Abstract
Transcriptional regulation of gene expression during development is critical for proper neuronal differentiation and migration. Alternative splicing and differential isoform expression have been demonstrated for most mammalian genes, but their specific contributions to gene function are not well understood. In mice, the transcription factor gene Pitx2 is expressed as three different isoforms (PITX2A, PITX2B, and PITX2C) which have unique amino termini and common DNA binding homeodomains and carboxyl termini. The specific roles of these isoforms in neuronal development are not known. Here we report the onset of Pitx2ab and Pitx2c isoform-specific expression by E9.5 in the developing mouse brain. Using isoform-specific Pitx2 deletion mouse strains, we show that collicular neuron migration requires PITX2AB and that collicular GABAergic differentiation and targeting of hypothalamic projections require unique Pitx2 isoform dosage. These results provide insights into Pitx2 dosage and isoform-specific requirements underlying midbrain and hypothalamic development.
Keywords: migration, transcription factor, midbrain, isoform, differentiation, axon
1. Introduction
Gene expression is a tightly controlled process known to direct critical aspects of neuronal migration and differentiation (Briscoe and Novitch, 2008; Dessaud et al., 2008; Wilson and Maden, 2005). Alternative splicing adds an additional layer of gene regulation, wherein a single gene gives rise to multiple protein isoforms with distinct functions, greatly increasing functional capacity. Splicing occurs in up to 98% of human genes with multiple exons (Dessaud et al., 2008; Pan et al., 2008; Wang et al., 2008). Recent data on mouse gene splicing is not available, but previous studies found that the mouse genome undergoes slightly less splicing than the human genome (Chacko and Ranganathan, 2009; Kim et al., 2007; Modrek and Lee, 2003). Organs with increased cellular and functional complexity, such as the central nervous system (CNS), utilize gene splicing (Modrek et al., 2001; Yeo et al., 2004), nonetheless, there are few detailed studies of protein isoform functions in the developing brain. The morphogen fibroblast growth factor 8 (Fgf8) gene is expressed as eight unique isoforms with variable receptor binding properties and roles in midbrain/hindbrain development (Guo et al., 2010). Several transcription factor genes expressed in the brain, including the forkhead-domain containing gene FOXP2 and the basic helix-loop helix domain containing gene TCF4 (mutated in human Pitt-Hopkins syndrome) exhibit alternative splicing, but the specific roles of individual isoforms for these two genes in neuronal development are also unclear (Santos et al., 2011; Sepp et al., 2011). A critical unanswered question is whether different transcription factor isoforms also exhibit unique functions during brain development.
PITX2 is a bicoid-like homeodomain transcription factor gene. Heterozygous PITX2 mutations in humans result in Rieger Syndrome, characterized by developmental defects in the eyes, teeth, umbilicus, heart, and brain (Amendt et al., 2000; Childers and Wright, 1986; Cunningham et al., 1998; Idrees et al., 2006; Semina et al., 1997). Mouse models for Pitx2 deficiency exhibit ocular, tooth, and brain phenotypes similar to humans with PITX2 mutations, but the underlying molecular mechanisms of these defects are only partially understood (Gage et al., 1999; Kitamura et al., 1999; Lin et al., 1999; Liu et al., 2003; Lu et al., 1999; Martin et al., 2004; Skidmore et al., 2012; Waite et al., 2011). In the mouse CNS, Pitx2 is expressed in discrete populations of neurons in the hypothalamus, midbrain, rhombomere 1, and spinal cord. In the hypothalamus, Pitx2 is necessary for formation of the mammillothalamic tract (MTT) and midbrain Pitx2 is critical for neuronal migration and GABAergic differentiation (Skidmore et al., 2012; Waite et al., 2011). In the midbrain, Pitx2 is expressed downstream of a GABAergic cell-fate signaling cascade involving Helt and Gata2 (Cazorla et al., 2000; Miyoshi et al., 2004; Nakatani et al., 2007). In vitro studies have shown that Pitx2 is capable of activating Gad1 expression for GABA synthesis (Chen et al., 2011; Westmoreland et al., 2001), suggesting Pitx2 may act indirectly or directly as a terminal GABAergic differentiation factor.
In chick, mouse, and rat, Pitx2 gives rise to three unique isoforms (PITX2A, PITX2B, and PITX2C) that arise from alternative promoter usage and exon splicing. These isoforms have distinct N-termini which are necessary for modulation of gene expression and exhibit dosage and tissue-specific requirements (Kioussi et al., 2002; Simard et al., 2009). In mouse, PITX2C (but not PITX2AB) is required for left-sided morphogenesis of the heart, lungs, and ovaries, as well as for looping of the gut (Guioli and Lovell-Badge, 2007; Liu et al., 2001; Liu et al., 2002). Conversely, PITX2A is the only isoform expressed in and required for heart development in zebrafish (Essner et al., 2000). In vitro, PITX2C is necessary for retention of myoblasts in an undifferentiated state and for continued proliferation (Martinez-Fernandez et al., 2006), whereas PITX2A regulates actin-myosin changes in HeLa cells to promote cell spreading and migration (Wei and Adelstein, 2002). Interestingly, no unique in vivo requirements for PITX2A or PITX2B have been identified in the mouse, although PITX2AB appears to be sufficient for tooth development (Liu et al., 2003).
All three Pitx2 isoforms appear to be equally expressed in the mature rodent brain (Smidt et al., 2000). Therefore, we hypothesized that PITX2 isoforms may have unique functions during brain development. To test this hypothesis, we characterized the onset of Pitx2 isoform expression in the brain and the effects of global, conditional, or isoform-specific Pitx2 deficiency on hypothalamic and midbrain neuronal development. Our results suggest the presence of brain-region, dosage, and isoform-specific roles for Pitx2 in neuronal migration, differentiation, and axon tract formation.
2. Materials and Methods
2.1 Mice
C57BL/6J mice were obtained from the Jackson Laboratory (JAX #000664). Mouse alleles used in this study are shown in Figure 1. Pitx2Δab/+ and Pitx2Δc/+ mice were as previously described (Liu et al., 2001; Liu et al., 2002). Pitx2c-lacZ transgenic mice were created by Hiroshi Hamada and express lacZ under the control of the Pitx2c promoter (manuscript in preparation). To generate Pitx2+/−;ZsGrn mice, ZsGrn/ZsGrn reporter mice obtained from Jackson Laboratories (JAX #007006) (Madisen et al., 2010) were crossed with Pitx2+/− mice (Gage et al., 1999). To generate Pitx2Cre/−;ZsGrn embryos, Pitx2Cre/+ mice (Liu et al., 2002; Skidmore et al., 2008; Waite et al., 2011) were crossed to Pitx2+/−;ZsGrn mice. Pitx2tlz/+ mice were as previously described (Skidmore et al., 2012). Nestin-Cre (NCre) transgenic mice (Tronche et al., 1999) were crossed with Pitx2tlz/+ (Skidmore et al., 2012) to produce NCre;Pitx2tlz/+ mice. NCre;Pitx2tlz/+ mice were then crossed with Pitx2flox/flox mice (Gage et al., 1999) to generate NCre;Pitx2tlz/flox embryos.
Figure 1.
Pitx2 isoforms and alleles. (A) Map of the Pitx2 gene showing exons, introns, and isoforms. Arrows indicate alternate transcription start sites. (B) Summary of exon usage and size of Pitx2 isoforms. (C) List of mouse Pitx2 alleles used to generate unique Pitx2 deficient embryos. Pitx2 isoforms that remain intact are listed on the right.
2.2 Tissue Preparation
The morning of plug identification was designated as E0.5. Pregnant females underwent cervical dislocation and hysterectomy and embryos were dissected into PBS. Embryos were then fixed in 2–4% paraformaldehyde for 15 minutes to 4 hours, depending on the age and genotype. For frozen sections, embryos were cryoprotected overnight in 30% sucrose-PBS, flash frozen in O.C.T. embedding compound (Tissue Tek, Torrance, CA), and stored at −80°C until being sectioned at 12–30 μm. For paraffin sections, embryos were dehydrated in an ethanol gradient, embedded in paraffin, and sectioned at 7–9 μm. From each embryo, amniotic sac or tail tissue was used for genotyping. All procedures were approved by the University Committee on Use and Care for Animals at the University of Michigan.
2.3 ES cell isolation and chimera generation
On Day 1, Pitx2tlz/+ females, aged 28 days, were treated with 5 IU of pregnant mare’s serum gonadotropin. On Day 3, pregnant females were treated with 5 IU human chorionic gonadotropin and subsequently crossed to Pitx2+/− males overnight. On Day 7, pregnant females were sacrificed and blastocysts were collected. ES cell lines were prepared from blastocysts, genotyped, and cryopreserved. 3 clones each of Pitx2tlz/+ and Pitx2tlz/− ES cells were expanded, checked for chromosomal euploidy, and one clone of each genotype was injected into wild type blastocysts to generate chimeric mice with assistance from The Transgenic Animal Model Core at the University of Michigan. At E14.5, chimeric embryos were dissected from the females, cryoprepared as described below, and sectioned at 30 μm for X-gal staining. Midbrain X-gal staining was scored as normal or medially mislocalized and performed blind to the genotype.
2.4 Immunofluorescence, immunohistochemistry, and in situ hybridization
Immunofluorescence on paraffin embedded tissues was done as previously described (Martin et al., 2002; Martin et al., 2004). In preparation for frozen-section immunofluorescence, sections were fixed for 5 minutes in 4% PFA, rinsed in PBS, and washed in 0.1% PBS-Tween. Immunofluorescence was then performed as for paraffin sections. Antibodies used were rabbit anti-phosphohistone H3 at 1:200 (Upstate Biotechnology, Inc., Lake Placid, NY), rabbit anti-PITX2 at 1:8000 (provided by Dr. Thomas Jessell, Columbia University), rabbit anti-BRN3A at 1:800 (provided by Dr. Eric Turner, University of California-San Diego), and rabbit anti-GABA (Sigma). DAB immunohistochemistry was performed using a mouse anti-Neurofilament at 1:100 (2H3, Developmental Studies Hybridoma Bank) (Skidmore et al., 2008) and processed for immunohistochemistry using the Vectastain ABC reagent (Vector labs) and DAB (3,3′-Diaminobenzidine, Sigma). In situ hybridization on frozen sections was done as previously described (Martin et al., 2002; Martin et al., 2004) using cRNA probes created from PCR-amplified cDNA for Pitx2.
2.5 β-galactosidase and cresyl violet histochemistry
To generate embryonic tissues for X-gal staining, Pitx2Δab/+, Pitx2Δc/+, or Pitx2+/− female mice were crossed with Pitx2Δab/+, Pitx2+/−, or Pitx2tlz/+ males. E9.25-E14.5 whole embryos and E18.5 brains were isolated and fixed in 2–4% paraformaldehyde for 10 minutes to 4 hours, depending on age. Samples for cryosectioning were washed with PBS, cryoprotected in 30% sucrose-PBS with 2 mM MgCl2 overnight, and frozen in O.C.T. embedding medium (Tissue Tek, Torrance, CA). Frozen sections were postfixed in 0.5% glutaraldehyde fixative, washed in X-Gal Wash Buffer, and stained with X-Gal Staining Solution overnight at 37 C as previously described (Sclafani et al., 2006). Stained slides were washed in PBS, followed by eosin counterstaining, and then mounted using Permount (Fisher). For vibratome sections, whole embryos were washed in X-Gal Wash Buffer, incubated at 37º C for 3–7 days in X-Gal Staining Solution, then fixed in 4% PFA for up to 7 days. Stained embryos were embedded in 4% low-melt agarose and vibratome sectioned at 150 μm. To visualize tract formation, paraffin sections were stained with cresyl violet.
2.6 Microscopy
Confocal fluorescent images were taken using a Leica TCS SP5 X Supercontinuum Confocal System with Upright Fluorescent Microscope. For neighboring merged images, non-fluorescent sections were photographed in brightfield and converted into pseudo-fluorescent color, then overlaid in Photoshop. Brightfield and some fluorescent sections were imaged on a Leica DM500B upright microscope. For vibratome sections, wells were photographed in brightfield on a Leica MZ10F dissecting microscope. Digital images were processed with Adobe Photoshop CS3 software.
2.7 RNA isolation and real-time PCR
The midbrain of E14.5 and hypothalamus of E14.5 and E18.5 littermate mice were microdissected and RNA was isolated using the RNAqueous-Micro RNA Isolation Kit (Ambion, Austin, TX, USA). Isolated RNA was treated with DNase I prior to cDNA synthesis. cDNA was generated using the Superscript First-Strand cDNA Synthesis system for quantitative real-time PCR with random primers (Invitrogen, Carlsbad, CA, USA). Relative gene expression levels were assayed using TaqMan Gene Expression Master Mix and TaqMan probes (Applied Biosystems, Foster City, CA, USA) for Gapdh, Pitx2abc, Pitx2a, Pitx2b and Pitx2c. Each sample was run in triplicate using an Applied Biosystems StepOne-Plus Real-Time qPCR System. The gene expression level of Gapdh was used as an internal, positive control. The difference in threshold cycle (CT) between the assayed gene and Gapdh for any given sample was defined as the change in threshold cycle (ΔCT ). The difference in ΔCT between two samples was defined as ΔΔCT which represents a relative difference in expression of the assayed gene. Fold change of Pitx2a, Pitx2b, or Pitx2c relative to total Pitx2abc was defined as 2−ΔΔCT(Livak and Schmittgen, 2001).
3. Results
3.1 Pitx2 isoforms and alleles
The mouse Pitx2 gene is composed of two promoters and six exons (Fig. 1A). Alternative splicing and promoter usage generates three different Pitx2 isoforms, PITX2A, PITX2B, and PITX2C (Fig. 1A,B). All three isoforms have unique N-termini, but share the same C-terminus composed of exons 5 and 6. Exon 5 contains the homeodomain which is required for proper DNA binding, specificity, and transactivation potential of Pitx2 (Amendt et al., 1998; Saadi et al., 2001). PITX2C is the largest isoform at 324 amino acids due to the large size of exon 4, whereas PITX2A is the smallest with 271 amino acids.
To determine the functions and expression patterns of Pitx2 isoforms in the developing mouse brain, we used various combinations of mouse Pitx2 alleles (Fig. 1C). Pitx2Δab is a Pitx2ab-specific knockout allele, wherein part of exon 2 and all of exon 3 are replaced by the lacZ gene, rendering PITX2AB non-functional and leaving PITX2C intact (Liu et al., 2001). Conversely, the Pitx2Δc allele lacks exon 4, rendering PITX2C non-functional and leaving PITX2AB intact (Liu et al., 2002). The Pitx2flox allele contains two loxP sites flanking exon 5, where Cre recombination acts to excise exon 5 and create a null allele (Gage et al., 1999). The Pitx2null (or Pitx2−) allele is missing exon 5 and is functionally null (Gage et al., 1999). The Pitx2Cre allele contains a Cre sequence in place of exon 5, rendering Pitx2 non-functional with Cre expression under the control of both Pitx2 promoters (Liu et al., 2002; Liu et al., 2003; Skidmore et al., 2008). The Pitx2tlz allele contains an IRES-TauLacZ (tlz) sequence in place of exon 5, resulting in disrupted Pitx2 function and expression of β-galactosidase under control of Tau bovine neurofilament in neuronal axons (Skidmore et al., 2012).
3.2 Pitx2ab and Pitx2c are expressed in the mouse midbrain by E9.25
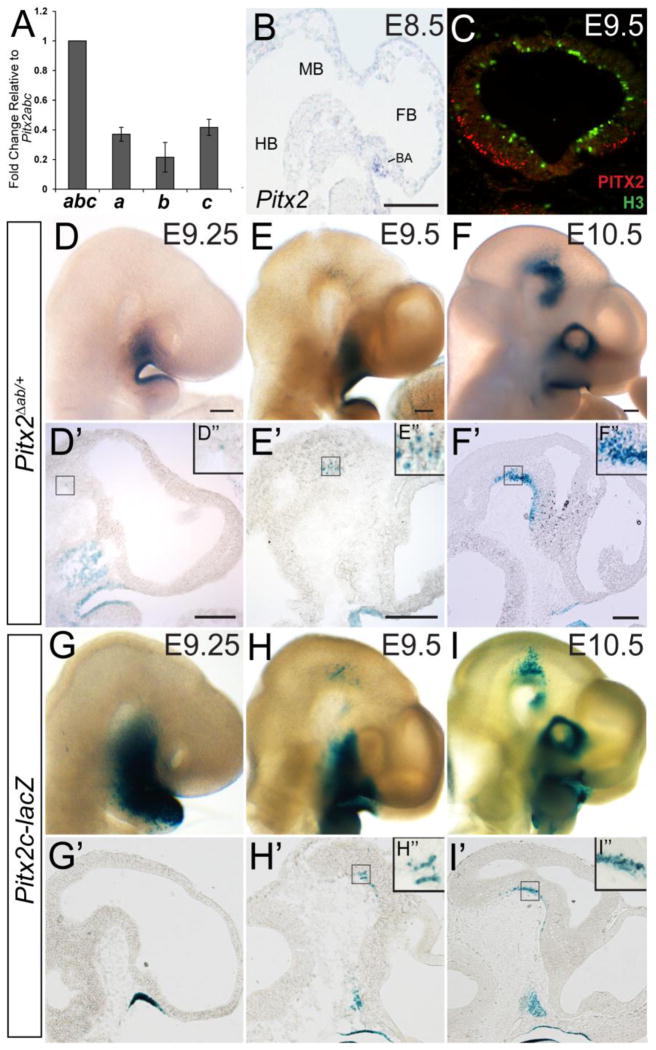
Pitx2 expression in the developing mouse embryo begins at E8.0 in the lateral plate mesoderm and is later expressed in multiple tissues, including brain, craniofacial structures, eyes, heart, and thoracic/abdominal viscera (Campione et al., 1999; Liu et al., 2001; Mucchielli et al., 1997; Porter et al., 2001). Pitx2 expression continues through adulthood in the brain, eyes, and heart (Kirchhof et al., 2011; Porter et al., 2001; Smidt et al., 2000). To determine which Pitx2 isoforms are expressed in the developing mouse brain, we performed quantitative RT-PCR (QPCR) on RNA obtained from E14.5 midbrain tissue samples (Fig. 2A). All three Pitx2 isoforms were expressed in the midbrain at E14.5 (N=3 embryos) (Fig. 2A). Pitx2b cDNA was present at half the level of Pitx2a and Pitx2c. In situ hybridization using a cRNA probe that detects all three isoforms (Fig. 1A) showed that Pitx2 mRNA is present at E8.5 in the branchial arches, but not in the neuroepithelium (Fig. 2B). Consistent with this, X-gal staining of E8.5 Pitx2Δab/+ tissues (in which βgal is expressed as a knock-in under the control of the Pitx2ab promoter) revealed no Pitx2ab-positive cells in the neuroepithelium (data not shown). However, by E9.5, PITX2 immunofluorescence was detected in post-mitotic ventral midbrain neurons, as determined by co-staining with anti-PITX2 and anti-phosphohistone H3 (Fig. 2C). Therefore, Pitx2 is expressed very early in the neuroepithelium, and by E14.5 all isoforms are expressed, albeit at different levels. Moreover, the expression level of Pitx2c decreases by 21% in midbrain and increases by 9% in hypothalamus with loss of one Pitx2ab allele, suggesting there may be mild dosage compensation by the remaining isoforms that influence our findings (Supplemental Fig. 1).
Figure 2.
Pitx2 is expressed in early post-mitotic midbrain neurons. (A) QPCR for Pitx2a, Pitx2b, and Pitx2c from E14.5 midbrain RNA shows that Pitx2a and Pitx2c are more abundant than Pitx2b. (B) Sagittal section of an E8.5 wild type embryo processed for in situ hybridization shows Pitx2 mRNA in the branchial arch (BA). (C) Coronal section of an E9.5 wild type midbrain immunostained for PITX2 (red) and H3 (green). Pitx2Δab/+ (D–F) and Pitx2c-lacZ (G–I) embryos (E9.25–E10.5) processed for wholemount X-gal staining. (D’–I’) Sagittal sections from embryos shown in D–I. Boxes in D’–I’ are enlarged in D”–I”. Pitx2ab expression is visible in the ventral midbrain in rare cells at E9.25, and is easily detected at E9.5 and E10.5. Pitx2c expression is first visible in the ventral midbrain at E9.5, and is more abundant at E10.5. Abbreviations: BA, branchial arch; FB, forebrain; HB, hindbrain; MB, midbrain. Scale bar in B is 100 μm. Scale bars in D, E, and F are 200 μm and apply to panels D–I. Scale bars in D’, E’, and F’ are 250 μm and apply to panels D’:G’, E’:H’, and F’:I’.
To determine the onset and isoform specificity of Pitx2 expression in the brain, we performed X-gal staining on E9.25–E10.5 Pitx2Δab/+ (knock-in) and Pitx2c-lacZ transgenic embryos. At E9.25, occasional βgal-positive Pitx2ab-positive cells were observed in the ventral midbrain (Fig. 2D–D”); at E9.5, numerous βgal-positive cells were detected (Fig. 2E–E”). At E10.5, βgal-positive cells in Pitx2Δab/+ embryos were visible throughout the forebrain and midbrain (Fig. 2F–F”). In Pitx2c-lacZ embryos, βgal-positive cells were absent from the ventral midbrain at E9.25 (Fig. 2G’) but present at E9.5–E10.5 (Fig. 2H–I’), indicating an onset of Pitx2c expression in the brain that is similar to Pitx2ab. Interestingly, Pitx2ab- and Pitx2c-positive cells in E9.25–E10.5 embryos were always localized to the outer regions of the ventral midbrain neuroepithelium, and did not intermingle with cells closer to the ventricle, suggesting that Pitx2 isoforms are expressed early in brain development in post-mitotic neurons.
3.3 Pitx2ab and Pitx2c are expressed in E14.5 collicular neurons
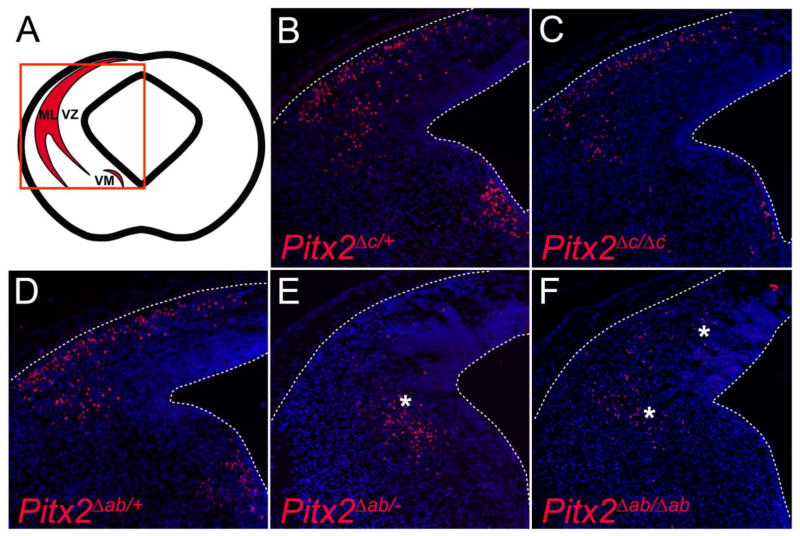
To determine which Pitx2 isoforms are present at the protein level in the superior colliculus, we analyzed PITX2 immunofluorescence in the midbrains of various Pitx2 isoform-knockout mice. Given that the immunofluorescence was performed using tyramide signal amplification, it was not possible to assign significance to apparent differences in staining intensity between the various embryos. However, some conclusions were drawn based on presence or absence of positively labeled cells. At E14.5, PITX2 protein in wild type mice localized to cells at the collicular surface and to a ventromedial (VM) neuronal population (Fig. 3B) (Martin et al., 2002; Waite et al., 2011). Pitx2Δc/Δc embryos, which lack the PITX2C isoform but produce two alleles of PITX2AB protein, exhibited a pattern of PITX2 immunofluorescence similar to wild type (Fig. 3C). In Pitx2Δab/− and Pitx2Δab/Δab embryos, which produce only PITX2C protein, PITX2 immunofluorescence was shifted medially and deep into the neuroepithelium (Fig. 3E,F), consistent with prior reports of delayed collicular neuron migration in Pitx2 null embryos (Martin et al., 2004; Waite et al., 2011). Interestingly, PITX2 is normally present in the VM population (Fig. 3B); however, ventromedial PITX2 was not present in embryos lacking PITX2AB (Pitx2Δab/− and Pitx2Δab/Δab) and appeared reduced in embryos lacking Pitx2c (Fig. 3C, E,F). This suggests that Pitx2c is either not expressed in the VM population and/or that Pitx2ab is required for the formation of the VM population. Further analyses are necessary to distinguish between these possibilities. Additionally, medial mislocalization of Pitx2-expressing neurons in Pitx2Δab/− and Pitx2Δab/Δab embryos (Fig. 3E,F and Fig. 4), suggests roles for Pitx2ab in neuronal migration.
Figure 3.
Pitx2ab and Pitx2c are expressed in midbrain neurons. (A) Schematic of coronal midbrain section highlighting Pitx2 expression as shown in panels B–F. (B–F) E14.5 coronal midbrain sections processed for PITX2 immunofluorescence. (B) Pitx2Δc/+, (C) Pitx2Δc/Δc, and (D) Pitx2Δab/+ midbrains exhibit PITX2-positive cells at the collicular pial surface and in the ventromedial (VM) population. (E–F) Pitx2Δab/− midbrains exhibit medially mislocalized PITX2-positive cells (*), whereas Pitx2Δab/Δab collicular PITX2-positive cells exhibit an intermediate location (*).
Figure 4.
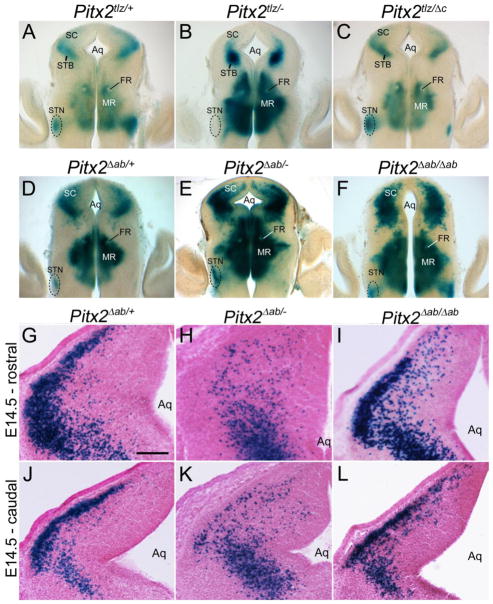
Pitx2 isoforms exhibit differential contributions to midbrain neuron migration. (A–F) Coronal midbrain sections of E14.5 X-gal stained, vibratome-sectioned (150 μm) embryos. (A,D) Embryos heterozygous for Pitx2 (Pitx2tlz/+) and Pitx2ab (Pitx2Δab/+) exhibit X-gal staining in the superior colliculus (SC), mammillary region (MR), and subthalamic nucleus (STN). (B) Pitx2tlz/− mutants exhibit medial mislocalization of collicular βgal-positive neurons and absence of label in the subthalamic nucleus. (C) Pitx2tlz/Δc embryos display normal βgal-positive neuron localization in both midbrain and hypothalamus. (E) Pitx2Δab/− embryos exhibit medially denser label. (F) Pitx2Δab/Δab embryos exhibit an intermediate phenotype, with some collicular neurons reaching the pial surface and others occupying deeper locations. X-gal stained coronal cryosections of E14.5 Pitx2Δab/+ (G,J), Pitx2Δab/− (H,K), or Pitx2Δab/Δab (I,L) colliculi. Panels are arranged rostral (G–I) to caudal (J–L) with Pitx2Δab/− (H,K) and Pitx2Δab/Δab (I,L) rostral sections showing more severe mislocalization phenotypes than caudal sections. Scale bar in G is 150 μm and applies to panels G–L. Other abbreviations: Aq, aqueduct; FR, fasciculus retroflexus; MR, mammillary region; SC, superior colliculus; STB, subtectal band; STN, subthalamic nucleus.
3.4 Distinct dosage requirements for Pitx2 isoforms in collicular neuronal migration
Previous studies showed that Pitx2 is necessary for superior colliculus neuronal migration (Martin et al., 2004; Waite et al., 2011); however, the Pitx2 isoforms required for this function were not known. Embryos heterozygous for Pitx2 tlz (Pitx2tlz/+) and Pitx2ab null (Pitx2Δab/+) alleles displayed normal localization of Pitx2-expressing cells at the collicular surface (N=7 embryos) (Fig. 4A,D), whereas loss of all Pitx2 isoforms (in Pitx2tlz/− embryos) resulted in medial or deep mislocalization of Pitx2-expressing cells (Fig. 4B). Pitx2tlz/Δc embryos, which have no functional Pitx2c and a single allele of Pitx2ab, exhibited normal collicular neuron localization (Fig. 4C). Thus, a single allele of Pitx2ab appears sufficient for superior collicular neuronal migration, whereas Pitx2c has a minor role. Interestingly, many collicular cells in Pitx2Δab/− midbrains were also mislocalized (N=7 embryos) compared to controls (Pitx2Δab/+), although the phenotype was not as severe as in Pitx2 null embryos (compare Fig. 3E and 4E to Fig. 3D, 4A, and 4B). Pitx2Δab/Δab midbrains also exhibited intermediate phenotypes (N=6 embryos), where some neurons were medially mislocalized although less severely than in Pitx2Δab/− embryos (Fig. 4F and Fig. 3F). These data suggest that Pitx2 isoforms and their dosage are both important in collicular neuron migration.
To determine whether Pitx2ab is required for the timing of collicular neuron migration, we analyzed conditional- and isoform-specific Pitx2 knockout embryos at a later gestational age (E18.5), when collicular layering is nearing completion (Edwards et al., 1986). We assessed collicular lamination using anti-BRN3A and Pitx2 expression, since Brn3a and Pitx2 mark neighboring laminae (Waite et al., 2011). At E18.5, βgal-positive neurons in Pitx2Δab/+, Pitx2Δab/Δab, and Pitx2Δab/− colliculi were properly localized between BRN3A-positive layers (Fig. 5A–I), although several βgal-positive neurons were present in deeper layers in Pitx2Δab/Δab and Pitx2Δab/− embryos (*) (Fig. 5 D–I). To determine the migrational phenotype of E18.5 midbrains in the absence of all Pitx2 isoforms, NCre;Pitx2tlz/flox embryos were analyzed by Pitx2 in situ hybridization instead of βgal staining due to faint βgal staining from the Pitx2tlz allele at this stage. In NCre;Pitx2tlz/flox E18.5 conditional mutants, most Pitx2-expressing neurons were mislocalized to the deep BRN3A-positive layer (*), although a few neurons were properly localized between BRN3A-positive layers (Fig. 5J–L). Thus, complete loss of Pitx2 leads to severely disrupted collicular neuron localization, whereas isoform-specific deletion results in milder phenotypes.
Figure 5.
Pitx2ab regulates the timing of midbrain neuronal migration. (A–L) Pseudocolored and merged images of neighboring coronal midbrain cryosections (orientation similar to panel A in figure 3) of E18.5 Pitx2Δab/+, Pitx2Δab/−, Pitx2Δab/Δab, and NCre;Pitx2tlz/flox alleles processed for X-gal (A, D, G) or Pitx2 in situ (J) and adjacent sections processed for BRN3A immunofluorescence (B, E, H, K). Merged images show some relatively normal βgal positive neuron localization (C, F, I) with some genotypes exhibiting a number of medially mislocalized neurons (*) (F, I, L). Scale bar in A is 250 μm and applies to panels A–L.
3.5 Evidence against extrinsic influences on migration of Pitx2-deficient collicular neurons
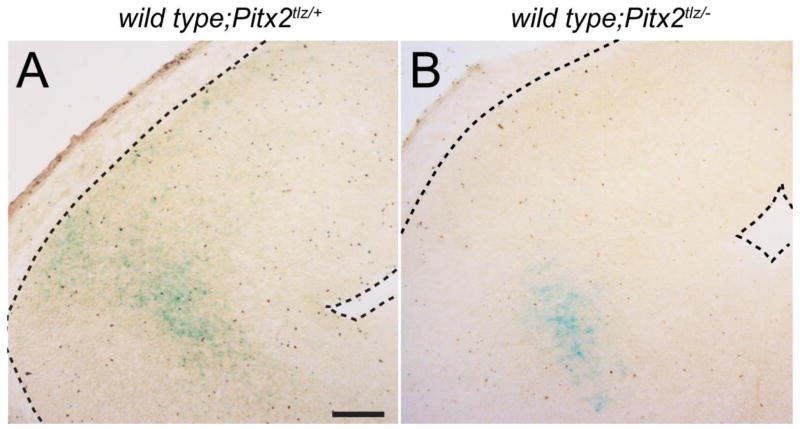
Pitx2 exhibits both cell autonomous and non-cell autonomous requirements during tissue development. For example, Pitx2 is required non-cell autonomously in the thalamus for formation of the mammillothalamic tract and in the eye for optic stalk development (Evans and Gage, 2005; Skidmore et al., 2012), but cell autonomously for survival of extraocular muscle (Zacharias et al., 2011). The cell autonomous nature of Pitx2 functions in migration and differentiation of collicular neurons has not been studied. To address this, we generated chimeric embryos by injecting wild type blastocysts with either Pitx2tlz/+ or Pitx2tlz/− embryonic stem cells. Chimeric embryos were harvested at E14.5 and brain sections analyzed by X-gal staining to visualize locations of Pitx2-expressing cells. In wild type;Pitx2tlz/+ midbrains (N=4 embryos), βgal-positive cells were properly localized to the collicular surface (Fig. 6A), whereas βgal-positive cells in wild type;Pitx2tlz/− embryos (N=4 embryos) were shifted deeper in the neuroepithelium, consistent with migratory delay or arrest (Fig. 6B). The lack of βgal-expressing cells at more superficial locations in the wild type;Pitx2tlz/− colliculus argues against non-cell autonomous functions for Pitx2.
Figure 6.
Evidence for cell autonomous effects of Pitx2 deficiency on collicular neuronal migration. Coronal midbrain cryosections from E14.5 wild type;Pitx2tlz/+ (A) or wild type;Pitx2tlz/− (B) chimeras produced from mouse embryonic stem (ES) cells and processed for X-gal histochemistry. (A) wild type;Pitx2tlz/+ sections display proper patterns of collicular βgal-positive neurons, whereas neurons in the wild type;Pitx2tlz/− colliculus are mislocalized deeper in the neuroepithelium (B). Scale bar in A is 200 μm and applies to panels A and B.
3.6 Collicular GABAergic differentiation is Pitx2 dosage-dependent but isoform-independent
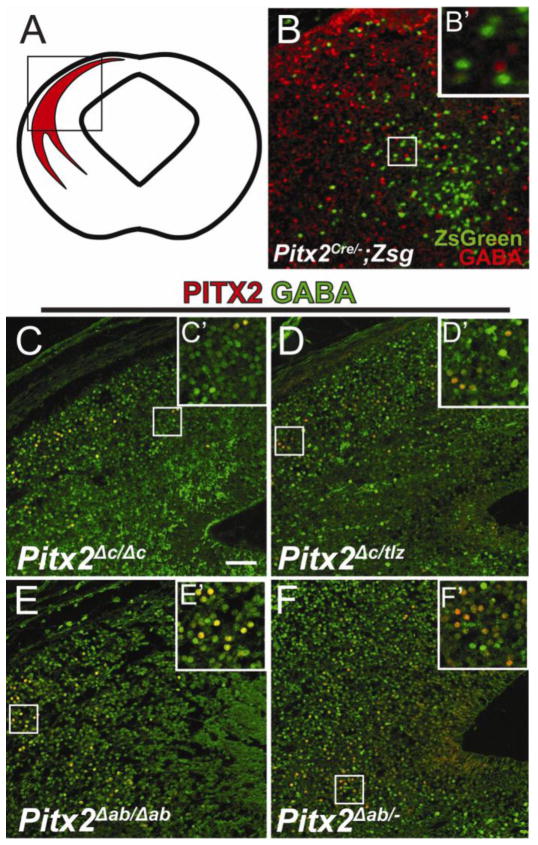
We previously showed that Pitx2 is necessary for GABAergic differentiation of a subpopulation of midbrain neurons (Waite et al., 2011), wherein loss of Pitx2 results in lack of GABAergic identity (Fig. 7B,B’). To determine whether specific Pitx2 isoforms were required for GABAergic differentiation, Pitx2 isoform-specific knockout embryos were analyzed for midbrain GABAergic differentiation. Here, we found that embryos null for Pitx2c (Pitx2Δc/Δc) and those with only a single allele of Pitx2ab (Pitx2Δc/tlz), display normal PITX2 co-localization with GABA (Fig. 7C–D’), suggesting a single allele of Pitx2ab is sufficient for GABAergic differentiation. Similarly, embryos null for Pitx2ab (Pitx2Δab/Δab) and those with a single allele of Pitx2c (Pitx2Δab/−) also display normal GABA co-localization, suggesting a single allele of Pitx2c is sufficient for GABAergic differentiation of PITX2-positive collicular neurons (Fig. 7E–F’). Because Pitx2Δab/− and Pitx2Δc/tlz Pitx2-positive neurons are GABAergic, a single allele of either Pitx2ab or Pitx2c appears sufficient for GABAergic differentiation of collicular Pitx2-positive neurons. Additionally, because Pitx2Δab/Δab and Pitx2Δc/Δc βgal-positive neurons are GABAergic, neither isoform is individually necessary.
Figure 7.
Collicular GABAergic differentiation requires a single allele dose of either Pitx2ab or Pitx2c. (A) Cartoon showing coronal view of an embryonic mouse midbrain identifying the dorsal Pitx2-positive population. Box indicates location of Pitx2-positive neurons magnified in panels B–F. (B) E14.5 Pitx2Cre/−;Zsg (green) coronal midbrain section processed for immunofluorescence against GABA (red). (C–F) E14.5 coronal midbrain sections processed for double-immunofluorescence against PITX2 (red) and GABA (green). (C) Pitx2Δc/Δc, (D) Pitx2Δc/tlz, (E) Pitx2Δab/Δab, and (F) Pitx2Δab/− colliculi exhibit similar co-localization of PITX2 and GABA. Scale bar in B is 50 μm and applies to panels B–F.
3.7 PITX2AB is necessary for tract formation in the developing brain
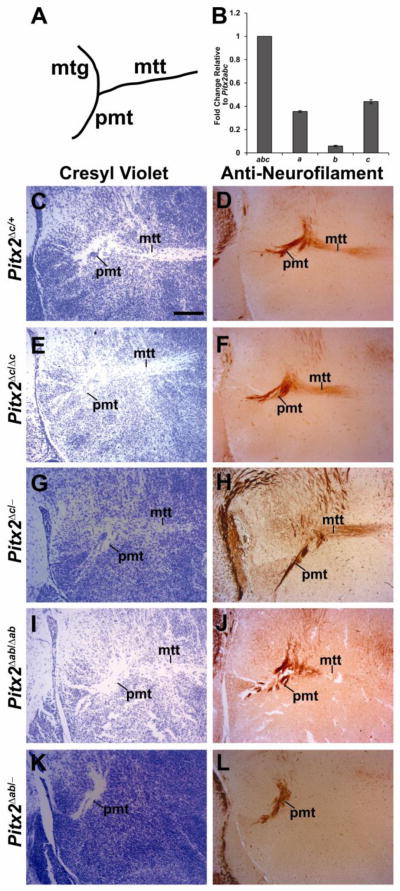
These studies suggest that unique Pitx2 isoforms are required for development of the midbrain through regulation of neuronal migration and differentiation. Previous studies showed that Pitx2 is expressed in hypothalamic neurons and is required non-cell autonomously for development of the mammillothalamic tract (MTT), which projects from the mammillary body to the anterior nucleus of the thalamus (Skidmore et al., 2012); however, the isoforms responsible were not identified. As in the midbrain, Pitx2b mRNA was less abundant than Pitx2a or Pitx2c in the E18.5 hypothalamus (Fig. 8B) . Embryos that were heterozygous or null for Pitx2c (Pitx2Δc/+,Pitx2Δc/Δc or Pitx2Δc/−) or null for Pitx2ab (Pitx2Δab/Δab) displayed normal MTTs (Fig. 8C–J). However, embryos with only a single allele of Pitx2c (Pitx2Δab/−) failed to form the MTT (Fig. 8K–L), similar to embryos with Nestin-Cre-mediated conditional Pitx2 deletion (Skidmore et al., 2012). Thus, a single allele of Pitx2c is not sufficient for MTT formation, which requires either two alleles of Pitx2c or one of Pitx2ab.
Figure 8.
Pitx2ab is necessary for formation of the mammillothalamic tract (MTT). (A) Cartoon of a sagittal section identifying tracks in the forebrain. (B) QPCR for Pitx2a, Pitx2b, and Pitx2c from E18.5 hypothalamus RNA shows that Pitx2b is more abundant than Pitx2a and Pitx2c. E18.5 sagittal brain sections were processed for cresyl violet staining (C, E, G, I, K) or immunohistochemistry for Neurofilament (D, F, H, J, L). (C–D) Pitx2Δc/+, (E–F) Pitx2Δc/Δc, (G–H) Pitx2Δc/−, and (I–J) Pitx2Δab/Δab embryos exhibit normal MTT. (K–L) Pitx2ab/− embryos lack the MTT stemming from principal mammillary tract (PMT). Scale bar in B is 200 μm and applies to panels B–I. Abbreviations: mtt, mammillothalamic tract; pmt, principle mammillary tract.
4. Discussion
4.1 Conclusion
Ours is the first study to identify unique Pitx2 transcription factor isoform requirements in the developing brain. This is also the first report of a requirement for PITX2AB in tissue development. We show that all three Pitx2 isoforms are expressed in the developing midbrain and hypothalamus, and that Pitx2a and Pitx2b isoforms are expressed at higher levels than Pitx2c. We also demonstrate that a subpopulation of collicular neurons requires Pitx2ab for proper migration, and a single allele of Pitx2ab or Pitx2c for GABAergic differentiation. Finally, we show that formation of the mammillothalamic tract requires a combination of two isoform-specific Pitx2 alleles.
4.2 Pitx2 isoforms exhibit unique dosage effects during brain development
Pitx2 isoforms exhibit differential dosages in the developing midbrain and hypothalamus, suggesting they have unique functions and dosage requirements during brain development. For example, GABAergic differentiation of collicular neurons requires only a single allele of Pitx2ab or Pitx2c, suggesting low dosage of Pitx2 may be sufficient (Table 1). In contrast, MTT formation requires either a single allele of Pitx2ab or two alleles of Pitx2c, suggesting it requires higher Pitx2 dosage than midbrain GABAergic differentiation. The highest dosage is required by collicular neurons undergoing migration which require one allele of Pitx2ab, although two Pitx2c alleles are partially sufficient. Pitx2 isoforms may also be partially functionally redundant, there may be isoform-specific gene regulation, or there may be a threshold level of Pitx2 isoform necessary for neuronal development. This situation is reminiscent to that in the developing branchial arches, where Pitx2 isoforms are interchangeable and contribute distinct dosages which translate into unique developmental functions (Liu et al., 2003).
Table 1.
Different functions during collicular development require unique Pitx2 dosage. Left side of table lists developmental functions. Mouse genotypes at top of table are in order from highest Pitx2 dose (left) to lowest (right). Green boxes indicate a normal phenotype, yellow indicates an intermediate (int) phenotype, and red boxes indicate abnormal phenotypes. The box referencing MTT formation in Pitx2−/− embryos refers to results from E18.5 conditional Pitx2 knockout embryos.
| Pitx2 dosage | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Function | Pitx2+/− | Pitx2Δc/+ | Pitx2Δc/Δc | Pitx2Δab/Δab | Pitx2Δab/− | Pitx2−/− |
| Midbrain GABAergic differentiation | nl | nl | nl | nl | nl | negative |
| MTT formation | nl | nl | nl | nl | absent | absent |
| Midbrain neuronal migration | nl | nl | nl | interm | disrupted | disrupted |
4.3 Pitx2a and Pitx2b are dominant PITX2 isoforms during mouse brain development
The embryonic brain may be unique from other organs in its requirement for Pitx2 isoforms. Loss of Pitx2a and Pitx2b results in more severe phenotypes than loss of Pitx2c. To date, there are no studies which have identified a requirement for Pitx2ab in tissue-specific development, and prior reports indicate that loss of Pitx2ab is not lethal in mice (Liu et al., 2001). Pitx2ab is co-expressed with Pitx2c in the developing eyes, craniofacial tissues, pituitary, liver hematopoietic stem cells, body wall, and weakly in the lungs (Gage and Camper, 1997; Kieusseian et al., 2006; Kitamura et al., 1999; Liu et al., 2001; Liu et al., 2003). Minor roles for Pitx2ab have been identified in lung development (Liu et al., 2001), and Pitx2ab is sufficient but dispensable for tooth development (Liu et al., 2003). However, Pitx2Δab/Δab embryos often have medially displaced eyes (unpublished observations) reminiscent of ocular defects observed in Pitx2−/− mice (Evans and Gage, 2005; Gage et al., 1999). The neuroepithelial origin of neural crest-derived ocular Pitx2-expressing cells may partly explain their sensitivity to reduced Pitx2ab function (Echelard et al., 1994; Gage et al., 2005), although the exact Pitx2 isoform-specific requirements for eye development are unknown.
In contrast to Pitx2 expression in the mouse brain, Pitx2c is the only Pitx2 isoform expressed in the zebrafish brain. Interestingly, Pitx2c exhibits asymmetric expression in the left dorsal diencephalon (pineal gland), although its function in this region is unknown (Essner et al., 2000; Liang et al., 2000). In vitro studies on PITX2A and PITX2B functions have provided some functional information. In cell lines, PITX2A regulates cellular migration and cell spreading through activation of RhoA and Rac1 (Liu et al., 2001; Wei and Adelstein, 2002). Additionally, PITX2A regulates cell cycle genes such as P21 and CyclinD1 in epithelial cells (Zhao et al., 1999). Both PITX2A and PITX2B are capable of transactivating the same genes as Pitx2c, but with different efficiencies which are dependent upon cell type and the presence of other proteins (Cox et al., 2002; Ganga et al., 2003; Smidt et al., 2000). Of the three PITX2 isoforms, PITX2B often has the lowest transactivation efficiency (Cox et al., 2002; Ganga et al., 2003; Smidt et al., 2000), but can heterodimerize with PITX2A and PITX2C for improved gene activation (Cox et al., 2002). Isoform heterodimerization is likely facilitated by the homeodomain or C-terminal tail, both of which have also been implicated in PITX2 homodimerization (Amendt et al., 1999; Green et al., 2001). However, the mechanism by which Pitx2 isoform heterodimerization influences gene expression is unknown.
4.4 Pitx2c may be redundant during mouse brain development
In both midbrain and hypothalamus, the Pitx2ab allele appears necessary and sufficient for proper migration of superior colliculus neurons and for extension of the MTT. In contrast, the Pitx2c allele is neither necessary nor sufficient for either process. One potential explanation for these findings is that the Pitx2ab mutant allele disrupts both PITX2A and PITX2B, whereas the Pitx2c mutant allele disrupts only PITX2C, such that it is the overall amount of Pitx2 isoforms present that is necessary for neuronal differentiation. If true, then loss of similar amounts of Pitx2 isoforms should lead to similar phenotypes. This does not appear to hold true in the midbrain, where preservation of both Pitx2a and Pitx2b is sufficient for proper neuronal migration (Fig. 4C) whereas preservation of both Pitx2c copies (Fig. 4F) is not. We interpret these findings to suggest that the differing Pitx2 isoforms exhibit unique properties and dosage sensitivities, and may have unique downstream targets.
In the brain, Pitx2ab appears to be more important than Pitx2c. This contrasts with other organs such as the heart, lungs, and gut, where Pitx2c is essential for normal development (Liu et al., 2001; Liu et al., 2002). Pitx2c is first expressed at E8.5 in the left lateral plate mesoderm (L-LPM) downstream of Shh and Nodal, and LPM induction of Pitx2c is necessary for later Pitx2c expression in left-sided organs (Brennan et al., 2002; Campione et al., 1999; Kahr et al., 2011; Pagan-Westphal and Tabin, 1998; Shiratori and Hamada, 2006). Pitx2c is required for left-sided heart and lung morphogenesis and for looping of the gut (Liu et al., 2001; Liu et al., 2002). Later in development, Pitx2c induces expression of atrial natriuretic factor (ANF) and Plod1 and the cardiac transcription factors Isl1, Mef2c and Gata4 (Lozano-Velasco et al., 2011). In vitro studies suggest that heart development requires synergism specifically between PITX2C and NKX2.5 to regulate downstream genes, and that other Pitx2 isoforms are inadequate (Ganga et al., 2003; Simard et al., 2009; Warren et al., 2011). Consistent with this, PITX2C/NKX2.5 synergism requires the unique PITX2C N-terminus (Simard et al., 2009). Interestingly, continued Pitx2c expression in the heart through adulthood appears to be required for cardiac fitness, as loss of Pitx2c in the cardiac atrium results in susceptibility to atrial fibrillations (Chinchilla et al., 2011; Kirchhof et al., 2011; Wang et al., 2010). Thus, Pitx2c appears to have unique requirements in mediastinal organs; its precise role in the brain remains unclear.
In addition to organ-specific functions, the various Pitx2 isoforms may exhibit unique transcriptional auto-regulation. Pitx2a has been shown to induce expression of Pitx2c (Guioli and Lovell-Badge, 2007; Kala et al., 2009), and in all tissues examined thus far, all three isoforms are expressed (Gage and Camper, 1997; Kieusseian et al., 2006; Liu et al., 2001; Liu et al., 2003). In our experiments, there were mild (9–21%) changes in Pitx2c mRNA levels with reduced Pitx2ab, but it is not clear whether this leads to changes in the amount of protein present.
4.5 Transcriptional regulation of collicular neuron migration and differentiation
In the superior colliculus, PITX2 is downstream of Helt and Gata2 and is necessary for GABAergic differentiation (Kala et al., 2009; Miyoshi et al., 2004; Waite et al., 2011). In vitro, Pitx2 is capable of inducing expression of Gad1, (glutamate decarboxylase), an enzyme that catalyzes GABA synthesis and is necessary for GABAergic identity (Chen et al., 2011; Westmoreland et al., 2001). Therefore, Pitx2 may be the first terminal differentiation factor identified in a subpopulation of GABAergic neurons in the superior colliculus. Other transcription factors such as Pax3/7, Gata2, Lhx1/5, and Brn3a are expressed during superior colliculus development and are required at various developmental stages; however, the spatiotemporal distribution of their expression and prior functional studies suggest they act earlier than terminal differentiation. Pax3 and Pax7 are expressed in progenitors, whereas Gata2, Lhx1/5, and Brn3a are expressed during or after neurogenesis. The paired-box transcription factors, Pax3 and Pax7, are expressed throughout the dorsal neural tube, and are important for dorsal brain identity and polarity (Jostes et al., 1990; Kawakami et al., 1997; Matsunaga et al., 2001; Thomas et al., 2004). Midbrain progenitors continue to express Pax3 but down-regulate Pax7 later in development (Thompson et al., 2008). Pax7 then becomes restricted to precursors and mature neurons, where it is thought to somehow establish regional identity neuronal maintenance (Jostes et al., 1990; Stoykova and Gruss, 1994; Thomas et al., 2004). While Pax7 is expressed during terminal differentiation, it is unknown whether Pax7 is involved in the terminal differentiation process.
Pax3/7 midbrain neural progenitors express Gata2 as they undergo neurogenesis and continue Gata2 expression as collicular precursors (Kala et al., 2009; Willett and Greene, 2011). Gata2 is necessary for GABAergic neuronal identity determination and migration of neural precursors, but its expression turns off prior to terminal differentiation (Kala et al., 2009; Willett and Greene, 2011). Lhx1 and Lhx5 (Lhx1/5) are LIM-homeodomain transcription factors that are expressed in Gata2-lineage collicular neurons (Kala et al., 2009). In the colliculus, Lhx1/5 are expressed downstream of Gata2 in progenitors undergoing neurogenesis and continue to be expressed in neuronal precursors and mature GABAergic neurons (Kala et al., 2009; Waite et al., 2011). Lhx1/5 are required for neurogenesis, precursor differentiation, and maintenance of neuronal identity, but their roles in terminal differentiation are unclear (Pillai et al., 2007; Taira et al., 1994; Zhao et al., 1999). Unlike Lhx1/5 and Gata2, the POU domain transcription factor Brn3a is expressed in post-mitotic glutamatergic precursors and mature glutamatergic neurons (Fedtsova and Turner, 1995; Lanier et al., 2009; Nakatani et al., 2007; Waite et al., 2011). No studies have identified the function of Brn3a in these collicular precursors. In trigeminal ganglion neurons, Brn3a is required for the expression of early fate markers and repression of alternate differentiation programs (Lanier et al., 2009), suggesting that it may also act earlier than terminal differentiation in the colliculus. Thus, unlike Pitx2, Gata2, Lhx1/5 and Brn3a have not been associated with GABAergic terminal differentiation.
4.6 Independent regulation of collicular neuron migration and differentiation by Pitx2
It is unknown whether Pitx2 regulation of midbrain neuronal migration and GABAergic differentiation are independent or linked processes. For example, the location of collicular neurons within the neuroepithelium may influence local inputs that direct terminal differentiation. If true, then Pitx2 requirements for collicular neuron migration could be linked to its requirements for GABAergic differentiation. Alternatively, Pitx2 could regulate a cell autonomous differentiation program independent of its migrational functions. Interestingly, the tumor suppressor p27Kip1 is capable of independently regulating both migration and differentiation by inhibiting RhoA/ROCK to promote neuronal migration and stabilizing Ngn2 to promote differentiation (Nguyen et al., 2006). Different termini of the p27Kip1 protein regulate neuronal migration (N-terminal) and differentiation (C-terminal) (Nguyen et al., 2006). PITX2A regulates RhoA signaling to facilitate migration in HeLa cells and activates Gad1 in developing neurons (Kirchhof et al., 2011; Morselli et al., 1999; Wei and Adelstein, 2002), suggesting that Pitx2 could regulate midbrain neuronal migration and differentiation as independent processes. Pitx2Δab/− midbrains exhibit medially mislocalized, yet GABA-positive neurons at E14.5, indicating that midbrain neurons can be medially mislocalized but still undergo GABAergic differentiation. Therefore, Pitx2 is capable of independently regulating different developmental processes in the midbrain.
As genetic sequencing techniques have improved, the ability to identify causative variant mutations and link these mutations to developmental brain phenotypes has also advanced. Accurate assignment of functionality for sequence variants, however, requires an understanding of the developmental consequences produced by sequence variation. Our results highlight the unique developmental requirements for Pitx2 isoforms, which could be critical for functional annotation of future sequence analyses in humans. Ultimately, this could improve our ability to diagnose and treat a variety of neurodevelopmental disorders.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Phil Gage for insightful discussions and critical reading of the manuscript. Thom Saunders and Elizabeth Hughes at the University of Michigan Transgenic Animal Model Core prepared ES cell lines and blastocyst injections to create chimeric mice. This work was supported by the NIH Cellular and Molecular Biology Training Grant (T32-GM007315), a Rackham Regents Fellowship, and a Rackham Predoctoral Fellowship to MRW, the NIH Hearing, Balance and Chemical Senses Training Grant (T32-DC00011) to JAM, NIH R01 HL093484 and Vivian L Smith Foundation to JFM, and NIH R01 NS054784 to DMM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amendt BA, Semina EV, Alward WL. Rieger syndrome: a clinical, molecular, and biochemical analysis. Cell Mol Life Sci. 2000;57:1652–1666. doi: 10.1007/PL00000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendt BA, Sutherland LB, Russo AF. Multifunctional role of the Pitx2 homeodomain protein C-terminal tail. Molecular and cellular biology. 1999;19:7001–7010. doi: 10.1128/mcb.19.10.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendt BA, Sutherland LB, Semina EV, Russo AF. The molecular basis of Rieger syndrome. Analysis of Pitx2 homeodomain protein activities. The Journal of biological chemistry. 1998;273:20066–20072. doi: 10.1074/jbc.273.32.20066. [DOI] [PubMed] [Google Scholar]
- Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes & development. 2002;16:2339–2344. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Novitch BG. Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos Trans R Soc Lond B Biol Sci. 2008;363:57–70. doi: 10.1098/rstb.2006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campione M, Steinbeisser H, Schweickert A, Deissler K, van Bebber F, Lowe LA, Nowotschin S, Viebahn C, Haffter P, Kuehn MR, Blum M. The homeobox gene Pitx2: mediator of asymmetric left-right signaling in vertebrate heart and gut looping. Development. 1999;126:1225–1234. doi: 10.1242/dev.126.6.1225. [DOI] [PubMed] [Google Scholar]
- Cazorla P, Smidt MP, O'Malley KL, Burbach JP. A response element for the homeodomain transcription factor Ptx3 in the tyrosine hydroxylase gene promoter. J Neurochem. 2000;74:1829–1837. doi: 10.1046/j.1471-4159.2000.0741829.x. [DOI] [PubMed] [Google Scholar]
- Chacko E, Ranganathan S. Comprehensive splicing graph analysis of alternative splicing patterns in chicken, compared to human and mouse. BMC Genomics. 2009;10(Suppl 1):S5. doi: 10.1186/1471-2164-10-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dong E, Grayson DR. Analysis of the GAD1 promoter: trans-acting factors and DNA methylation converge on the 5' untranslated region. Neuropharmacology. 2011;60:1075–1087. doi: 10.1016/j.neuropharm.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Childers NK, Wright JT. Dental and craniofacial anomalies of Axenfeld-Rieger syndrome. J Oral Pathol. 1986;15:534–539. doi: 10.1111/j.1600-0714.1986.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Chinchilla A, Daimi H, Lozano-Velasco E, Dominguez JN, Caballero R, Delpon E, Tamargo J, Cinca J, Hove-Madsen L, Aranega AE, Franco D. PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circulation Cardiovascular genetics. 2011;4:269–279. doi: 10.1161/CIRCGENETICS.110.958116. [DOI] [PubMed] [Google Scholar]
- Cox CJ, Espinoza HM, McWilliams B, Chappell K, Morton L, Hjalt TA, Semina EV, Amendt BA. Differential regulation of gene expression by PITX2 isoforms. J Biol Chem. 2002;277:25001–25010. doi: 10.1074/jbc.M201737200. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Eliott D, Miller NR, Maumenee IH, Green WR. Familial Axenfeld-Rieger anomaly, atrial septal defect, and sensorineural hearing loss: a possible new genetic syndrome. Arch Ophthalmol. 1998;116:78–82. doi: 10.1001/archopht.116.1.78. [DOI] [PubMed] [Google Scholar]
- Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–2503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Edwards MA, Caviness VS, Jr, Schneider GE. Development of cell and fiber lamination in the mouse superior colliculus. J Comp Neurol. 1986;248:395–409. doi: 10.1002/cne.902480308. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Branford WW, Zhang J, Yost HJ. Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development. 2000;127:1081–1093. doi: 10.1242/dev.127.5.1081. [DOI] [PubMed] [Google Scholar]
- Evans AL, Gage PJ. Expression of the homeobox gene Pitx2 in neural crest is required for optic stalk and ocular anterior segment development. Hum Mol Genet. 2005;14:3347–3359. doi: 10.1093/hmg/ddi365. [DOI] [PubMed] [Google Scholar]
- Fedtsova NG, Turner EE. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Camper SA. Pituitary homeobox 2, a novel member of the bicoid-related family of homeobox genes, is a potential regulator of anterior structure formation. Hum Mol Genet. 1997;6:457–464. doi: 10.1093/hmg/6.3.457. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Rhoades W, Prucka SK, Hjalt T. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208. doi: 10.1167/iovs.05-0691. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Ganga M, Espinoza HM, Cox CJ, Morton L, Hjalt TA, Lee Y, Amendt BA. PITX2 isoform-specific regulation of atrial natriuretic factor expression: synergism and repression with Nkx2.5. J Biol Chem. 2003;278:22437–22445. doi: 10.1074/jbc.M210163200. [DOI] [PubMed] [Google Scholar]
- Green PD, Hjalt TA, Kirk DE, Sutherland LB, Thomas BL, Sharpe PT, Snead ML, Murray JC, Russo AF, Amendt BA. Antagonistic regulation of Dlx2 expression by PITX2 and Msx2: implications for tooth development. Gene Expr. 2001;9:265–281. doi: 10.3727/000000001783992515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guioli S, Lovell-Badge R. PITX2 controls asymmetric gonadal development in both sexes of the chick and can rescue the degeneration of the right ovary. Development. 2007;134:4199–4208. doi: 10.1242/dev.010249. [DOI] [PubMed] [Google Scholar]
- Guo Q, Li K, Sunmonu NA, Li JYH. Fgf8b-containing spliceforms, but not Fgf8a, are essential for Fgf8 function during development of the midbrain and cerebellum. Dev Biol. 2010;338:183–192. doi: 10.1016/j.ydbio.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrees F, Bloch-Zupan A, Free SL, Vaideanu D, Thompson PJ, Ashley P, Brice G, Rutland P, Bitner-Glindzicz M, Khaw PT, Fraser S, Sisodiya SM, Sowden JC. A novel homeobox mutation in the PITX2 gene in a family with Axenfeld-Rieger syndrome associated with brain, ocular, and dental phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:184–191. doi: 10.1002/ajmg.b.30237. [DOI] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990;33:27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Kahr PC, Piccini I, Fabritz L, Greber B, Scholer H, Scheld HH, Hoffmeier A, Brown NA, Kirchhof P. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS One. 2011;6:e26389. doi: 10.1371/journal.pone.0026389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala K, Haugas M, Lillevali K, Guimera J, Wurst W, Salminen M, Partanen J. Gata2 is a tissue-specific post-mitotic selector gene for midbrain GABAergic neurons. Development. 2009;136:253–262. doi: 10.1242/dev.029900. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H. Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev. 1997;66:119–130. doi: 10.1016/s0925-4773(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Kieusseian A, Chagraoui J, Kerdudo C, Mangeot PE, Gage PJ, Navarro N, Izac B, Uzan G, Forget BG, Dubart-Kupperschmitt A. Expression of Pitx2 in stromal cells is required for normal hematopoiesis. Blood. 2006;107:492–500. doi: 10.1182/blood-2005-02-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Magen A, Ast G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Res. 2007;35:125–131. doi: 10.1093/nar/gkl924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–685. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- Kirchhof P, Kahr PC, Kaese S, Piccini I, Vokshi I, Scheld HH, Rotering H, Fortmueller L, Laakmann S, Verheule S, Schotten U, Fabritz L, Brown NA. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circulation Cardiovascular genetics. 2011;4:123–133. doi: 10.1161/CIRCGENETICS.110.958058. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, Yanazawa M, Katoh-Fukui Y, Suzuki R, Ohuchi H, Suehiro A, Motegi Y, Nakahara Y, Kondo S, Yokoyama M. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Lanier J, Dykes IM, Nissen S, Eng SR, Turner EE. Brn3a regulates the transition from neurogenesis to terminal differentiation and represses non-neural gene expression in the trigeminal ganglion. Dev Dyn. 2009;238:3065–3079. doi: 10.1002/dvdy.22145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O'Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Liu C, Liu W, Lu MF, Brown NA, Martin JF. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development. 2001;128:2039–2048. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- Liu C, Liu W, Palie J, Lu MF, Brown NA, Martin JF. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development. 2002;129:5081–5091. doi: 10.1242/dev.129.21.5081. [DOI] [PubMed] [Google Scholar]
- Liu W, Selever J, Lu MF, Martin JF. Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development. 2003;130:6375–6385. doi: 10.1242/dev.00849. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lozano-Velasco E, Chinchilla A, Martinez-Fernandez S, Hernandez-Torres F, Navarro F, Lyons GE, Franco D, Aranega AE. Pitx2c modulates cardiac-specific transcription factors networks in differentiating cardiomyocytes from murine embryonic stem cells. Cells Tissues Organs. 2011;194:349–362. doi: 10.1159/000323533. [DOI] [PubMed] [Google Scholar]
- Lu MF, Pressman C, Dyer R, Johnson RL, Martin JF. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Skidmore JM, Fox SE, Gage PJ, Camper SA. Pitx2 distinguishes subtypes of terminally differentiated neurons in the developing mouse neuroepithelium. Dev Biol. 2002;252:84–99. doi: 10.1006/dbio.2002.0835. [DOI] [PubMed] [Google Scholar]
- Martin DM, Skidmore JM, Philips ST, Vieira C, Gage PJ, Condie BG, Raphael Y, Martinez S, Camper SA. PITX2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev Biol. 2004;267:93–9108. doi: 10.1016/j.ydbio.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Martinez-Fernandez S, Hernandez-Torres F, Franco D, Lyons GE, Navarro F, Aranega AE. Pitx2c overexpression promotes cell proliferation and arrests differentiation in myoblasts. Dev Dyn. 2006;235:2930–2939. doi: 10.1002/dvdy.20924. [DOI] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Role of Pax3/7 in the tectum regionalization. Development. 2001;128:4069–4077. doi: 10.1242/dev.128.20.4069. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Bessho Y, Yamada S, Kageyama R. Identification of a novel basic helix-loop-helix gene, Heslike, and its role in GABAergic neurogenesis. J Neurosci. 2004;24:3672–3682. doi: 10.1523/JNEUROSCI.5327-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrek B, Lee CJ. Alternative splicing in the human, mouse and rat genomes is associated with an increased frequency of exon creation and/or loss. Nat Genet. 2003;34:177–180. doi: 10.1038/ng1159. [DOI] [PubMed] [Google Scholar]
- Modrek B, Resch A, Grasso C, Lee C. Genome-wide detection of alternative splicing in expressed sequences of human genes. Nucleic Acids Res. 2001;29:2850–2859. doi: 10.1093/nar/29.13.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli M, Luppi M, Barozzi P, Dominici M, Temperani P, Campione D, Lanza F, Trovato R, Marasca R, Longo G, Emilia G, Torelli G. Lack of confirmation of an association between HTLV-I infection and myelodysplastic syndrome. Br J Haematol. 1999;105:1146–1147. doi: 10.1111/j.1365-2141.1999.01525.x. [DOI] [PubMed] [Google Scholar]
- Mucchielli ML, Mitsiadis TA, Raffo S, Brunet JF, Proust JP, Goridis C. Mouse Otlx2/RIEG expression in the odontogenic epithelium precedes tooth initiation and requires mesenchyme-derived signals for its maintenance. Dev Biol. 1997;189:275–284. doi: 10.1006/dbio.1997.8672. [DOI] [PubMed] [Google Scholar]
- Nakatani T, Minaki Y, Kumai M, Ono Y. Helt determines GABAergic over glutamatergic neuronal fate by repressing Ngn genes in the developing mesencephalon. Development. 2007;134:2783–2793. doi: 10.1242/dev.02870. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Heng JIT, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan-Westphal SM, Tabin CJ. The transfer of left-right positional information during chick embryogenesis. Cell. 1998;93:25–35. doi: 10.1016/s0092-8674(00)81143-5. [DOI] [PubMed] [Google Scholar]
- Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- Pillai A, Mansouri A, Behringer R, Westphal H, Goulding M. Lhx1 and Lhx5 maintain the inhibitory-neurotransmitter status of interneurons in the dorsal spinal cord. Development. 2007;134:357–366. doi: 10.1242/dev.02717. [DOI] [PubMed] [Google Scholar]
- Porter JD, Khanna S, Kaminski HJ, Rao JS, Merriam AP, Richmonds CR, Leahy P, Li J, Andrade FH. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:12062–12067. doi: 10.1073/pnas.211257298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi I, Semina EV, Amendt BA, Harris DJ, Murphy KP, Murray JC, Russo AF. Identification of a dominant negative homeodomain mutation in Rieger syndrome. The Journal of biological chemistry. 2001;276:23034–23041. doi: 10.1074/jbc.M008592200. [DOI] [PubMed] [Google Scholar]
- Santos ME, Athanasiadis A, Leitao AB, DuPasquier L, Sucena E. Alternative splicing and gene duplication in the evolution of the FoxP gene subfamily. Mol Biol Evol. 2011;28:237–247. doi: 10.1093/molbev/msq182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semina EV, Reiter RS, Murray JC. Isolation of a new homeobox gene belonging to the Pitx/Rieg family: expression during lens development and mapping to the aphakia region on mouse chromosome 19. Hum Mol Genet. 1997;6:2109–2116. doi: 10.1093/hmg/6.12.2109. [DOI] [PubMed] [Google Scholar]
- Sepp M, Kannike K, Eesmaa A, Urb M, Timmusk T. Functional diversity of human basic helix-loop-helix transcription factor TCF4 isoforms generated by alternative 5' exon usage and splicing. PLoS One. 2011;6:e22138. doi: 10.1371/journal.pone.0022138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratori H, Hamada H. The left-right axis in the mouse: from origin to morphology. Development. 2006;133:2095–2104. doi: 10.1242/dev.02384. [DOI] [PubMed] [Google Scholar]
- Simard A, Di Giorgio L, Amen M, Westwood A, Amendt BA, Ryan AK. The Pitx2c N-terminal domain is a critical interaction domain required for asymmetric morphogenesis. Dev Dyn. 2009;238:2459–2470. doi: 10.1002/dvdy.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore JM, Cramer JD, Martin JF, Martin DM. Cre fate mapping reveals lineage specific defects in neuronal migration with loss of Pitx2 function in the developing mouse hypothalamus and subthalamic nucleus. Mol Cell Neurosci. 2008;37:696–707. doi: 10.1016/j.mcn.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore JM, Waite MR, Alvarez-Bolado G, Puelles L, Martin DM. A novel TaulacZ allele reveals a requirement for Pitx2 in formation of the mammillothalamic tract. Genesis. 2012;50:67–73. doi: 10.1002/dvg.20793. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Cox JJ, van Schaick HS, Coolen M, Schepers J, van der Kleij AM, Burbach JP. Analysis of three Ptx2 splice variants on transcriptional activity and differential expression pattern in the brain. J Neurochem. 2000;75:1818–1825. doi: 10.1046/j.1471-4159.2000.0751818.x. [DOI] [PubMed] [Google Scholar]
- Stoykova A, Gruss P. Roles of Pax-genes in developing and adult brain as suggested by expression patterns. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:1395–1412. doi: 10.1523/JNEUROSCI.14-03-01395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M, Otani H, Saint-Jeannet JP, Dawid IB. Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature. 1994;372:677–679. doi: 10.1038/372677a0. [DOI] [PubMed] [Google Scholar]
- Thomas M, Lazic S, Beazley L, Ziman M. Expression profiles suggest a role for Pax7 in the establishment of tectal polarity and map refinement. Exp Brain Res. 2004;156:263–273. doi: 10.1007/s00221-003-1775-z. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Zembrzycki A, Mansouri A, Ziman M. Pax7 is requisite for maintenance of a subpopulation of superior collicular neurons and shows a diverging expression pattern to Pax3 during superior collicular development. BMC developmental biology. 2008;8:62. doi: 10.1186/1471-213X-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Waite MR, Skidmore JM, Billi AC, Martin JF, Martin DM. GABAergic and glutamatergic identities of developing midbrain Pitx2 neurons. Dev Dyn. 2011;240:333–346. doi: 10.1002/dvdy.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Klysik E, Sood S, Johnson RL, Wehrens XH, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SA, Terada R, Briggs LE, Cole-Jeffrey CT, Chien WM, Seki T, Weinberg EO, Yang TP, Chin MT, Bungert J, Kasahara H. Differential role of Nkx2-5 in activation of the atrial natriuretic factor gene in the developing versus failing heart. Mol Cell Biol. 2011;31:4633–4645. doi: 10.1128/MCB.05940-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Adelstein RS. Pitx2a expression alters actin-myosin cytoskeleton and migration of HeLa cells through Rho GTPase signaling. Mol Biol Cell. 2002;13:683–697. doi: 10.1091/mbc.01-07-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland JJ, McEwen J, Moore BA, Jin Y, Condie BG. Conserved function of Caenorhabditis elegans UNC-30 and mouse Pitx2 in controlling GABAergic neuron differentiation. J Neurosci. 2001;21:6810–6819. doi: 10.1523/JNEUROSCI.21-17-06810.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett RT, Greene LA. Gata2 is required for migration and differentiation of retinorecipient neurons in the superior colliculus. J Neurosci. 2011;31:4444–4455. doi: 10.1523/JNEUROSCI.4616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L, Maden M. The mechanisms of dorsoventral patterning in the vertebrate neural tube. Dev Biol. 2005;282:1–13. doi: 10.1016/j.ydbio.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Yeo G, Holste D, Kreiman G, Burge CB. Variation in alternative splicing across human tissues. Genome Biol. 2004;5:R74. doi: 10.1186/gb-2004-5-10-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias AL, Lewandoski M, Rudnicki MA, Gage PJ. Pitx2 is an upstream activator of extraocular myogenesis and survival. Dev Biol. 2011;349:395–405. doi: 10.1016/j.ydbio.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sheng HZ, Amini R, Grinberg A, Lee E, Huang S, Taira M, Westphal H. Control of hippocampal morphogenesis and neuronal differentiation by the LIM homeobox gene Lhx5. Science. 1999;284:1155–1158. doi: 10.1126/science.284.5417.1155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.