Abstract
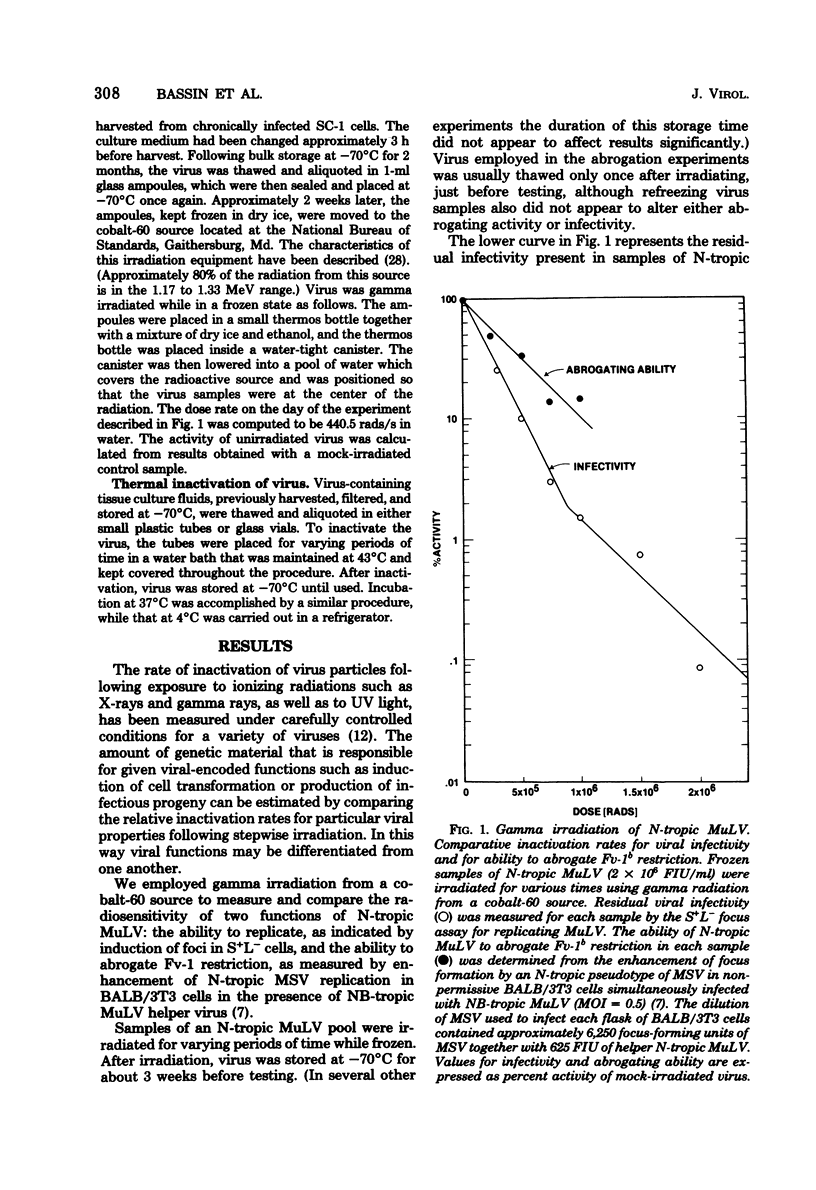
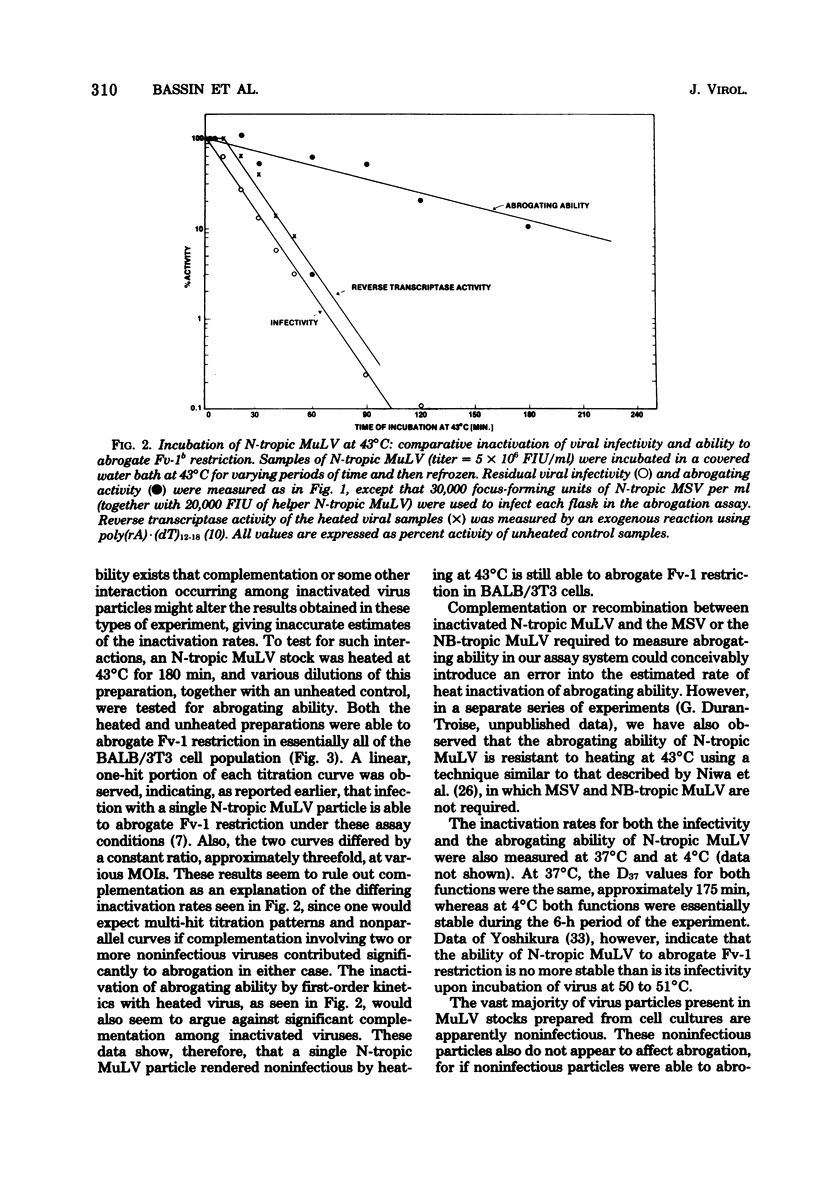
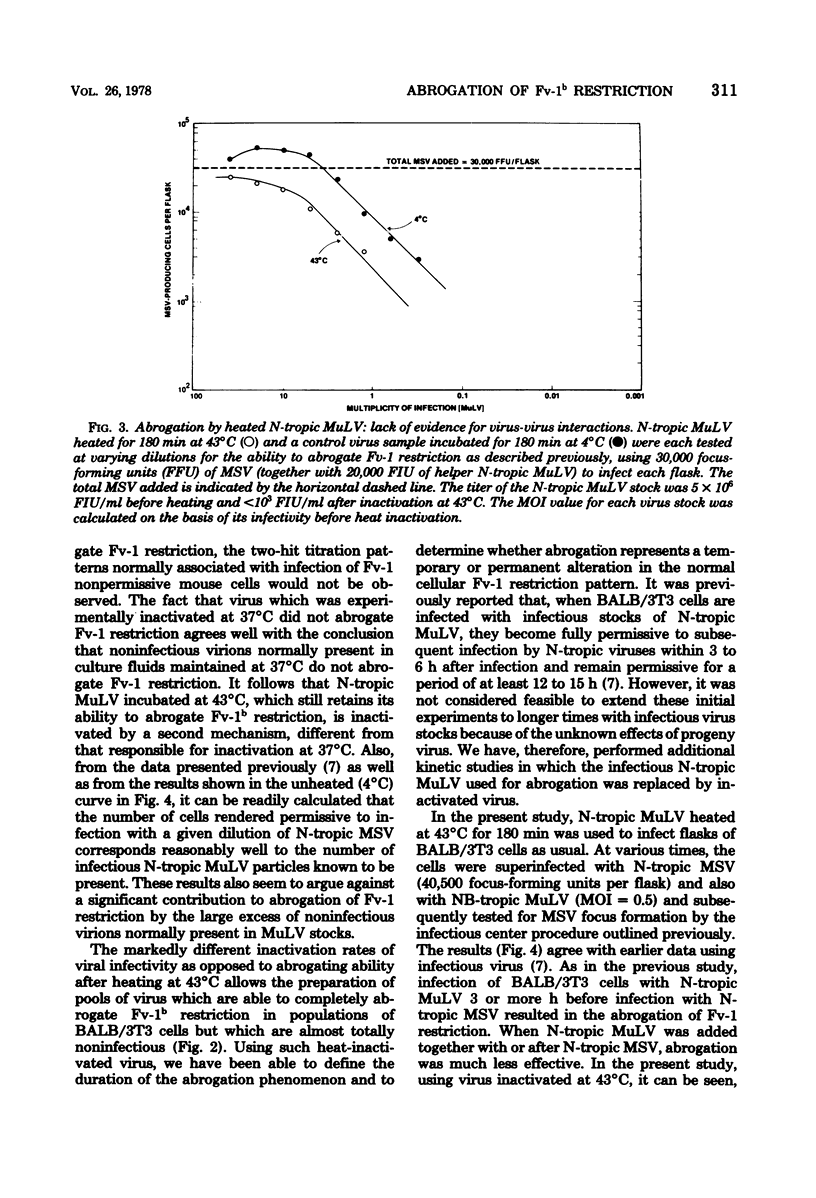
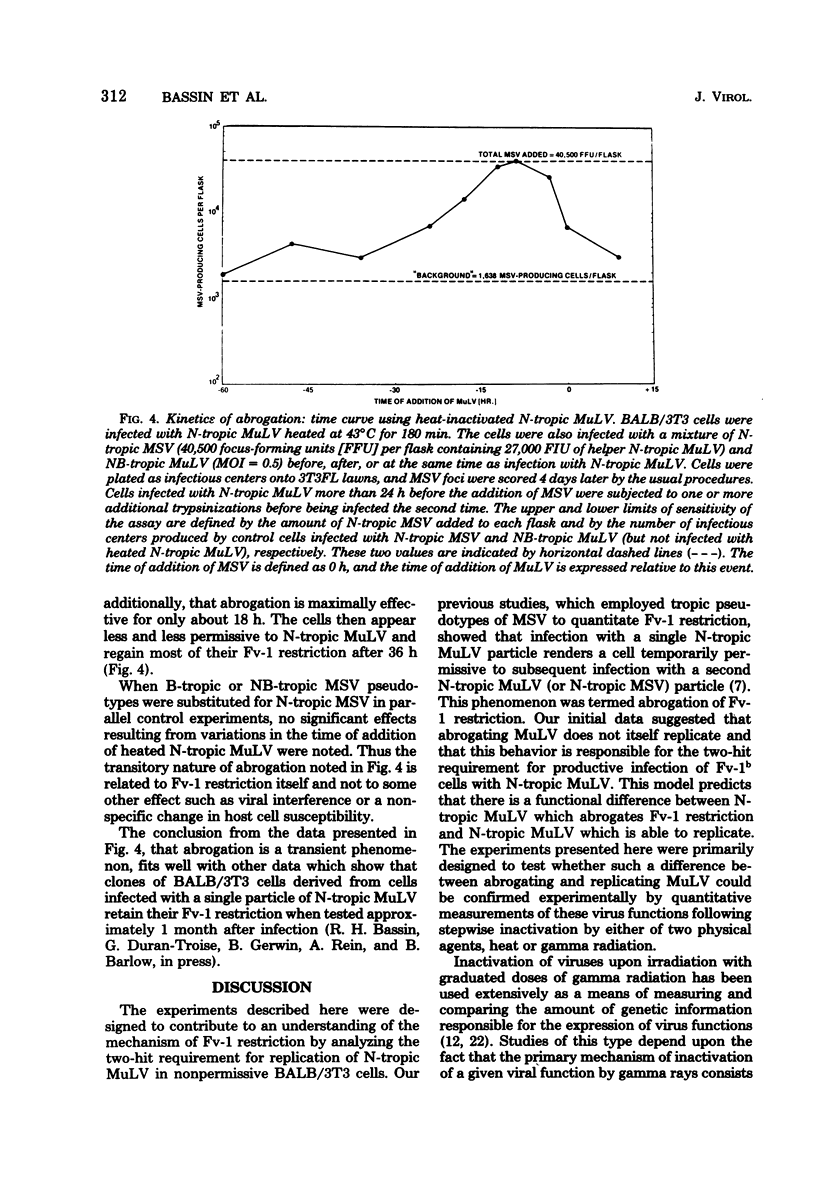
Fv-1b restriction in BALB/3T3 cells is temporarily abrogated following infection with N-tropic murine leukemia virus. The mechanism of this phenomenon was investigated by comparing the inactivation rates for viral infectivity and for the ability of the same virus to abrogate Fv-1 restriction. Inactivation of the abrogating ability of N-tropic murine leukemia virus following graduated doses of gamma radiation proceeded at half the rate of that for viral infectivity. This result indicates that viral RNA must function in abrogating Fv-1b restriction but that only a portion of the viral genome is required. The inactivation kinetics of N-tropic murine leukemia virus were also determined following incubation of virus at 43 degrees C. Abrogating ability of N-tropic murine leukemia virus was found to be about six times as stable under these conditions as was viral infectivity. Interestingly, virion-associated reverse transcriptase activity was inactivated at the same rate as was viral infectivity, indicating that this enzyme may not need to function during abrogation. Virus heated at 43 degrees C was used to study the kinetics of the abrogation phenomenon itself. Abrogation was shown to be transient, requiring 6 to 9 h after virus infection to become maximally effective and beginning to disappear after about 18 h. The data reported here confirm the idea that abrogation of Fv-1 restriction can be separated experimentally from virus replication, and they raise the possibility that a separate biochemical pathway exists for incoming viral RNA in Fv-1 restrictive cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Gerwin B. I., Duran-Troise G., Gisselbrecht S., Rein A. Murine sarcoma virus pseudotypes acquire a determinant specifying N or B tropism from leukaemia virus during rescue. Nature. 1975 Jul 17;256(5514):223–225. doi: 10.1038/256223a0. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bassin R. H., Tuttle N., Fischinger P. J. Rapid cell culture assay technic for murine leukaemia viruses. Nature. 1971 Feb 19;229(5286):564–566. doi: 10.1038/229564b0. [DOI] [PubMed] [Google Scholar]
- Beemon K. L., Faras A. J., Hasse A. T., Duesberg P. H., Maisel J. E. Genomic complexities of murine leukemia and sarcoma, reticuloendotheliosis, and visna viruses. J Virol. 1976 Feb;17(2):525–537. doi: 10.1128/jvi.17.2.525-537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Varmus H. E., Stavnezer E., Hunter E., Vogt P. K. Biological and biochemical studies on the inactivation of avian oncoviruses by ultraviolet irradiation. Virology. 1977 Apr;77(2):689–704. doi: 10.1016/0042-6822(77)90492-5. [DOI] [PubMed] [Google Scholar]
- Declève A., Niwa O., Gelmann E., Kaplan H. S. Replication kinetics of N- and B-tropic murine leukemia viruses on permissive and nonpermissive cells in vitro. Virology. 1975 Jun;65(2):320–332. doi: 10.1016/0042-6822(75)90038-0. [DOI] [PubMed] [Google Scholar]
- Duran-Troise G., Bassin R. H., Rein A., Gerwin B. I. Loss of Fv-1 restriction in Balb/3T3 cells following infection with a single N tropic murine leukemia virus particle. Cell. 1977 Mar;10(3):479–488. doi: 10.1016/0092-8674(77)90035-6. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Meier H. A short-term quantitative XC assay for murine leukemia virus. Virology. 1976 Jul 15;72(2):509–513. doi: 10.1016/0042-6822(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Gerwin B. I., Levin J. G. Interactions of murine leukemia virus core components: characterization of reverse transcriptase packaged in the absence of 70S genomic RNA. J Virol. 1977 Nov;24(2):478–488. doi: 10.1128/jvi.24.2.478-488.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashmiri S. V., Rein A., Bassin R. H., Gerwin B. I., Gisselbrecht S. Donation of N- or B-tropic phenotype to NB-tropic murine leukemia virus during mixed infections. J Virol. 1977 Jun;22(3):626–633. doi: 10.1128/jvi.22.3.626-633.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latarjet R. Inactivation du virus leucémogène de gross par les radiations UV, X et gamma. Int J Cancer. 1970 Jul 15;6(1):31–39. doi: 10.1002/ijc.2910060106. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Rosenak M. J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger G. G., Ling H. P., Gilden R. V., Hatanaka M. Effect of UV Light on RNA-Directed DNA Polymerase Activity of Murine Oncornaviruses. J Virol. 1975 May;15(5):1273–1275. doi: 10.1128/jvi.15.5.1273-1275.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owada M., Ihara S., Toyoshima K., Sugino Y., Kozai Y. Ultraviolet inactivation of avian sarcoma viruses: biological and biochemical analysis. Virology. 1976 Feb;69(2):710–718. doi: 10.1016/0042-6822(76)90499-2. [DOI] [PubMed] [Google Scholar]
- Schuh V., Blackstein M. E., Axelrad A. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: titration patterns in strains SIM and SIM.R congenic at the Fv-1 locus. J Virol. 1976 May;18(2):473–480. doi: 10.1128/jvi.18.2.473-480.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveda M. M., Soeiro R. Host restriction of Friend leukemia virus: synthesis and integration of the provirus. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2356–2360. doi: 10.1073/pnas.73.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]


