, and Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Gestational age at birth was assessed from the first day of the last menstrual period, confirmed by ultrasound biometry of either crown-rump length measurements (first trimester) or biparietal diameter, head circumference, abdominal circumference, and femoral diaphysis length (second trimester). If there was a discrepancy of more than 1 week, sonographic age was used to date the pregnancy. Gestational ages are expressed in completed weeks estimated to the day.
Neonatal fat and lean body mass were assessed using ADP (PEA POD®, Life Measurement, Concord, CA). This approach measures body density and calculates a two-compartment model of body composition. Previous research has validated the reliability and accuracy of the system (Ellis et al., 2007; Ma et al., 2004). In this study, 256 infants were measured within the first 24 h (84% of the sample), the remaining 16% within 28 h, and one infant on day 3 of life. There was no significant difference in the distribution of measurement day by ethnicity and no significant association of time to measurement with gestational age at birth. The neonates were evaluated without clothing, and care was taken to compensate for the volume and mass of the plastic umbilical cord clip and name bracelet. Artifacts in volume measurement of neonates with excessive amounts of hair were minimized with the use of baby oil. After body weight was obtained, the neonate was placed into the temperature-controlled, enclosed chamber for the ADP. A body volume measurement was attained within ~ 2 min. Body density was calculated from these data and applied to the two-compartment model estimate of percent fat and lean mass (Ellis et al., 2007). Total fat and lean mass (g) were calculated by multiplying these percentages by the concurrent total neonatal body mass.
Statistical Analysis
The normality of study variables was assessed with Shapiro-Wilk. The samples were compared for neonatal sex proportion and for maternal weight, age, pregnancy complications, and self-reported smoking. Group comparisons used χ2 for categorical variables, t-test for Gaussian variables, and K-sample median test for non-Gaussian variables. Kolmogorov-Smirnov compared the distributions of weight among neonatal samples by sex and ethnicity. Kruskal-Wallis compared non-Gaussian variables for homogeneity of population, and Levene’s test assessed the equality of variances.
Linear and parametric models to the third degree were compared for goodness-of-fit to describe the relationship between body mass parameters and weeks of gestational age at birth as a continuous variable (Royston and Altman, 1994). Ordinary least squares regression models for lean and fat body mass were stratified by ethnicity and sex. The homogeneity of relationships by ethnicity and sex was specifically addressed by an interaction term between ethnicity and gestational age in the first step and sex by gestational age within ethnic in the second step. The slopes were compared with the F-test. The primary regression model considered only neonates delivered of pregnancies free from pathologic conditions that affect body size and composition (i.e., gestational diabetes and preeclampsia) (Catalano et al., 2003; Rasmussen and Irgens, 2003). The check for robustness of the model, a second regression included all neonates with categorical variables for the pregnancy-associated complications.
Regression analyses considered maternal age, prepregnancy weight, pregnancy weight gain, and self-reported smoking as covariates. Maternal age, prepregnancy weight, and pregnancy weight gain positively predicted neonatal lean and fat mass and were retained in the analyses. The interactions between gestational age at birth and maternal age, prepregnancy weight, and weight gain were investigated; they were not significant and were not retained. All statistical analyses used STATA version 11 (StataCorp., 2009).
RESULTS
Maternal characteristics
Among women without pregnancy complications, Caucasians weighed less than African Americans before pregnancy and gained more weight during pregnancy (Table 1). Caucasian women were significantly older than African American women. Two African American women experienced chronic hypertension, and pregnancy complications included gestational diabetes (10 Caucasians and 12 African Americans) and preeclampsia (four Caucasians and 10 African Americans). No information on population of origin, family socioeconomic situation, maternal education, or parity was available for the study sample.
Table 1.
Median values (interquartile range) of the maternal characteristics of the sample
| Caucasian (n = 206) | African American (n = 71) | P for difference | |
|---|---|---|---|
| Maternal age (years) | 30 (27–34) | 25 (21–31) | 0.001 |
| Prepregnancy weight (kg) | 63.3 (56.7–73.9) | 68.2 (57.6–88.0) | 0.02 |
| Pregnancy weight gain (kg) | 14.0 (10.3–18.2) | 11.3 (5.4–15.9) | 0.003 |
| Maternal weight after delivery (kg) | 79.7 (70.3–88.5) | 81.6 (66.8–97.1) | 0.79 |
| Maternal smoking | n = 14 | n = 1 | 0.08 |
Birth weight and gestational age at birth
Neonates from pregnancies complicated by gestational diabetes weighed more on average at birth than neonates from uncomplicated pregnancies (P = 0.002). Neonates from pregnancies complicated by preeclampsia weighed less than neonates from uncomplicated pregnancies (P < 0.001). Caucasian neonates had a higher mean birth weight compared with African American neonates (P < 0.001, Table 2). There were no significant sex differences in birth weight in either ethnic group. There were no ethnic differences in the length of gestation or the proportion of preterm births.
Table 2.
Mean (SD) or median (interquartile range) of neonatal characteristics of the sample
| Caucasian (n = 206) | African American (n = 71) | P for difference | |
|---|---|---|---|
| Neonatal sex (% males) | 51% | 49% | 0.81 |
| Gestational age at delivery (weeks) | 39.0 (37.6–39.9) | 38.7 (37.6–40.0) | 0.88 |
| Males | 39.0 (37.4–39.9) | 38.5 (37.1–39.9) | 0.89 |
| Females | 39.0 (37.9–39.9) | 39.1 (38.1–39.7) | 0.87 |
| Preterm (<37 gestational weeks) | 17.0% | 16.9% | 0.99 |
| Males | 21.9% | 17.1% | 0.55 |
| Birthweight (g) | 3,392 (549.7) | 3,086 (466.8) | 0.001 |
| Males | 3,451 (572.1) | 3,155 (486.4) | 0.008 |
| Females | 3,330 (521.1) | 3,018 (443.2) | 0.003 |
| Neonatal body mass (g) | 3,269 (535.9) | 3,009 (454.4) | 0.001 |
| Males | 3,327 (550.7) | 3,068 (470.4) | 0.015 |
| Females | 3,209 (515.8) | 2,951 (437.1) | 0.015 |
| Lean mass (g) | 2,924 (407.9) | 2,691 (378.9) | 0.0001 |
| Males | 3,003 (431.7) | 2,772 (380.9) | 0.007 |
| Females | 2,842 (365.9) | 2,613 (365.4) | 0.002 |
| Fat mass (g) | 313 (216.7–459.8) | 319 (240.7–396.9) | 0.51 |
| Males | 290 (206.9–440.4) | 282 (220.8–361.1) | 0.51 |
| Females | 331 (226.6–491.5) | 338 (269.3–419.9) | 0.73 |
Neonatal fat and lean mass
Neonates of pregnancies complicated by gestational diabetes had both greater fat mass (P < 0.001 and lean mass (P = 0.01) than those from uncomplicated pregnancies, and neonates of pregnancies complicated by preeclampsia had less lean mass (P = 0.006) (neonatal sex and gestational age at birth as covariates).
Among neonates delivered of pregnancies free from pathologic conditions, males had greater average lean mass than females among both Caucasian (3,003 vs. 2,842 g, P = 0.004) and African American (2,772 vs. 2,613 g, P = 0.08) neonates (Table 2). No significant sex differences were found for neonatal total mass or fat mass. Caucasian neonates had greater average lean mass (P < 0.001), but there were no significant differences in mean fat mass.
Ethnic differences in neonatal body composition by gestational age at birth
Neonates from pregnancies complicated by gestational diabetes weighed more on average at birth than neonates from uncomplicated pregnancies (P = 0.002). Neonates from pregnancies complicated by preeclampsia weighted less than neonates from uncomplicated pregnancies (P < 0.001). Caucasian neonates had a higher mean birth weight compared with African American neonates (P < 0.001, Table 2). There were no significant sex differences in birth weight in either ethnic group. There were no ethnic differences in the length of gestation or the proportion of preterm births.
Neonatal fat and lean mass
Neonates of pregnancies complicated by gestational diabetes had both greater fat mass (P < 0.001) and lean mass (P = 0.01) than those from uncomplicated pregnancies, and neonates of pregnancies complicated by preeclampsia had less lean mass (P = 0.006) (neonatal sex and gestational age at birth as covariates).
Among neonates delivered of pregnancies free from pathologic conditions, males had a greater average lean mass than females among both Caucasian (3,003 vs. 2,842 g, P = 0.004) and African American (2,772 vs. 2,613 g, P = 0.08) neonates (Table 2). No significant sex differences were found for neonatal total mass or fat mass. Caucasian neonates had greater average lean mass (P < 0.001), but there were no significant differences in mean fat mass.
Ethnic differences in neonatal body composition by gestational age at birth
The higher lean mass of Caucasian neonates was established by 36 weeks of gestation (Fig. 1). Among neonates delivered at 36 gestational weeks, the average lean mass of Caucasian neonates was 2,515 g (SD 45.3 g) compared with that of 2,319 g of African American neonates (SD 70.3 g, difference P = 0.02). After that time, the changes in lean mass with increasing gestational age at birth were similar in the two ethnic groups.
Figure 1. Ethnicity and late gestation body composition.
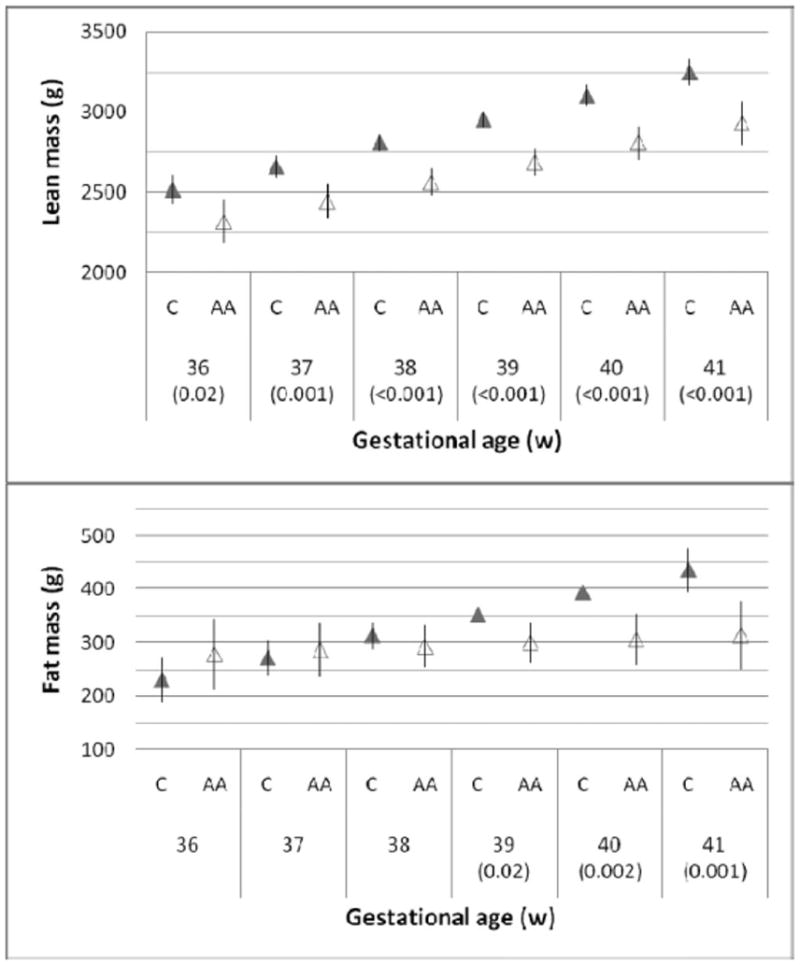
Lean and fat mass according to completed weeks of gestation at birth (mean and SEM). Figures in parentheses are P-values for group differences <0.05 among African Americans (open triangles) and Caucasians (shaded triangles) (neonatal sex, maternal age, prepregnancy weight, and pregnancy weight gain were covariates).
By contrast, there was no significant difference in fat mass at 36 weeks, with an average of 230.6 g (SD 33.5 g) among African Americans (difference P = 0.24). Thereafter, the changes in size for gestational age at birth diverged so that fat mass among Caucasian neonates changed more with increasing gestational age at birth than among African American neonates. The slope of the linear regression of fat mass vs. gestational weeks of age at birth was 5.8 times greater (P = 0.009) for Caucasian neonates (41.0 g/week) than for African American neonates (7.0 g/week). This represents an accrual to Caucasian neonates of 34 g/week or about 10% of the median fat mass at birth (313 g).
By 41 gestational weeks of age at birth, the Caucasian were 319 g (SD 80.6 g) heavier in lean body mass (P < 0.001) but were also 123 g (SD 38.4 g) heavier in fat mass (P = 0.001). Thus, increasing gestational age at birth was associated with decreasing fat to lean ratio among African American neonates compared with Caucasian neonates. The patterns were similar in the two sexes.
Testing the robustness of the regression analyses by including the neonates of maternal gestational diabetes and preeclampsia, with categorical covariates for these pathologies of pregnancy, did not change the pattern of the results. Adding time between birth and ADP as a covariate also did not change the results.
DISCUSSION
Compared with Caucasian neonates, African American neonates had a lower mean birth weight with lower average lean mass, but no difference in average fat mass. This relationship was previously shown using anthropometry (Singh et al., 2010) and suggests that at any birth weight and age Caucasian neonates will have greater lean mass but similar amounts of fat, compared with African American neonates. What we have now further shown is that the similarity in mean fat mass among Caucasian and African Americans conceals ethnically different relationships of body composition at age at delivery, suggesting divergent paths of accumulation in late gestation.
When we compared neonates born after increasing duration of gestation, lean mass at birth between 36 and 41 gestational weeks of age changed similarly in the two ethnic groups, although the African Americans had less lean mass at each age. In contrast, there was a progressive divergence in the amount of fat mass from 36 weeks onward, so that increasing gestational age at birth brought a progressively larger distinction in fat mass between the two groups.
The lower lean mass of African American neonates born at 36 weeks of gestation indicates that they had grown more slowly than Caucasian neonates at some previous time during gestation. For neonates born after 36 weeks of gestation, however, the differences in lean mass with increasing gestational age found among the African American neonates were similar to Caucasians. This implies that lean mass accrual was accelerated among African Americans, relative to Caucasians, at some point in gestation. In contrast, the African Americans had accumulated fat at the same rate as Caucasians up to 36 weeks, but thereafter their fat accumulation declined relative to Caucasians of similar gestational age at birth.
Implications of neonatal body composition variability
The underlying mechanisms responsible for these observations are not known. The description of ethnic variability in body composition by age at birth, provided by the cross-sectional outcome data, provides a viewpoint on early development that is not available from serial ultrasounds of the whole fetal body. The data are hypothesis generating and raise questions of both theoretical and practical importance regarding differential risks for health sequelae.
Developmental timing
Recent models of the development of fetal body composition tend toward a late-gestation fat-deposition paradigm (Wells et al., 2007) based on relatively small sample sizes with little ethnic differences to date (Cooke and Griffin, 2009; Rigo et al., 1998). The data from this study suggest that the late-gestation fat-deposition paradigm may be biased toward a description of Caucasian developmental patterns. Likewise, reporting on DXA results taken within 48 h of birth from 70 newborns appropriate-for-gestational-age (32–41 gestational weeks of age), Lapillonne et al. (1997) reflected that ideas about the importance of the third trimester for fat and calcium include studies that may not reflect universal developmental patterns. Similar conclusions have been reported among full-term appropriate-for-gestational-age infants assessed by anthropometry (Luque et al., 2009). Taken together with observations that variability in birth weight among African Americans is associated with maternal weight gain in the first half of pregnancy, whereas that among Caucasians is associated with maternal weight gain in the second half of pregnancy (Misra et al., 2010), further consideration of ethnic variability in developmental timing is warranted.
Nature of lean and fat mass tissue
In this study, the ADP results do not permit a complete understanding of the nature of lean mass and provide no precision regarding he specific anatomical distribution of fat mass. What is clear is that similar body compartment masses are not achieved at equivalent gestational age at birth. It is possible that the differential timing of body composition development inferred here reflects distinctive differences in the nature of the tissues themselves (Costello et al., 2008), only expressed at later ages. For example, an unfavorable metabolic profile among African American children of lower adiponectin levels and decreased insulin sensitivity (Osei et al., 2005), independent of total adiposity (Lee et al., 2006) compared with their Caucasian peers, has been suggested to reflect the expression of population-specific polymorphic variations associated with body composition, lipids, and insulin sensitivity (Ukkola et al., 2005). A multiethnic cohort of prepubertal children identified variability in metabolic health consequences of body fat deposition, such that African Americans had less intra-abdominal fat but an increased risk for type 2 diabetes unrelated to BMI (Casazza et al., 2009). The possibility that these patterns may be related to distinctive early growth in body composition deposition remains unexplored.
Developmental diversity: hypotheses
The cross-sectional nature of the present data prevents direct assessment of growth. Nonetheless, a hypothesis of different developmental patterns among African American and Caucasian fetuses emerges. As size for age is a summary of previous growth, the African American neonates in this sample kept pace with their Caucasian peers in terms of growth of fat body mass before 36 gestational weeks at birth. This is in contrast to less rapid growth of African American infants in earlier gestation in terms of lean body mass (they are lighter at every gestational age at birth). These relationships altered during late gestation, as increasing age at birth was associated with similar lean mass accrual with advancing age but less fat mass among African Americans compared with Caucasian neonates. It appears that African American and Caucasian neonates may have experienced different fetal developmental relationships among body mass compartments. In view of data supporting genetic bases for population differences in body composition (Shaffer et al., 2007), potential sources of these differences are worth considering.
Divergent growth strategies
Regarding a biological basis for divergent somatic trajectories, we posit two hypothetical selection scenarios: energetics and disease ecology. Although the precise energetics of building fetal tissue remain to be described, the caloric costs estimated from biochemical ATP requirements are about 4.8 times greater for protein compared with fat synthesis (Hall, 2010). Thus, a body that has proportionately less lean mass, compared with fat tissue, is less costly to construct. Furthermore, oxidative metabolism and body composition are linked, and lean mass is the principal factor contributing to metabolic requirements (Gallagher et al., 1998). Lean tissue-specific energy costs assessed among adults range from the limited costs associated with bone and skeletal muscle (13 kcal/kg/day) to the mid-range costs of liver and brain (200–240 kcal/kg/day) and the high metabolic expenses of heart and kidney tissues (440 kcal/kg/day) (Elia, 1992). Comparable fetal estimates remain to be described. Previous reports have suggested an increase of 1.37 in VO2/kg/day/g of high metabolic rate organ weight during the final 3 months of gestation (Holliday, 1997). Theoretically, a diminution in lean tissue mass, particularly high metabolic rate organs, would be an energetically thrifty fetal life strategy in terms of both tissue construction and maintenance. It is notable that a smaller mass of high metabolic rate truncal organs characterizes both the adolescent and adult habitus of African Americans, accompanied by a lower resting energy expenditure, compared with Caucasians (Gallagher et al., 2006; Sun et al., 2001).
The exact nature of the lean mass tissue that distinguishes African American and Caucasian neonates in our sample is not known. Previous studies have reported no significant differences in biparietal diameter, but longer limbs relative to trunk among the African American fetus compared with Caucasians, despite an overall reduced body weight (Garn, 1972). Thus, despite less lean mass, this may not be a diminution of the musculoskeletal system among the African Americans. Indeed, the African American neonates kept pace in changes in lean mass with age found among the Caucasians during eh late third trimester. One possibility is that African Americans did not augment fat stores to sustain accelerated growth in lean mass during late gestation, which may be the time to clad the skeleton with muscle, albeit in a trade-off with the further acquisition of fat-based tissues. Such a trade-off implies that fetal growth was a greater energetic challenge among the African American neonates compared with their Caucasian peers in the late third trimester. Perhaps, the well-described African American habitus of greater bone and skeletal mass, and smaller organs with high metabolic rate compared with Caucasians (Gallagher et al., 2006; Wang et al., 2000), has its origins during tissue growth in utero. Such an energetically conservative growth strategy may be one source of ethnic differences in subsequent health risk.
Fetal growth as the foundation for health
Increasing knowledge of variability in early body composition is important to further mechanistic understanding of the observation that low birth weight may not predict identical health risks in all settings (Huxley et al., 2004). Distinctions in body composition development, such as lower lean body mass, may contribute to ethnic disparities in health, including, for example, a shortfall in organ growth during early gestation among African Americans compared with Caucasians. In the United States, people whose birth weights are toward the lower end of the normal range are at increased risk of end-stage renal failure if they develop hypertension or type 2 diabetes (Lackland et al., 2000). The highest US rates of end-stage renal disease (ESRD) are in the south (Burrows et al., 2010) and are concentrated in the African American population (Centers for Disease Control and Prevention, 2010; Klag et al., 1997). Rates of ESRD are higher among African Americans than Caucasians at all levels of baseline-estimated glomerular filtration rate, and ESRD occurs at an earlier age (Hughson et al., 2007; Young and Kew, 2005) This may reflect a greater vulnerability of the African American kidney to the effects of hypertension and diabetes. Fetal ultrasound has identifies that renal growth between 23 and 32 weeks of gestation scales to birth weight and ponderal index (Lampl et al., 2002). In fetal life, kidney size is correlated with nephron number (Barker et al., 2006). The number of nephorns is established for life by around 34 weeks (Konje et al., 1996). There is a wide variation in the number among individuals (Puelles et al., 2011). In animal experiments, maternal undernutrition often reduces kidney size (Barker et al., 2006) and nephrogenesis (Magee et al., 2011). The kidney is an energetically high-cost (Elia, 1992), low-priority organ during intrauterine life because the mother performs its functions. In our study, the lower lean neonatal mass among African American neonates by 36 weeks of gestation is consistent with, among other reductions in visceral size, a reduced kidney mass.
Theoretical advantages posited for increased neonatal adipose tissue have included survival benefits in face of the prenatal to postnatal transition in terms of both nutrition and infection (Kuzawa, 1998; Wells, 2009). Considering the rapid turnover of body fat mass in the first 5 postnatal days (Roggero et al., 2010), survival may depend on prenatally acquired resources. Research identifying that greater energy deficits are required per unit of weight loss among individuals with higher body fat (Hall, 2008) suggests that the earlier timing of fat mass accrual would be a strategic advantage in the context of earlier gestational ages at birth characteristic of African American neonates (Behrman and Butler, 2007).
Population history and the early fat, late lean phenotype
The lower birth weight of African Americans compared with Caucasians in the United States is well documented (Behrman and Butler, 2007). Our findings show that lower birth weight is established before 36 weeks of age. Preterm birth rates are ~30% higher among African Americans than Caucasians in the United States (Spong et al., 2011). The cause is multifactorial, with interactions between race-specific expression of fetal and maternal genes (Menon et al., 2009) and environmental influences (York et al., 2010) that remain to be clarified. This study does not address sources for these disparities in birth timing and growth rates that result in smaller size at birth. Numerous studies have investigated a wide range of potential environmental, behavioral, and genetic factors, which remain controversial in their overall explanatory power (Muglia and Katz, 2010). Longer term historical circumstances that may influence infant outcomes (Jasienska, 2009) await mechanistic documentation among humans, and preconceptional stress effects, directly or in interaction with genetic susceptibility or infection, require further research (Kramer et al., 2011).
This study adds body composition differences by gestational age to investigative inquiries. In addition to contemporaneous influences, ethnic diversification in fetal growth patterns can be posited to result from natural selection on genes that govern growth timing and resulting fetal size to promote survival in the face of different environmental challenges. A specific example of an ecological niche in which the early fat/late lean pattern of body composition development described here might be beneficial is endemic Falciparum malaria, which occurs in Africa. There, a thrifty body replete with resources to survive malarial-associated challenges, including both an early birth and a postnatal environment of low subsequent weight gain (Walther et al., 2010), would be favored for survival. Moreover, fetal fitness in the face of placental malaria has been documented to be associated with fetal genetic variants of vascular endothelial growth factor receptor 1 (Muehlenbachs et al., 2008), an angiogenesis inhibitor factor, also known to slow fetal growth rate associated with altered uterine blood flow (Chaiworapongsa et al., 2008). A fetal growth pattern favoring slower growth rates of metabolically active tissues would be selectively advantageous in these ecological circumstances. Thus, one hypothesis regarding the meaning of the present observations is that altered timing of tissue differentiation may be an evolutionary consequence of maternal/fetal collaboration in the face of endemic disease.
Study strengths and limitations
These data are from the first study to assess non-Caucasian neonatal body composition with ADP and are the largest sample of Caucasian neonates yet reported (Carberry et al., 2010; Eriksson et al., 2010; Fields et al., 2011; Lee et al., in press). The average values of the Caucasian neonates are similar to the previous reports. Our study has several advantages in comparison to previously published work comparing Caucasian and African American neonates. We were able to identify distinctive trajectories in tissue growth for the first time because we specifically investigated age-based size and body composition relationships. Divergent paths of growth timing are not identifiable from a comparison of averages. Our measure of gestational age, fetal size, and other variables were reliable, and any potential differential reporting by ethnic group of these variables cannot explain the results observed. Differences in maternal weight by ethnicity were controlled in the regression models. The mean size of the babies in our study is similar to national reference values for both Caucasian and African American neonates (Alexander et al., 2003). As a limitation, it should be noted that although the PeaPod™ has been validated against four compartment models and deuterium dilution, these previous studies examined infants between 3 days and 24 weeks of age (Ellis et al., 2007; Ma et al., 2004), not 0–3 days as in the study. The validity of ADP in this critical transition period remains to be clarified.
Our study took advantage of variability in length of gestation to understand growth in body composition during late gestation. Assessment methods to carry out a longitudinal study of whole-body composition in the fetus are not available, so our approach to gaining a window on the trajectories of fat and lean tissue is innovative. Nevertheless, we were able to definitively distinguish whether gestational age is driving body composition differentially (as we have interpreted it) or whether differential body composition is driving the gestational age at birth (i.e., triggering the timing of birth). An example of the latter scenario in theory would be if African American fetuses with relatively more fat at a given age tend to be born earlier than African American fetuses with less fat at that age.
CONCLUSION
Our findings show that the lower birth weight of African Americans is established before 36 weeks of gestation. At this time, the African American fetus has developed similar fat mass but less lean body mass than the Caucasian fetus. Thereafter, the African American fetus sustained differences in lean mass at increasing gestational age similar to the Caucasian fetus, but incurred little change in fat mass with increasing gestational age at birth compared with the Caucasian fetus. We hypothesize that these observations reflect ethnically diverse patterns of tissue growth related to developmental strategies involving energy and posit body composition differences according to neonatal body size as a mediator of risk profiles for health and disease in later life.
Acknowledgments
The authors acknowledge the technical assistance of Melissa Powell, RDMS; Beverley McNie, BS, CCRP; Elizabeth Kring, RNC, BSN, CCRC; and Jennifer DeRidder, RN, BSN.
References
- Alexander GR, Kogan M, Bader D, Carlo W, Allen M, Mor J. US birthweight/gestational age-specific neonatal mortality: 1995–1997 rates for whites, Hispanics and blacks. Pediatrics. 2003;111:e61–e66. doi: 10.1542/peds.111.1.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Liebeskind DS, Pineda S, Yun SW, Ovbiagele B. Impact of metabolic syndrome on distribution of cervicocephalic atherosclerosis: data from a diverse race-ethnic group. J Neurol Sci. 2009;284:40–45. doi: 10.1016/j.jns.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Fetal origins of coronary heart disease. BMJ. 1995;311:171– 174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Bagby SP, Hanson MA. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat Clin Pract Nephrol. 2006;2:700–707. doi: 10.1038/ncpneph0344. [DOI] [PubMed] [Google Scholar]
- Beckles GL, Zhu J, Moonesinghe R. Diabetes US, 2004 and 2008. MMWR Surveill Summ. 2011;60:90–93. [PubMed] [Google Scholar]
- Behrman RE, Bulter AL, editors. Preterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press; 2007. [PubMed] [Google Scholar]
- Burrows NR, Hora I, Cho P, Gerzoff RB, Geiss LS. Incidence of endstage renal disease attributed to diabetes among persons with diagnosed diabetes—Unites States and Puerto Rico, 1996–2007. MMWR Morb Mortal Wkly Rep. 2010;59:1361–1366. [PubMed] [Google Scholar]
- Carberry AE, Colditz PB, Lingwood BE. Body composition from birth to 4.5 months in infants born to non-obese women. Pediatr Res. 2010;68:84–88. doi: 10.1203/PDR.0b013e3181df5421. [DOI] [PubMed] [Google Scholar]
- Casazza K, Dulin-Keita A, Gower BA, Fernandez JR. Intra-abdominal fat is related to metabolic risk factors in Hispanic Americans, African Americans and in girls. Acta Paediatr. 2009;98:1965–1971. doi: 10.1111/j.1651-2227.2009.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–1704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Google Scholar]
- Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, Pineles BL, Papp Z, Hassan S. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke RJ, Griffin I. Altered body composition in preterm infants at hospital discharge. Acta Paediatr. 2009;98:1269–1273. doi: 10.1111/j.1651-2227.2009.01354.x. [DOI] [PubMed] [Google Scholar]
- Costello PM, Rowlerson A, Astaman NA, Anthony FEW, Sayer AA, Cooper C, Hanson MA, Green LR. Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol. 2008;586:2371–2379. doi: 10.1113/jphysiol.2008.150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, Couch W, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity. 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen D, Manocha P, Patel RS, Kassas I, Nanjundappa R, Sher S, Uphoff I, Liu YX, Janjua U, Koduru S, Syed H, Mohammed A, Menon V, Khan AS, Neuman R, Poole J, Sperling L, Quyyumi A. Percent body fat is not an independent predictor of arterial stiffness in South Asians unlike in Caucasians. J Am Coll Cardiol. 2011;57:1583. [Google Scholar]
- Elia M. Organ and tissue contribution to metabolic rate. In: Kinney JM, Tucker HN, editors. Energy metabolism: tissue determinants and cellular corollaries. New York: Raven Press; 1992. pp. 61–80. [Google Scholar]
- Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85:90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- Eriksson B, Lof M, Forsum E. Body composition in full-term healthy infants measured with air displacement plethysmography at 1 and 12 weeks of age. Acta Paediatr. 2010;99:563–568. doi: 10.1111/j.1651-2227.2009.01665.x. [DOI] [PubMed] [Google Scholar]
- Fields DA, Gilchrist JM, Catalano PM, Gianni ML, Roggero PM, Mosca F. Longitudinal body composition data in exclusively breast-fed infants: a multicenter study. Obesity. 2011;19:1887–1891. doi: 10.1038/oby.2011.11. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Albu J, He Q, Heshka S, Boxt L, Krasnow N, Elia M. Small organs with a high metabolic rate explain lower resting energy expenditure in African American than in white adults. Am J Clin Nutr. 2006;83:1062–1067. doi: 10.1093/ajcn/83.5.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Belmonte D, Deurenberg P, Wang ZM, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of resting energy expenditure and metabolically active tissue mass. Am J Physiol Endocrinol Metab. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- Garn SM. The course of bone gain and the phases of bone loss. Orthop Clin North Am. 1972;3:503–520. [PubMed] [Google Scholar]
- Hall KD. What is the required energy deficit per unit weight loss? Int J Obes. 2008;32:573–576. doi: 10.1038/sj.ijo.0803720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD. Mathematical modeling of energy expenditure during tissue deposition. Br J Nutr. 2010;104:4–7. doi: 10.1017/S0007114510000206. [DOI] [PubMed] [Google Scholar]
- Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gestation and early infancy. Pediatrics. 1971;47:169–179. [PubMed] [Google Scholar]
- Hughson Samuel T, Hoy WE, Bertram JF. Glomerular volume and clinicopathologic features related to disease severity in renal biopsies of African Americans and whites in the southeastern United States. 2007. Arch Pathol Lab Med. 2007;131:1665–1672. doi: 10.5858/2007-131-1665-GVACFR. [DOI] [PubMed] [Google Scholar]
- Huxley R, Owen CG, Whincup PH, Cook D, Colman S, Collins R. Birth weight and subsequent cholesterol levels. Exploration of the “fetal origins” hypothesis. JAMA. 2004;292:2755–2764. doi: 10.1001/jama.292.22.2755. [DOI] [PubMed] [Google Scholar]
- Jasienska G. Low birth weight of contemporary African Americans: an intergenerational effect of slavery? Am J Hum Biol. 2009;21:16–24. doi: 10.1002/ajhb.20824. [DOI] [PubMed] [Google Scholar]
- Keenan NL, Shaw KM. Coronary heart disease and stroke deaths US 2006. MMWR Surveill Summ. 2011;60:62–66. [PubMed] [Google Scholar]
- Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men: 16- year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- Konje JC, Bell SC, Morton JJ, de Chazal R, Taylor DJ. Human fetal kidney morphometry during gestation and the relationship between weight, kidney morphometry and plasma active renin concentration at birth. Clin Sci (Lond) 1996;91:169–175. doi: 10.1042/cs0910169. [DOI] [PubMed] [Google Scholar]
- Koo WWK, Walters JC, Hockman EM. Body composition in human infants at birth and postnatally. J Nutr. 2000;130:2188–2194. doi: 10.1093/jn/130.9.2188. [DOI] [PubMed] [Google Scholar]
- Kramer MR, Hogue CJ, Dunlop AL, Menon R. Preconceptional stress and racial disparities in preterm birth: an overview. Acta Obstet Gynecol Scand. 2011;90:1307–1316. doi: 10.1111/j.1600-0412.2011.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW. Adipose tissue in human infancy and childhood: an evolutionary perspective. Am J Phys Anthropol Suppl. 1998;27:177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Lackland DL, Bendall HE, Osmond C, Egan BM, Barker DJP. Low birthweights contribute to the high rates of early onset chronic renal failure in the Southeast United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- Lampl M, Kuzawa C, Jeanty P. Infants thinner at birth exhibit smaller kidneys for their size in late gestation in a sample of fetuses with appropriate growth. Am J Hum Biol. 2002;14:398–406. doi: 10.1002/ajhb.10050. [DOI] [PubMed] [Google Scholar]
- Lancaster KJ, Watts SO, Dixon LB. Dietary intake and risk of coronary heart disease differ among ethnic subgroups of black Americans. J Nutr. 2006;136:446–451. doi: 10.1093/jn/136.2.446. [DOI] [PubMed] [Google Scholar]
- Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997;86:196–200. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth. Diabetes Care. 2006;29:51–56. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- Lee W, Riggs T, Koo W, Deter RL, Yeo L, Romero R. The relationship of newborn adiposity to fetal growth outcome based on birth weight or the modified neonatal growth assessment score. J Matern Fetal Neonatal Med. 2009 Apr 12; doi: 10.3109/14767058.2012.683084. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque V, Mendez G, Capdevila F, Closa R, Ferre N, Reina Garcia M, Escribano J. Subcutaneous fat stores related to weight in full-term neonates. Ann Hum Biol. 2009;36:88–97. doi: 10.1080/03014460802575633. [DOI] [PubMed] [Google Scholar]
- Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, Wong W, Nommsen-Rivers L, Dewey KG. Validation of a new pediatric air-displacement plethysmography for assessing body composition in infants. Am J Clin Nutr. 2004;79:653–660. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- Magee TR, Tafti SA, Desai M, Liu Q, Ross MG, Nast CC. Maternal undernourished fetal kidneys exhibit differential regulation of nephrogenic genes including downregulation of the notch signaling pathway. Reprod Sci. 2011;18:563–576. doi: 10.1177/1933719110393025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, Fortunato SJ, Thorsen P. Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Rep Biol Endocrinol. 2009;7:62–78. doi: 10.1186/1477-7827-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra VK, Hobel CJ, Sing CF. The effects of maternal weight gain patterns on term birth weight in African-American women. J Matern Fetal Neonatal Med. 2010;23:842–849. doi: 10.3109/14767050903387037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbachs A, Fried M, Lachowitzer J, Mutabingwa TK, Duffy P. Natural selection of FLT1 alleles and their association with malaria resistance in utero. Proc Natl Acad Sci USA. 2008;105:14488–14491. doi: 10.1073/pnas.0803657105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muglia LJ, Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- Muthayya S, Dwarkanath P, Thomas T, Vaz M, Mhaskar A, Mhaskar R, Thomas A, Bhat S, Kurpad A. Anthropometry and body composition of south Indian babies at birth. Public Health Nutr. 2006;9:896–903. doi: 10.1017/phn2006943. [DOI] [PubMed] [Google Scholar]
- Osei K, Gaillard T, Schuster D. Plasma adiponectin levels in high risk African-Americans with normal glucose tolerance, imparied glucose tolerance, and type 2 diabetes. Obesity Res. 2005;13:179–185. doi: 10.1038/oby.2005.23. [DOI] [PubMed] [Google Scholar]
- Puelles VH, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF. Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens. 2011;10:7–15. doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- Rasmussen S, Irgens LM. Fetal growth and body proportion in preeclampsia. Obstet Gynecol. 2003;101:575–583. doi: 10.1016/s0029-7844(02)03071-5. [DOI] [PubMed] [Google Scholar]
- Rigo J, Nyamugabo K, Picaud JC, Gerard P, Pieltain C, De Curtis M. Reference values of body composition obtained by dual energy X-ray absorptiometry in preterm and term neonates. J Pediatr Gastroenterol Nutr. 1998;27:184–190. doi: 10.1097/00005176-199808000-00011. [DOI] [PubMed] [Google Scholar]
- Roggero P, Gianni ML, Orsi A, Piemontese P, Amato O, Moioli C, Mosca F. Neonatal period: body composition changes in breast-fed full-term newborns. Neonatology. 2010;97:139–143. doi: 10.1159/000239767. [DOI] [PubMed] [Google Scholar]
- Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimoniousparametric modeling. Appl Stat. 1994;43:429–467. [Google Scholar]
- Shaffer JR, Kammerer CM, Reich D, McDonald G, Patterson N, Goodpaster B, Bauer DC, Li J, Newmand AB, Cauley JA, Harris TB, Tylavsky F, Ferrell RE, Zmuda JM Health ABC study. Genetic markers for ancestry are correlated with body composition traits in older African Americans. Osteoporos Int. 2007;18:733–741. doi: 10.1007/s00198-006-0316-6. [DOI] [PubMed] [Google Scholar]
- Singh KA, Huston-Presley LP, Mencin P, Thomas A, Amini SB, Catalano PM. Neonates born to Caucasian compared with African American mothers. Obstet Gynecol. 2010;115:998–1002. doi: 10.1097/AOG.0b013e3181da901a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spong CY, Iams J, Goldenberg R, Hauck FR, Willinger M. Disparities in perinatal medicine: preterm birth, stillbirth, and infant mortality. Obstet Gynecol. 2011;117:948–955. doi: 10.1097/AOG.0b013e318211726f. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- Sun M, Gower BA, Bartolucci AA, Hunter GR, Figueroa-Colon R, Goran MI. A longitudinal study of resting energy expenditure relative to body composition during puberty in African American and white children. Am J Clin Nutr. 2001;73:308–315. doi: 10.1093/ajcn/73.2.308. [DOI] [PubMed] [Google Scholar]
- Ukkola O, Santaniemi M, Rankinen T, Leon AS, Skinner JS, Wilmore JH, Rao DC, Bergman R, Kesäniemi Y, Bouchard C. Adiponectin polymorphisms, adiposity and insulin metabolism: HERITAGE family study and Oulu diabetic study. Ann Med. 2005;37:141–150. doi: 10.1080/07853890510007241. [DOI] [PubMed] [Google Scholar]
- Walther B, Miles DJ, Crozier S, Waight P, Palmero MS, Ojuola O, Touray E, van der Sande M, Whittle H, Rowland-Jones S, Flanagan KL. Placental malaria is associated with reduced early life weight development of affected children independent of low birth weight. Malar J. 2010;14:9–16. doi: 10.1186/1475-2875-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Heshka S, Gallagher D, Boozer CN, Kotler DP, Heymsfield SB. Resting energy expenditure-fat-free mass relationship: new insights provided by body composition modeling. Am J Physiol Endocrinol Metab. 2000;279:E539–E545. doi: 10.1152/ajpendo.2000.279.3.E539. [DOI] [PubMed] [Google Scholar]
- Weiss R. Fat distribution and storage: how much, where, and how? Eur J Endocrinol. 2007;157 (Suppl 1):S39–S45. doi: 10.1530/EJE-07-0125. [DOI] [PubMed] [Google Scholar]
- Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–434. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- Wells JC. Ethnic variability in adiposity and cardiovascular risk: the variable disease selection hypothesis. Ann Hum Biol. 2009;36:445–458. doi: 10.1093/ije/dyn183. [DOI] [PubMed] [Google Scholar]
- Yajnik CS, Fall CHD, Coyaji KJ, Hirve SS, Rao S, Barker DJP, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes. 2003;27:173–180. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- York TP, Strauss JF, III, Neale MC, Eaves LJ. Racial differences in genetic and environmental risk to preterm birth. PLoS One. 2010;25:e12391. doi: 10.1371/journal.pone.0012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CH, Kew C. Heath disparities in transplantation: focus on complexity and challenge of renal transplantation in African Americans. Med Clin North Am. 2005;89:1003–1031. doi: 10.1016/j.mcna.2005.05.002. [DOI] [PubMed] [Google Scholar]


