Abstract
Cyclin-dependent kinase 5 (Cdk5) is a serine/threonine kinase, and its kinase activity is dependent upon its association with either of the activating subunits p35 or p39, which are mainly expressed in neurons. We previously reported that Cdk5 knockout (KO) mice exhibit perinatal lethality, defective neuronal migration, and abnormal positioning of neurons in the facial motor nucleus and inferior olive in the hindbrain and Purkinje cells (PCs) in the cerebellum.. In this study, we focused on the analysis of the role of Cdk5 in cerebellar development. For this purpose we generated midbrain-hindbrain-specific Cdk5 conditional knockout (MHB-Cdk5 KO) mice because the cerebellum develops postnatally, whereas Cdk5 KO mice die perinatally. Histological analysis of the MHB-Cdk5 KO mice revealed a significant size reduction of the cerebellum. In addition, profound disturbance of inward migration of granule cells (GC) was observed in the developing cerebellum. A normal dendritic development of the Purkinje cells (PCs) was disturbed in MHB-Cdk5 KO mice. Cultured Cdk5-null PCs showed similar dendritic abnormalities. These results indicate that Cdk5/p35 plays an important role in neuronal migration of PCs and GCs and dendrite formation of PCs in cerebellar development.
Keywords: Cdk5, neuronal migration, midbrain-hindbrain, conditional KO, dendrite
Introduction
Precise identification of the various molecules that guide migration of neurons from their site of genesis to their final positions in the developing brain is important for our understanding of the development and function of the brain. We have identified Cyclin-dependent kinase 5 (Cdk5) as a key regulator of neuronal migration and positioning (Ohshima et al. 1996; Gilmore et al. 1998). Cdk5 is a serine/threonine kinase, and its kinase activity is detected mainly in postmitotic neurons because of the neuron-specific expression of its activating subunits, p35 and p39 (reviewed in Dhavan and Tsai 2001; Ohshima and Mikoshiba 2002). Cdk5 knockout (KO) mice exhibit perinatal lethality and defective positioning of several types of neurons (Ohshima et al. 1996; Gilmore et al. 1998). However, p35 KO mice show a milder phenotype of positioning defects of neurons and survive to adulthood because of redundant and overlapping expression of p39 (Chae et al. 1997; Ohshima et al. 2001). p39 KO mice exhibit normal brain histology, but p35 KO;p39 double KO mice show phenotypes similar to those of Cdk5 KO mice, indicating the functional redundancy among these 2 Cdk5 activating subunits (Ko et al. 2001). Cdk5/p35-dependent neuronal migration and positioning in the cerebral cortex were well characterized using Cdk5 KO and p35 KO mice (Ohshima et al. 1996; Chae et al. 1997; Gilmore et al. 1998, however their roles in neuronal migration in the midbrain-hindbrain (MHB) region have not been well-characterized because the MHB region continues to develop postnatally whereas Cdk5 KO mice die perinatally. We previously reported profound migration defects of the Purkinje cells (PCs) in the embryonic cerebellum of Cdk5 KO mice (Ohshima et al. 1999). Additionally, a mild degree of ectopic positioning of the PCs, along with defective inward migration of the granule cells (GCs) from the external GC layer (EGL) to the internal GC layer (IGL), was found in adult p35 KO mice (Chae et al. 1997; Ohshima et al. 2001). Our study of Cdk5+/+;Cdk5−/− chimeric mice further indicated that normal Cdk5 activity is important for migration of the PCs and the GCs in cell-autonomous manner (Ohshima et al. 1999).
Because Cdk5 KO mice die prenatally and cerebellum develops postnatally, we could not analyze the role of Cdk5 in postnatal cerebellar development. To overcome this disadvantage, we generated MHB-specific Cdk5-cKO mice and analyzed the cerebellar development. We also analyzed the dendritic development of PCs in p35 KO mice and cultured PCs from Cdk5 KO mice. Our results indicate that Cdk5/p35 is essential for proper development of the cerebellum, especially for neuronal migration of cerebellar cortical neurons and dendrite formation of PCs.
Materials and Methods
Mutant mice
Cdk5 KO mice were generated (Ohshima et al. 1996) and maintained in a C57BL/6J background and genotyped as described (Ohshima et al. 2001). Cdk5-loxP flanked mice (Cdk5f//f) were generated as described (Hirasawa et al. 2004). The loxP allele of Cdk5 was genotyped using the following primer sets: C5NF-3, 5′-TACCGATAATCCTAGTGGGGGCACA-3′ and lox P-R, 5′-CAGCCAAGAGCTGTGGAATGTAC-3′ using HotStar Taq (Qiagen, Valencia, CA). Wnt1-Cre mice were generated as described (Danielian et al. 1998) and obtained from The Jackson Laboratory (Bar Harbor, ME). In the Wnt1-Cre mouse line, Cre recombinase activity is restricted to the Wnt1-expression region and appears in the MHB region as early as E9.5 (Danielian et al. 1998; Chai et al. 2000; Rico et al. 2002; Baquet et al. 2005). To obtain MHB region-specific Cdk5 conditional KO (MHB-Cdk5 KO; Cdk5f/−;Wnt1-Cre) mice, we established Cdk5+/−;Wnt1-Cre founder males and mated them with Cdk5f/f females. MHB-Cdk5 KO mice were analyzed along with littermate controls (Cdk5f/+). Cre/loxP recombinant reporter mice carrying CAG-CAT-Z (Sakai and Miyazaki, 1997) were kindly provided by Dr Miyazaki. p35 KO mice were generated and genotyped as described (Ohshima et al., 2001). All experimental animal protocols were approved by the Institutional Animal Care and Research Advisory Committee at RIKEN BSI and Waseda University.
Bromodeoxyuridine injection and histological analysis
For bromodeoxyuridine (BrdU) experiments, BrdU (100 μg/g, i.p.) was injected at the indicated age, mice were sacrificed 1 hour later, and tissues were analyzed for the proliferation study or 72 hours later for the pulse-labeling study. Paraffin-embedded brain specimens were cut in 8-μm sections and stained with monoclonal anti-BrdU antibody as described (Gilmore et al. 1998). For histological analysis, paraffin sections were stained with hematoxylin and eosin (HE) or 0.5% thionine for Nissl staining. For the immunohistochemical analysis, 15-μm thick frozen sections were stained using the following primary antibodies as described (Gilmore et al. 1998; Ohshima et al. 2001): monoclonal rat antibody for IP3R1 (clone 4C11, Maeda et al. 1990), monoclonal antibody for NeuN (Chemicon, Temecula, CA), polyclonal antibody for Cdk5 (C-8, Santa Cruz Biotechnology, Santa Cruz, CA), monoclonal (Sigma, St. Louis, MO) and polyclonal (Swant, Germany) antibodies for calbindin, polyclonal anti-neurofilament (NF)-M (NF-150, Chemicon), anti-phospho-Histone H3 (Cell Signaling Technology, Beverly, MA), and anti-PCNA (Santa Cruz) antibodies. Biotinylated secondary antibodies were used, and the staining was visualized by diaminobenzidine as described (Ohshima et al. 1999). Secondary antibodies conjugated to FITC or Cy3 (Jackson ImmunoResearch, West Grove, PA) were used for double-staining experiments as described (Ohshima et al. 1999). For counting the number of PCs, parasagittal paraffin sections of the cerebella from Cdk5 KO and 2 MHB-Cdk5 KO mice at P0 and MHB-Cdk5 KO mice at P10 with their littermate controls were stained with polyclonal calbindin antibody and monoclonal anti-calbindin antibody, respectively. The number of the PCs in the PC layer (PCL) was counted in 4 consecutive sections to obtain the average percentage of PCs in the PCL.
in situ hybridization and LacZ staining
For in situ hybridization, 20-μm cryostat sections were used as described (Ohshima et al. 2002). Digoxigenin-labeled riboprobes directed against the following mRNA were used: Reelin (D’Arcangelo et al. 1996), TAG1 (Karagogeos et al. 1991), and doublecortin (DCX) (des Portes et al. 1998; Gleeson et al. 1998). Beta-gal expression in the brain tissue from CAG-CAT-Z;Wnt1-Cre mice was visualized by staining the sections with X-gal solution (5 mM K3FeCN6, 5 mM K4FeCN6, 2 mM MgCl2, 0.02% NP-40, 0.01% Na-deoxycholate, 1 mg/ml X-gal in 0.1 M PB) as described (Iwasato et al. 2004).
Western blot analysis
Protein extraction and Western blotting were performed as described (Ohshima et al. 2005) using the following primary antibodies: polyclonal rabbit anti-Cdk5 (1:500, C-8, Santa Cruz), monoclonal anti-β actin (1:1000, Sigma), and anti-NF-M (1:100, Chemicon), monoclonal anti-phospho-Thr-Pro antibody (Cell signal), polyclonal anti-pS732FAK (Thermo scientific), monoclonal anti-FAK (BD Transduction lab.), polyclonal pS522CRMP2 (Uchida et al., 2007), monoclonal CRMP2 (C4G, IBL), polyclonal anti-pT212Pak1 (Thermo scientific), polyclonal Pak1 (Santa Cruz), polyclonal anti-pS297DCX (Tanaka et al., 2004), monoclonal anti-DCX (BS Transduction lab.). Data were shown as the mean ± standard deviation (SD) and were analyzed by Student’s t-test.
Analysis of Purkinje cell morphology by introducing Dye by using Gene Gun
A Helios Gene Gun (Bio-Rad) was used to stain cells in fixed cerebellar slice preparations. DiO (3,3′-dioctadecyloxacarbocyanine perchlorate)-coated gold particles were prepared and delivered to fixed cerebellar slice preparations as described (Gan et al., 2009), unless otherwise mentioned. Cerebellar sections of 200 μm thickness were prepared with a vibratome-type slicer (DTK-1000, Dosaka EM, Kyoto, Japan) after transcardial fixation, as described in the histological analysis section. Labeled sections were suspended in PBS and dye was allowed to diffuse through neuronal membranes for 7 days in the dark at room temperature or 4°C. Slices were mounted using 50% glycerol in PBS and observed with a confocal microscope (FV-1000, Olympus, Tokyo, Japan, with a 40x objective lens). Morphometric analysis was conducted using Image J software.
Cerebellar neuronal culture and immunocytochemistry
Dissociated cerebellar neuronal cultures were prepared from E18 wild-type (WT) and Cdk5KO embryos according to Hisatsune et al. (Hisatsune et al., 2001), with some modifications. Briefly, cerebellar cells were dissociated using the Nerve-Cell culture system and were plated at 105 cells/ml in neuron culture medium (Sumitomo Bakelite Co.) on 35-mm polyethyleneimine-coated dishes. Cultured cells were fixed at DIV7 or DIV14 with 4% paraformaldehyde for 15 min at room temperature and then subjected to immunocytochemical staining with mouse anti-calbindin monoclonal antibody (1:500; Sigma) as described previously (Ohshima et al., 2007). Nuclei were stained with DAPI (Roche). Images of calbindin-stained PCs were captured using a BIOREVO immunefluorescence microscope (Keyence) and analyzed using Image J software. Maximum length of a primary dendrite, numbers of branch points on the primary dendrite, dendritic area, defined as the area surrounded by straight lines connecting the ends of all terminal dendritic tips of a single PC, were calculated. The first bifurcation point of the primary dendrite, defined as the length between the first bifurcation point and the exit point of the primary dendrite of PC, was also analyzed.
Results
MHB-specific deletion of Cdk5 resultes in a smaller cerebellum
To specifically analyze the functional roles of Cdk5/p35 in postnatal MHB development, we generated MHB-specific Cdk5 conditional KO (MHB-Cdk5 KO) mice by crossing Cdk5+/−;Wnt1-Cre and Cdk5f/f mice. We analyzed Cre activity in sagittal sections of Wnt1-Cre;CAG-CAT-Z mice by X-gal staining. As reported previously (Danielian et al. 1998; Chai et al. 2000; Rico et al. 2002; Baquet et al. 2005), Cre expression, seen as an X-gal-positive area, was detected in the MHB region (Sup. Fig. 1). In the cerebellum, the Purkinje cells, GC, and their precursors, as well as neurons in deep cerebellar nuclei, are X-gal-positive (Sup. Fig. 1B, C). Consistent with the Cre expression pattern (Fig. 1A), Cdk5 expression was eliminated in the cerebellum of newbornMHB-Cdk5 KO mice (Fig. 1B). At P0, the cerebellum of MHB-Cdk5 KO mice was slightly smaller in size and lacked foliation (Fig. 1C′), and few Cdk5-positive cells and axons were detected in the parasagittal sections (Fig. 1D′). Western blot analysis of the P10 cerebellum confirmed significantly decreased levels of Cdk5 protein (Fig. 4E, Cdk5 level normalized to actin: controls, 1.00 ± 0.159; MHB-Cdk5 KO, 0.076 ± 0.032, n=4, P<0.01). These results confirmed that Wnt1-Cre effectively deleted Cdk5-flox alleles, resulting in the loss of Cdk5 expression in the MHB region.
Fig. 1. MHB-specific Cdk5 conditional deletion of Cdk5 results in a smaller cerebellum.
A–D, Low magnification of the the MHB region of Cdk5-immunostained parasagittal section of MHB-Cdk5 KO mice at P0 (B) revealed loss of Cdk5 immunoreactivity in the MHB region where Cre is expressed, as tested in Wnt1-Cre;CAG-CAT-Z mice at P0 (A). Parasagittal sections of control (C, D) and MHB-Cdk5 KO (C′, D′) brains were stained for Nissl (C, C′) or anti-Cdk5 antibody (D, D′). Only axonal staining (arrow) was detected in this sagittal section from the MHB-Cdk5 KO brain (D′). E, Protein levels of Cdk5 and Actin in cerebellar tissue from control and MHB-Cdk5 KO mice at P10. Significant reduction of Cdk5/Actin was found in the cerebella from MHB-Cdk5KO mice. n=4, *, p< 0.01 F, F′. HE staining of sagittal sections of littermate control (F) and MHB-Cdk5 KO (F′) mice at P21. A smaller cerebellum (Cb, arrow) is evident in MHB-Cdk5 KO mice at P21 (F′). Scale bars, 100 um.
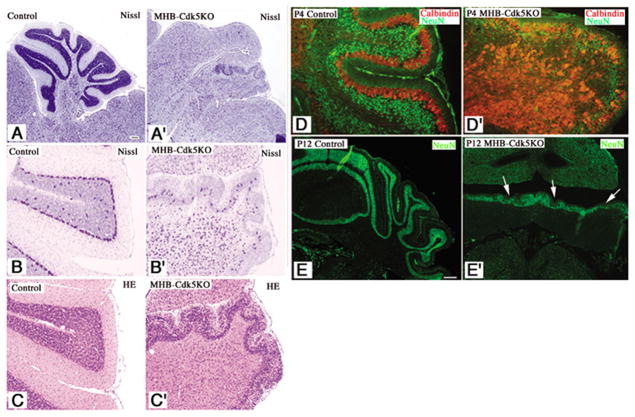
Fig. 4. Neuronal migration defects in the cerebellum of MHB-Cdk5 KO mice.
A–C. Nissl (A, A′, B, B′) and HE (C, C′) staining of sagittal sections from control (A, B, C) and MHB-Cdk5 KO (A′, B′, C′) cerebella at P21. A smaller cerebellum with few foliations is a feature of MHB-Cdk5 KO mice. Most PCs were localized ectopically, but a few formed the PCL (B′). In the cerebellum from MHB-Cdk5 KO mice, most of GCs accumulated in the molecular layer and failed to form the IGL (C′). D–E′. Immunostaining of the sagittal sections of cerebella from control (D, E) and MHB-Cdk5 KO (D′, E′) mice at P4 (D, D′) and coronal sections at P12 (E, E′) with indicated antibodies. In the control, the GCs migrated into the IGL and became NeuN-positive. In MHB-Cdk5 KO cerebellum, however, most of the GCs accumulated in the molecular layer and became NeuN-positive (arrows in E′). Scale bar, 100μm.
MHB-Cdk5 KO mice exhibited lower body weight than controls (at P8 controls, 6.83 ± 0.31 g vs. MHB-Cdk5 KO, 4.11 ± 0.35 g, n=4, P<0.01) as well as severe ataxia, and they died before 3 weeks of age. Gross examination revealed an abnormally small cerebellum. Sagittal sections of MHB-Cdk5 KO mice confirmed the size reduction of the cerebellum at P21 (Fig. 1F′).
Defective migration of cerebellar cortical neurons in MHB-Cdk5 KO mice
Because we previously identified significant defects in the PC migration into PCL in Cdk5 KO mice at E18.5 (Ohshima et al. 1999), we analyzed the position of the PCs in the cerebellum of MHB-Cdk5 KO at P0 (Fig. 2). The percentage of the PCs that reached the PCL was counted by calbindin-immunostaining of parasagittal sections as described in Materials and Methods. We found that 72% of PCs failed to reach the PCL and localized deep inside the cerebellum in MHB-Cdk5 KO mice at P0 (Fig. 2D, D′). On the other hand, 91% of the PCs failed to migrate to the PCL in Cdk5 KO mice (Fig. 2B). This difference may reflect an incomplete deletion of Cdk5 expression. Indeed, we found Cdk5-positive PCs in lateral areas of cerebellar hemispheres in MHB-Cdk5 KO mice (Fig. 3A, B). We then determined what percentage of the PCs lost Cdk5 expression and how loss of Cdk5 correlated with migration defects in the PCs at P10. 95% of the PCs lost Cdk5 expression at P10 (Fig. 3A, B; 642 out 671 PCs were Cdk5-negative, n=3), and All Cdk5-positive PCs were localized in the PCL, and 20% of PCs (controls 99.08 ± 1.14%, MHB-Cdk5 KO mice 20.0 ± 0.98%, n=4) were located in the PCL of the cerebella from MHB-Cdk5 KO mice. Among them, 75% of the PCs in the PCL were Cdk5-negative and 25% were Cdk5-positive. These results confirmed that Cdk5 is critical for migration of the PCs into the PCL. At the same time, these results indicate that some percentage of the PCs were able to reach the PCL without Cdk5. Despite the abnormal distribution of the PCs, the location of neurons in the deep cerebellar nuclei of MHB-Cdk5 KO mice was comparable to that of controls (data not shown). We also observed abnormal dendrite morphology of PCs both in the ectopic region and in the PCL of MHB-Cdk5 KO mice (Fig. 3C′). This abnormality was also observed in Cdk5-positive PCs in the PCL (indicated by arrowheads in Fig. 3E′), indicating a non-cell autonomous defect.
Fig. 2. Migration defects in the Purkinje cells in the cerebellum of MHB-Cdk5 KO mice at P0.

A–D. Calbindin staining of parasagittal sections of the cerebellum from newborn mutants of Cdk5+/+ (A), Cdk5−/− (B), control, Cdk5f/+, (C), and MHB-Cdk5 KO (D) mice. C′ and D′ are higher magnifications of C and D at the PCL, respectively. Most of the Purkinje cells (PCs) failed to migrate into the PCL in the cerebellum of Cdk5−/− and MHB-Cdk5 KO mice, but some percentage of the PCs were positioned in the PCL (arrows) or near the PCL (arrowheads) in these mutants.
Fig. 3. Defective dendritic structures of the Purkinje cells in MHB-Cdk5 KO mice.
A, B. Position of the PCs and its correlation to the presence or absence of Cdk5 protein. Coronal brain section of MHB-Cdk5 KO mouse at P4 was stained with Cdk5 and calbindin (CB) antibodies. Scale bar, 200 um. C, C′. Immunostaining of sagittal brain sections from MHB-Cdk5 KO (C′) and control (C) at P12 using anti-IP3R1 antibody, a marker for the PCs. Less complex dendrite trees of the PCs were observed in MHB-Cdk5 KO mice (C′). D–E. Sagittal brain sections from control (D, E) and MHB-Cdk5 KO mice at P8 (D′, E′) were stained with Cdk5 and anti-IP3R1. No clear difference in dendrite structure was detected between Cdk5-positive (arrow in D′ and E′) and Cdk5-negative PCs (arrowheads in E′). Scale bars are 100 um. F. Protein level of MAP2 but not NF-M was found to be decreased in the cerebella of MHB-Cdk5 KO mice at P10. n=4. *, p< 0.01. G. MAP2-immunostaining indicates a decreased level of protein in the MHB-Cdk5 KO cerebella. Cdk5-positive PCs (arrows) preserved MAP2 staining. Scale bars, 100 μm. H, Western blot analysis of phosphor-proteins in cerebellar homogenates from control and MHB-Cdk5 KO mice at P10 using anti-Thr-Pro antibody. a–d and asterisks indicate bands whose intensities were reduced in MHB-Cdk5KO cerebellar homogenates. I, Proteins corresponding to a–d were further analyzed for their phosphorylation levels by using phosphor-specific antibodies. Reduced phopshorylation levels were confirmed in FAK, CRMP, Pak1, and DCX at the Cdk5 site, although except for in the case of DCX, their protein levels were not changed. Protein level of DCX was found to be decreased. Actin was used for loading control.
Because the lower level of MAP2 was previously reported in the cerebral cortex of Cdk5 KO mice (Cicero and Heruup, 2005), we analyzed MAP2 protein levels of cerebellum in MHB-Cdk5 KO mice. We found that the protein level of MAP2 is also lower in the cerebellum of MHB-Cdk5 KO mice at P10 (48.7 +/− 11.0 % of littermate controls, n=4, p<0.01 Fig. 3F). On the other side, NF-M protein levels were not changed. Decreased immunoreactivities of MAP2 were found in the Cdk5-negative PCs in the sections from P8 MHB-Cdk5 KO cerebella. As expected, Cdk5-positive PCs reversed MAP2 immunostaining (Fig. 3G), indicating that the loss of Cdk5 is associated with decreased levels of MAP2 in PCs. We also compared phospho-proteins between cerebellar homogenates from controls and MHB-Cdk5 KO mice at P10 by using an anti-phospho-Ser/Thr-Pro antibody. We previously used this antibody to conduct a substrate screening of Cdk5 using brain homogenate from Cdk5 KO mice and identified CRMP2 as a Cdk5 substrate (Uchida et al., 2007). We observed different intensities for ten bands by Western blotting. Among them, differences in four bands (a to d in Fig. 3H) were later confirmed as reported Cdk5 substrates, FAK (Xie et al., 2003), CRMP2 (Uchida et al., 2007), Pak1 (Nikolic et al., 1998; Rashid et al., 2001), and DCX (Tanaka et al., 2004), respectively (Fig. 3I). These results indicate, however, the presence of at least 6 unidentified Cdk5 substrate proteins in the developing mouse cerebellum.
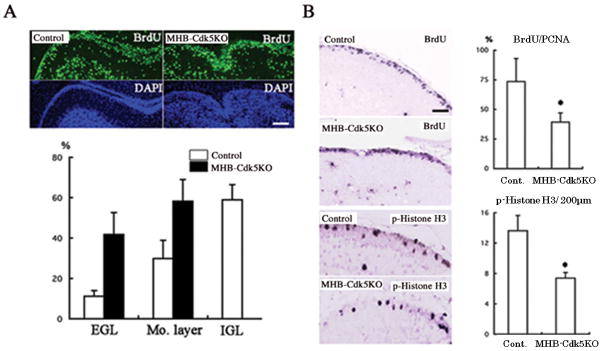
In addition to the smaller cerebellum and the ectopic location of the PCs, the striking feature of the cerebella of MHB-Cdk5 KO mice is the accumulation of GCs within the molecular layer (Fig. 4C′). After reaching the IGL, GC became NeuN-positive in the control cerebellum (Fig. 4D–F) (Weyer and Schilling 2003). In the cerebellum of MHB-Cdk5 KO mice, most of the GCs in the molecular layer were NeuN-positive (Fig. 4D′, E′), suggesting that the migration of GC from EGL to IGL was arrested and became NeuN-positive. To exclude the possibility that defective differentiation or maturation of the GC precursors caused the migration defects of GC, we characterized GC development in the cerebellum of MHB-Cdk5 KO mice using in situ hybridization. After their final division, GCs translocate from the outer layer to the inner layer of the EGL, which is also called the premigratory zone, and then express mRNAs of several genes, including TAG1, Reelin, and DCX. Therefore, we analyzed mRNA expression of these genes in the cerebellum from MHB-Cdk5 KO mice and littermate controls at P4 and P8. Expression patterns of these genes in the premigratory zone of the cerebella of MHB-Cdk5 KO mice were comparable with those of controls, indicating no alternation in the differentiation and early maturation of GCs (Sup. Fig. 2). To study GC migration in the mutant cerebella, we injected BrdU intraperitoneally at P9 and analyzed the localization of BrdU-positive cells after 72 hours. Sixty percent of BrdU-positive GC was found in the IGL of the control mouse cerebellum, whereas most of the BrdU-positive granule cells were localized in the molecular layer and EGL of MHB-Cdk5 KO mice (Fig. 5A). These observations further confirmed the migration defect in GC from the EGL to the IGL in MHB-Cdk5 KO mice, and exclude the possibility of a migratory disturbance caused by secondary effects of defective differentiation of GCs by loss of Cdk5/p35.
Fig. 5. EGL to IGL migration defects and decreased proliferation of the GC precursors in MHB-Cdk5 KO mice.
A. Evaluation of GC migration from the EGL to the IGL by BrdU pulse-labeling. 72 hours after BrdU injection at P9, average percentage of the BrdU-positive GC localized in the EGLand the molecular layer, and IGL was calculated. n=4. B. Immunostaining of BrdU and phospho-Histone H3 (p-Histone H3) of the cerebellum from control (Cont.) and MHB-Cdk5 KO mice at P8. Mouse cerebellum was fixed 1 hour after BrdU injection. BrdU-positive per PCNA-positive cells in the EGL and phospho-Histone H3-positive cells per 200 μm length of EGL were significantly decreased in the MHB-Cdk5 KO cerebellum. n=4. *, P< 0.05.
Next, we studied the proliferation of GC precursors in MHB-Cdk5 KO mice at P8 using BrdU uptake and immunostaining for mitotic markers phospho-Histone H3 and PCNA. For all the stages analyzed, proliferation of the granule cells was low in the EGL of MHB-Cdk5 KO mice as compared to that of control mice (Fig. 5B). There was no increase in TUNEL-positive apoptosis in EGL of MHB-Cdk5 KO mice (data not shown). These results indicate that the decreased number of GCs is caused by decreased proliferation of GC precursors.
Decreased dendritic density and branching of Purkinje cells in p35 KO mice
To determine the role of Cdk5/p35 in dendritic development, we first re-examined dendrite structures of PCs in p35 KO mice (Ohshima et al., 2001). To visualize the detailed morphology of PCs, we introduced DiO by Gene Guns into cerebellar slices from p35 KO mice and their littermate controls (Fig. 6A). We analyzed dendritic morphology in several parameters including the maximum dendritic length, the first bifurcation point of the primary dendrite, the dendritic area, and the highness and wideness of the dendritic tree and its ratio as well as the dendritic density and number of branching points per area. Among these parameters, we found decreased dendritic density and branching points per area (Fig. 6B), indicating PCs in p35 KO mice have sparse dendrite formation.
Fig. 6. Sparse dendrite formation of Purkinje cells in p35 KO mice.
A. Representative images of a single PC of cerebellar slices from p35 wild-type (WT) and p35KO mice. DiO was introduced by Gene Gun and the Z-stack image was captured by FV-1000. Scale bar =50 μm. B. Higher magnified images of indicated areas in A. Scale bar = 25μm. C. Reduced branching points and branching density of PC were observed in p35 KO mice. **, p< 0.01.
Cdk5/p35 is required for normal development of dendritic development of Purkinje cells in vitro
We next analyzed dendrite morphology in cultured PCs with Cdk5 inhibitor in vitro. Because PCs start to extend their dendrite from DIV7 and form typical dendritic tree by DIV14 in dissociated culture, (Furuya et al., 1998), we cultured PCs with the Cdk inhibitor Roscovitine (Ros) during DIV7 to DIV14. We analyzed the dendritic morphology at DIV14 by immunocytochemical staining of PCs with the PC marker calbindin antibody (Fig.7A). Compared to untreated control PCs, we observed a decreased number of dendritic branching in Ros-treated PCs in a dose-dependent manner. The dendritic area was found to be decreased in Ros-treated PCs. We also found first bifurcation points in Ros-treated PCs were apart from their exit point from the soma of the PCs.
Fig. 7. Defective dendrite development in Purkinje cells-treated with Cdk inhibitor and in Purkinje cells from Cdk5 KO mice.
Morphometric analysis including maximum length of primary dendrite, first bifurcation point of primary dendrite, number of branching points on the primary dendrite and dendritic area of cultured wild-type PC with Roscovitine (Ros) (A) and Cdk5 KO PC (B) were conducted at DIV14. A. Upper panel shows representative calbindin-stained PC cultured without Ros (Cont) and with 5 μM Ros and 10 μM Ros. Scale bar = 100 μm. Lower panel shows results of morphometric analysis. Maximum length of primary dendrite was comparable in Ros-treated PC cells. Dose-dependent (5μM and 10μM) reduction was observed in Ros-treated PCs in the number of branching points in primary dendrite and dendritic area. First bifurcation point of primary dendrite was extended in Ros-treated PC. *, p< 0.05. **, p< 0.01. B. Upper panel shows representative calbindin-staining PC from wild-type (WT) and Cdk5KO mice. Scale bar = 100 μm. Lower panel shows results of morphometric analysis. These parameters were similarly altered in PCs from Cdk5KO mice. *, p< 0.05. **, p< 0.01.
We next cultured PCs from wild-type and Cdk5 KO embryos at E18.5 and compared dendritic development (Fig. 7B). We observed decreased branching of PC dendrites and dendritic areas. An elongated distance between the soma to the first bifurcation point of dendrites was also observed in PCs from Cdk5 KO embryos. These results are consistent with those obtained in a study using Cdk inhibitor Ros, as described above, and suggest the requirement of Cdk5/p35 in proper dendritic development of PCs in vivo and in vitro.
Discussion
Cdk5/p35 has been shown to be a key player in many types of neuronal migration and positioning (Ohshima et al. 1996; Gilmore et al. 1998; Ohshima et al. 1999). Without Cdk5/p35, however, some types of neuronal migration occur normally, such as tangential migration of GC precursors from the upper rhombic lip (Ohshima et al. 1999). In the present study, we focused our attention on the lack of Cdk5 expression in the cereballar development and the effects of this, including the dendrite development of PCs. We found that a lack of Cdk5 resulted in a smaller cerebellum..These findings demonstrated the importance of Cdk5/p35 in cerebellar development during the embryonic and postnatal periods.
Our previous studies based on Cdk5 KO embryos and Cdk5+/+;Cdk5 −/− chimera mice indicated that the migration of all PCs was Cdk5-dependent (Ohshima et al. 1999). Interestingly, our present study clearly demonstrates that about 10% of the PCs are able to migrate to the PCL without Cdk5 at P0. Similarly a percentage of PCs was able to migrate to the PCL in Reelin-deficient reeler mice (Tissir and Goffinet, 2003). We observed less complex dendrite tree PCs in the PCL of MHB-Cdk5 KO mice compared to those of littermate controls. We do not believe this phenotype is a cell-autonomous defect of Cdk5-deficient PCs because Cdk5-positive PCs also have similar defects. The large number of GC packed within the molecular layer may be distorting dendrite development mechanically. We cannot exclude the possibility that the loss of Cdk5 in the PCs causes defective development of their dendrites, because Cdk5 is known to be involved in neurite extension (Nikolic et al. 1996; Cheung et al., 2007). Therefore, we examined PC dendrite structure of p35 KO mice. We also analyzed cultured PCs with Cdk inhibitors as well as PC-derived Cdk5 KO mice. Interestingly, PCs in p35 KO mice have sparse dendritic formation and PCs treated with Cdk inhibitors and PCs from Cdk5 KO mice also have decreased branching. These results indicate that Cdk5/p35 is required for the dendrite branching to make complex dendrite trees in vivo and in vitro. Previously, Cdk5 was shown to be required for BDNF-induced dendritic development in cultured hippocampal neurons (Cheung et al., 2007). Therefore, disturbed BDNF signaling is one of the possible explanations for sparse dendritic trees of PCs in p35 KO mice and decreased branching of PCs derived from Cdk5 KO mice.
PCs secrete mitotic factors such as Shh for the granule precursors to induce their prominent proliferation in the outer EGL (Dahmane and Ruiz-I-Altaba 1999; Wechsler-Reva and Scott 1999; Lewis et al. 2004). In reeler mice, defective positioning of the PCs in the PCL is thought to result in an insufficient amount of mitotic factors for the granule precursor cells, producing a small cerebellum and an ataxic phenotype (Tissir and Goffinet, 2003). A similar process may be taking place in MHB-Cdk5 KO mice. Indeed, the proliferation of the GC precursors was compromised in the EGL of MHB-Cdk5 KO through the analyzed periods, which may have a significant impact on the final size of the cerebellum.
The present study also confirmed the absolute requirement of Cdk5/p35 for the inward migration of GCs from the EGL to the IGL. Without Cdk5, GCs obtain normal differentiation and maturation. Several genes involved in neuronal migration are expressed in the migrating GCs of the cerebellum, including DCX (des Portes et al. 1998; Gleeson et al. 1998; Tanaka et al. 2004) and FAK (Xie te al. 2003). We also found a high expression of DCX mRNA in the premigratory zone and in migrating GC. Defective phosphorylation of DCX by Cdk5 is found in the cerebellum of MHB-Cdk5 KO mice (Fig. 3I). Because the introduction of phosphorylation-defective DCX into the GC caused migration defects in these neurons in vitro (Tanaka et al. 2004), DCX is considered to be one of the key Cdk5 downstream molecules involved in GC migration. At P10, the protein level of DCX was found low in MHB-Cdk5 KO cerebellum. Because DCX was highly expressed in immature GCs, a decrease of DCX protein in MHB-Cdk5 KO cerebellum may reflect a reduction in the number of GCs in this mutant mouse. We also observed hypo-phosphorylations of FAK, CRMP2, and Pak1. Phosphorylation of these proteins by Cdk5 may be involved in GC migration because their roles in the migration of cortical neurons were shown previously (Xie et al., 2003; Ip et al., 2011; Causeret et al., 2009). In addition, the results of our Western blot in MHB-Cdk5 KO mice demonstrated the presence of other candidates of Cdk5-substrates. Phosphorylation of these proteins by Cdk5 is also implicated in GC migration and in dendritic development of PCs in developing cerebellum. In this regard, further identification of Cdk5 substrates relating to cerebellar development will provide us with new insights into brain development.
Supplementary Material
Acknowledgments
We thank Dr J. Miyazaki for the gift of the CAG-CAT-Z mice. We thank Dr D. Karagogeos for the in situ probe and J. Gleeson for the in situ probe as well as the anti-phospho-DCX antibody. We also thank Dr. M. Ogawa and E. Utreras for critical reading of the manuscript and Shelagh Johnson for editorial assistance. This work was partially supported by Grants-in-Aid from the Ministry of Education, Science, and Culture, Japan, to Toshio Ohshima.
Abbreviations
- Cdk5
cyclin-dependent kinase 5
- MAP2
microtubule-associated protein 2
- EGL
external granule cell layer
- IGL
internal granule cell layer
- GC
granule cell
- PCR
polymerase chain reaction
- PFA
paraformaldehyde
- PB
phosphate buffer
- PBS
phosphate buffer saline
- BrdU
5-bromo-2′-deoxy uridine
- P
postnatal day
- E
embryonic day
- PC
Purkinje cell
- the PCL
the Purkinje cell layer
- DCN
deep cerebellar nuclei
- SHH
sonic hedgehog
- DCX
doublecortin
- NF-M
neurofilament-medium
References
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factors is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;29:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causeret F, Terao M, Jacobs T, Nishimura YV, Yanagawa Y, Obata K, Hoshino M, Nikolic M. The p21-activated kinase is required for neuronal migration in the cerebral cortex. Cereb Cortex. 2009;19:861–75. doi: 10.1093/cercor/bhn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae T, Kwon YT, Bronson R, Dikkes P, Li E, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures and adult lethality. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural Crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Cheung ZH, Chin WH, Chen Y, Ng YP, Ip NY. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007;5:e. doi: 10.1371/journal.pbio.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero S, Heruup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9659–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Ruiz-i-Altaba A. Sonic Hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowich DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of Cdk5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Furuya S, Makino A, Hirabayashi Y. An improved method for culturing cerebellar Purkinje cells with differentiated dendrites under a mixed monolayer setting. Brain Res Brain Res Protoc. 1998;3:192–8. doi: 10.1016/s1385-299x(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong RO, Lichtman JW. Ballistic delivery of dyes for structural and functional studies of the nervous system. Cold Spring Harb Protoc. 2009 doi: 10.1101/pdb.prot5202. 2009:pdb.prot5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in the cerebralcortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME, Walsh CA. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Ohshima T, Takahashi S, Longenecker G, Honjo Y, Veeranna, Pant HC, Mikoshiba K, Brady RO, Kulkarni AB. Perinatal abrogation of Cdk5 expression in brain results in neuronal migration defects. Proc Natl Acad Sci USA. 2004;101:6249–6254. doi: 10.1073/pnas.0307322101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatsune C, Kuroda Y, Akagi T, Torashima T, Hirai H, Hashikawa T, Inoue T, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor type 1 in granule cells, not in Purkinje cells, regulates the dendritic morphology of Purkinje cells through brain-derived neurotrophic factor production. J Neurosci. 2006;26:10916–24. doi: 10.1523/JNEUROSCI.3269-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip JP, Shi L, Chen Y, Itoh Y, Fu WY, Betz A, Yung WH, Gotoh Y, Fu AK, Ip NY. α2-chimaerin controls neuronal migration and functioning of the cerebral cortex through CRMP-2. Nat Neurosci. 2011;15:39–47. doi: 10.1038/nn.2972. [DOI] [PubMed] [Google Scholar]
- Iwasato T, Nomura R, Ando R, Ikeda T, Tanaka M, Itohara S. Dorsal telencephalon-specific expression of Cre bicombinase in PAC transgenic mice. Genesis. 2004;38:130–138. doi: 10.1002/gene.20009. [DOI] [PubMed] [Google Scholar]
- Karagogeos D, Morton SB, Casano F, Dodd J, Jessell TM. Developmental expression of the axonal glycoprotein TAG-1: differential regulation by central and peripheral neurons in vitro. Development. 1991;112:51–67. doi: 10.1242/dev.112.1.51. [DOI] [PubMed] [Google Scholar]
- Ko J, Humbert S, Brorson T, Takahashi S, Kulkarni AB, Li E, Tsai LH. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci. 2001;21:6758–6771. doi: 10.1523/JNEUROSCI.21-17-06758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PM, Gritil-Linde A, Smeyne R, Kottmann A, McMahon AP. Sonic Hedgehog signaling required for exoansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol. 2004;270:393–410. doi: 10.1016/j.ydbio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Maeda N, Niinobe M, Mikoshiba K. A cerebellar the Purkinje cell marker P400 protein is an inositol 1,2,5-triphosphate (InsP3) receptor protein. Purification and characterization of InsP3 receptor complex. EMBO J. 1990;9:61–67. doi: 10.1002/j.1460-2075.1990.tb08080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M, Chou MM, Lu W, Mayer BJ, Tsai LH. The p35/Cdk5 kinase is a neuron-specific Rac effector that inhibits Pak1 activity. Nature. 1998;395:194–8. doi: 10.1038/26034. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/p35 kinase is essential for neurite outgrowth during neuronal differentiation. Gene Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Mikoshiba K. Reelin signaling and Cdk5 in the control of neuronal positioning. Mol Neurobiol. 2002;26:153–166. doi: 10.1385/MN:26:2-3:153. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Gilmore EC, Longenecker G, Jacobowitz DM, Brady RO, Herrup K, Kulkarni AB. Migration defects of cdk5(−/−) neurons in the developing cerebellum is cell autonomous. J Neurosci. 1999;19:6017–6026. doi: 10.1523/JNEUROSCI.19-14-06017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Hirasawa M, Tabata H, Mutoh T, Adachi T, Suzuki H, Saruta K, Iwasato T, Itohara S, Hashimoto M, Nakajima K, Ogawa M, Kulkarni AB, Mikoshiba K. Cdk5 is required for multipolar-to-bipolar transition during radial neuronal migration and proper dendrite development of pyramidal neurons in the cerebral cortex. Development. 2007;134:2273–2282. doi: 10.1242/dev.02854. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ogawa M, Takeuchi K, Takahashi S, Kulkarni AB, Mikoshiba K. Cdk5/p35 contributes synergistically with Reelin/Dab1 to the positioning of facial branchiomotor and inferior olive neurons in the developing mouse hindbrain. J Neurosci. 2002;22:4036–4044. doi: 10.1523/JNEUROSCI.22-10-04036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Ogawa M, Veeranna, Hirasawa M, Longenecker G, Ishiguro K, Pant HC, Brady RO, Kulkarni AB, Mikoshiba K. Synergistic contribution of Cdk5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain. Proc Natl Acad Sci USA. 2001;98:2764–2769. doi: 10.1073/pnas.051628498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T, Ogura H, Tomizawa K, Hayashi K, Suzuki H, Saito T, Kamei H, Nishi A, Bibb JA, Hisanaga S, Matsui H, Mikoshiba K. Impairment of hippocampal long-term depression and defective spatial learning and memory in p35−/− mice. J Neurochem. 2005;94:917–925. doi: 10.1111/j.1471-4159.2005.03233.x. [DOI] [PubMed] [Google Scholar]
- Ohshima T, Ward JM, Huh CG, Longnecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid T, Banerjee M, Nikolic M. Phosphorylation of Pak1 by the p35/Cdk5 kinase affects neuronal morphology. J Biol Chem. 2001;276:49043–52. doi: 10.1074/jbc.M105599200. [DOI] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the Cre transgenic transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. Cdk5 phosphorylation of doublecortin ser 297 regulates its effect on neuronal migration. Neuron. 2004;41:215–227. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and Brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Ohshima T, Sasaki Y, Suzuki H, Yanai S, Yamashita N, Nakamura F, Takei K, Ihara Y, Mikoshiba K, Kolattukudy P, Honnorat J, Goshima Y. Semaphorin-3A signaling is mediated via sequential Cdk5-GSK3β phosphorylation of CRMP2: implication of common phosphorylating mechanism underlying axon guidance and Alzheimer’s disease. Genes to Cells. 2005;10:165–179. doi: 10.1111/j.1365-2443.2005.00827.x. [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Weyer A, Schilling K. Developmental and cell type-specific expression of the neuronal marker NeuN in the murine cerebellum. J Neurosci Res. 2003;73:400–409. doi: 10.1002/jnr.10655. [DOI] [PubMed] [Google Scholar]
- Xie Z, Sanada K, Samuels BA, Shih H, Tsai LH. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.