Abstract
A subject's baseline FA composition may influence the ability of dietary highly unsaturated omega-3 FAs (n3-HUFA) to change circulating profiles of esterified FAs and their oxygenated metabolites. This study evaluates the influence of basal n3-HUFA and n3-oxylipin status on the magnitude of response to n3-HUFA consumption. Blood was collected from fasting subjects (n = 30) before and after treatment (4 weeks; 11 ± 2 mg/kg/day n3-HUFA ethyl esters). Esterified FAs were quantified in erythrocytes, platelets, and plasma by GC-MS. Esterified oxylipins were quantified in plasma by LC-MS/MS. Treatment with n3-HUFAs increased n3-HUFAs and decreased n6-HUFAs in all reservoirs and increased plasma n3-oxylipins without significantly changing n6-oxylipin concentrations. As subject basal n3-HUFAs increased, treatment-associated changes decreased, and this behavior was reflected in the percentage of 20:5n3 + 22:6n3 in red blood cell membrane FAs (i.e., the omega-3 index). To maintain an omega-3 index of 8% and thus reduce cardiovascular disease risk, our analyses suggest a maintenance dose of 7 mg/kg/day n3-HUFA ethyl esters for a 70-kg individual. These results suggest that the basal n3 index may have clinical utility to establish efficacious therapeutic experimental feeding regimens and to evaluate the USDA Dietary Guidelines recommendations for n3-HUFA consumption.
Keywords: omega-3 fatty acids, oxylipins, targeted metabolomics, baseline status, highly unsaturated fatty acid
Health consequences associated with low intakes of the long-chain, marine omega-3 (n3) FAs have become a central issue in nutritional lipid research. The United States Department of Agriculture's 2010 Dietary Guidelines recommend consumption of 8 ounces per week of fish, providing an average of 250 mg eicosapentaenoic acid (20:5n3) and docosahexaenoic acid (22:6n3) per day for prevention of heart disease (1). Moreover, public awareness regarding the potential health benefits of n3 FAs has spurred an increase in fatty fish and fish oil consumption (2).
Although the mechanisms by which n3-HUFAs improve health are still being explored, it is clear that increasing n3-HUFA intake can decrease the risk of cardiovascular disease (CVD) in at-risk individuals (3–5). In hyperlipidemic subjects, treatment with high doses of n3-HUFAs lowers triglycerides (6) and improves total:HDL cholesterol ratios (6, 7), a surrogate marker associated with a reduction in CVD risk. The n3-HUFAs 20:5n3 and 22:6n3 also reduce inflammatory responses in a range of conditions (8–10). A well-accepted effect of n3-HUFA supplementation is a reduction in thrombin-stimulated platelet aggregation due to decreased platelet cyclooxygenase metabolism (11). More recently, a cyclooxygenase-independent diminution of platelet sensitivity to collagen has been reported after P-OM3 treatment (12). Thus, an increase in the anti-inflammatory n3-HUFAs, which lowers the more proinflammatory n6-HUFAs (13–15), at least partially explains the health benefits of n3-HUFA consumption (8–10).
Recently, the red blood cell (RBC) sum percent of 20:5n3 and 22:6n3 (i.e., the n3 index) has appeared as an indicator of CVD risk (16, 17) that also correlates with obesity, depression, and diet quality (18–20). However, investigations of metabolic covariates of the n3 index are limited. Although the n3 index may have clinical utility (21), we have little information regarding the variability of the index's response to intervention. Therefore, to better interpret the n3 index in clinical settings, the associated changes in other lipid metabolites and the variability of this biomarkers’ response to n3-HUFA intake need to be clarified.
In the current study, a cohort of apparently healthy individuals (n = 30) was treated with prescription n3 acid ethyl esters (P-OM3) for 4 weeks, extending an earlier study in a smaller cohort (n = 10) (22). The earlier study suggested that the magnitude of changes in plasma achieved with a P-OM3 challenge were dependent on metabolite baseline concentrations. In this study, P-OM3 impacts on plasma, erythrocyte, and platelet esterified FAs, as well as a suite of n3 and n6 oxylipins in the plasma esterified lipid pool, were assessed. Our findings support the observed baseline-dependent responses in subject lipid pools and suggest response thresholds in the study population.
MATERIALS AND METHODS
Subjects and sample collection
Thirty apparently healthy adults volunteered for this study and met inclusion criteria previously described (23). Participants (9 male; 21 female) had an average age of 32.9 years (range, 21–59 years) and body mass index of 25.7 kg/m2 (range, 21–32 kg/m2). Oxylipin and FA pools were measured before and after a 4 week challenge with 4 g/day P-OM3 (Lovaza; GlaxoSmithKline, Philadelphia, PA) containing 465 mg 20:5n3, 365 mg 22:6n3, and at least 900 mg of total n3 ethyl esters. This range of n3-HUFA intake has been associated with a protective antioxidant effect, whereas higher levels are accompanied by risks of oxidative stress (24, 25). Individual P-OM3 doses were calculated considering bodyweights and were 47 ± 9 mg/kg on average (men: 37–54 mg/kg; women: 34–58 mg/kg). After a 10-h overnight fast, blood was drawn into sodium EDTA, and plasma was isolated. Red blood cells (RBCs) and platelets were isolated as previously reported (22, 26). The study was approved by the University of South Dakota Institutional Review Board, and written consent was obtained from each subject.
Fatty acid analysis
Plasma, RBC, and platelet FA composition was measured using gas chromatography as previously described (22, 26). Briefly, lipids were extracted using methylene chloride, methanol, and water (2:2:1) followed by treatment with 14% boron trifluoride in methanol at 100°C for 10 minutes. Margaric acid (17:0) was used as an internal standard for determination of plasma FA concentrations. The FA methyl esters were analyzed in a GC2010 (Shimadzu, Columbia, MD) using a 100 m SP2560 capillary column (Supelco, Bellefonte, PA).
Oxylipin analysis
The International Union of Pure and Applied Chemistry (IUPAC)-adopted systematic abbreviations for oxidized FAs are used within this manuscript, and a translation of this naming structure can be found in the Supplementary data. Plasma oxylipins were determined by LC-MS/MS after their release from the esterified pools with mineral base using a previously reported method with slight modifications (22). Briefly, plasma aliquots (100 μl) were spiked with 11 deuterated oxylipins surrogates, including FA prostaglandins, thromboxanes, leukotrienes, primary alcohols, secondary alcohols, and epoxides. A complete list of analytical surrogates and internal standards and target analytes can be found in Supplementary Tables I–V. Spiked samples were then enriched with 12 M sodium hydroxide to yield a 2 M methanolic sodium hydroxide solution. This mixture was incubated for 1.5 h at 60°C. Subsequently, the hydrolyzed samples were diluted with water to 16% methanol, and analytes were trapped on conditioned 60 mg Oasis™ HLB SPE columns (Waters, Milford, MA). Columns were washed and dried, and residues were eluted with 0.5 ml methanol followed by 2 ml of ethyl acetate (22) into 6 μl 30% glycerol in methanol. Residues were brought to dryness under vacuum and reconstituted in methanol containing 100 nM each of 1-cyclohexylurea-3-dodecanoic acid (Sigma-Aldrich, St. Louis, MO) and 1-phenylurea-3-hexanoic acid as internal standards. The sample was vortexed, cooled, and filtered by centrifugation for 3 min using 0.1 μm Amicon® Ultrafree-135 MC Durapore PVDF filters (Millipore, Billerica, MA). Analytes were separated by reverse phase on an ultra-performance liquid chromatograph with a 1.7 μm Acquity BEH column (Waters, Milford, MA) using a 16 min gradient (Solvent A = 0.1% acetic acid; Solvent B = 90:10 v/v acetonitrile/isopropanol; see Supplementary Table V for details). Oxylipins were detected by negative mode electrospray ionization, tandem quadrupole mass spectroscopy ionization, and fragmentation energies for the reported analyte precursor-product ions were optimized for analysis on an AP 4000QTrap (AB SCIEX, Foster City, CA). Collision-induced dissociation mass transitions for all target analytes are reported in Supplementary Tables I–IV. Representative total ion chromatograms of calibration solutions and plasma samples are shown in Supplementary Fig. I. The total area under the sample m/z 353.2 > 193.1 ion trace between 4.4 and 5.7 min was quantified using the PGF2α calibration curve and used as an estimate of the sample F2-isoprostane concentrations (Supplementary Fig. II). Average surrogate recoveries were deemed acceptable across the study. Although recoveries were analyte specific, they ranged between 58 ± 15% and 90 ± 7% for all reported results (Supplementary Table VI).
Data analysis and statistics
Differences in measured lipid concentrations and mol% compositions between pre- and post P-OM3 challenge were tested by two-tailed paired t-tests after data transformation to normality. Although the amount of P-OM3 delivered was constant, body mass and thus dose varied considerably within the cohort. Changes in plasma concentrations of FAs and oxylipins were expressed with respect to subject P-OM3 mg/kg/day dose. Complete data sets were available for all assays except for three subjects whose platelet FAs were not available. All data were log-transformed to achieve normal distributions. Differences in means identified at α < 0.05 were considered significant after adjusting for false positives due to multiple comparisons (q = 0.2) using the procedures of Benjamini and Hochberg (27).
RESULTS
Changes in cellular and plasma esterified FAs
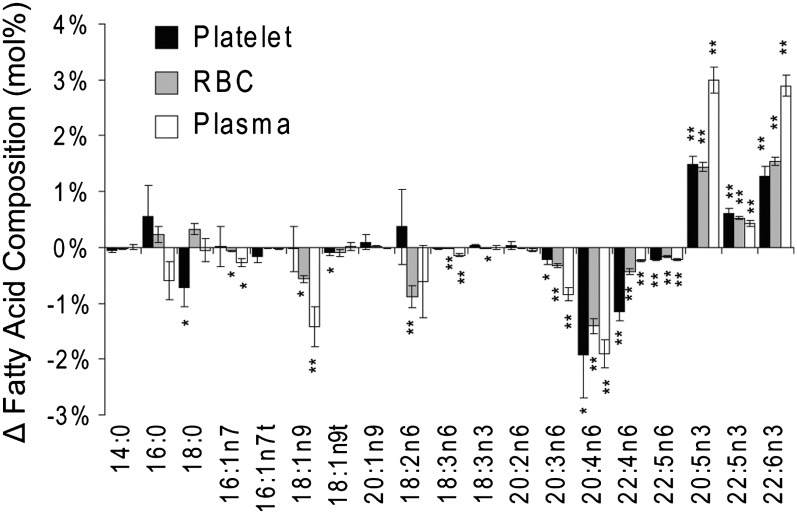
Plasma, platelets, and RBCs showed distinct FA profiles before and after treatment (supplementary Table VII), with treatment-induced changes summarized in Fig. 1. In general, n3-HUFAs were incorporated into all three compartments at the expense of the n6-HUFAs. Changes in RBC and platelet 20:5n3 and 22:6n3 composition was ∼50% of those observed in plasma.
Fig. 1.
Changes in the mol% composition of platelet, RBC, and plasma lipid pools after 4 g/day n3 FAs ethyl esters. The n6-HUFAs were reduced and n3-HUFAs were enriched in all measured compartments. MUFAs were reduced in plasma and RBCs, and minor but significant changes in platelet stearate (C18:0) were observed. Data are presented as mean ± SEM. Significant changes in composition were assessed with paired two-tailed t-tests and are indicated at *P < 0.05 and **P < 0.001.
Treatment with P-OM3 did not affect saturated FA composition. The relative abundances of monounsaturated FAs (MUFAs), including palmitoleic acid (16:1n7) and oleic acid (18:1n9), were reduced in plasma and RBCs but were unchanged in platelets. Among the n6-polyunsaturated FAs (PUFAs), the composition of linoleic acid (18:2n6) was reduced in RBCs, whereas γ-linolenic acid (18:3n6) was reduced in RBCs and plasma. On the other hand, 20- and 22-carbon HUFAs were changed in all lipid pools analyzed. Arachidonic acid (20:4n6) showed the greatest decrease in relative abundance, with changes in mol% being roughly equivalent at ∼2% across all three sample types (Fig. 1). Dihomo-γ-linolenic acid (20:3n6) was reduced in plasma > RBCs > platelets. Conversely, reductions in adrenic acid (22:4n6) were in the order of platelets > RBCs > plasma.
On a concentration basis, a slight increase in the total plasma-HUFAs was detected after treatment (Table 1). Although 20:4n6 concentrations were unchanged, levels of 22:4n6 and osbonic acid (22:5n6) were decreased, whereas 20:5n3, 22:5n3 and 22:6n3 were increased.
TABLE 1.
Plasma concentrations of HUFAs and their oxylipin products (mean ± SEM) before and after 4 weeks of 4 g/day n3 FA ethyl esters
| Pre | Post | Fold Change | P* | |
| Parent fatty acid (µM) | ||||
| ΣHUFAs | 1,680 ± 150 | 2,370 ± 290 | <+2 | 0.04 |
| 20:4n6 | 1,240 ± 99 | 1,140 ± 140 | — | >0.05 |
| 22:4n6 | 41.9 ± 3.0 | 23.9 ± 2.9 | −2 | <0.001 |
| 22:5n6 | 33.6 ± 3.2 | 15.3 ± 1.9 | −2 | <0.001 |
| 20:5n3 | 74.5 ± 17 | 512 ± 69 | +7 | <0.001 |
| 22:5n3 | 73 ± 10 | 130 ± 20 | +2 | <0.01 |
| 22:6n3 | 222 ± 26 | 557 ± 66 | +3 | <0.001 |
| Oxylipin (nM) | ||||
| ΣHUFA alcohols (n = 10) | 789 ± 92 | 756 ± 47 | — | >0.05 |
| 20:4n6 alcohols (n = 6) | 732 ± 87 | 562 ± 87 | <−2 | >0.05 |
| 20:5n3 alcohols (n = 3) | 17.7 ± 3.2 | 101 ± 8.0 | +6 | <0.0001 |
| 22:6n3 alcohols (n = 1) | 39.3 ± 3.7 | 94.0 ± 7.1 | +2 | <0.0001 |
| ΣHUFA epoxides (n = 10) | 58.9 ± 14 | 65 ± 13 | — | >0.05 |
| 20:4n6 epoxides (n = 3) | 50.6 ± 11.37 | 40.6 ± 7.86 | — | >0.05 |
| 20:5n3 epoxides (n = 2) | 1.13 ± 0.37 | 9.12 ± 2.03 | +9 | <0.001 |
| 22:6n3 epoxides (n = 2) | 7.13 ± 2.02 | 15.4 ± 3.29 | +2 | 0.07 |
| ΣHUFA vic-diols (n = 10) | 62.9 ± 4.0 | 68.5 ± 5.5 | — | >0.05 |
| 20:4n6 diols (n = 3) | 23.8 ± 2.0 | 20.0 ± 1.5 | — | 0.13 |
| 20:5n3 diols (n = 2) | 38.0 ± 2.6 | 47.3 ± 4.1 | <+2 | 0.09 |
| 22:6n3 diols (n = 1) | 0.48 ± 0.3 | 1.17 ± 0.20 | +2 | <0.001 |
* Differences in means were assessed by paired two-tailed t-tests.
Changes in plasma esterified oxylipins
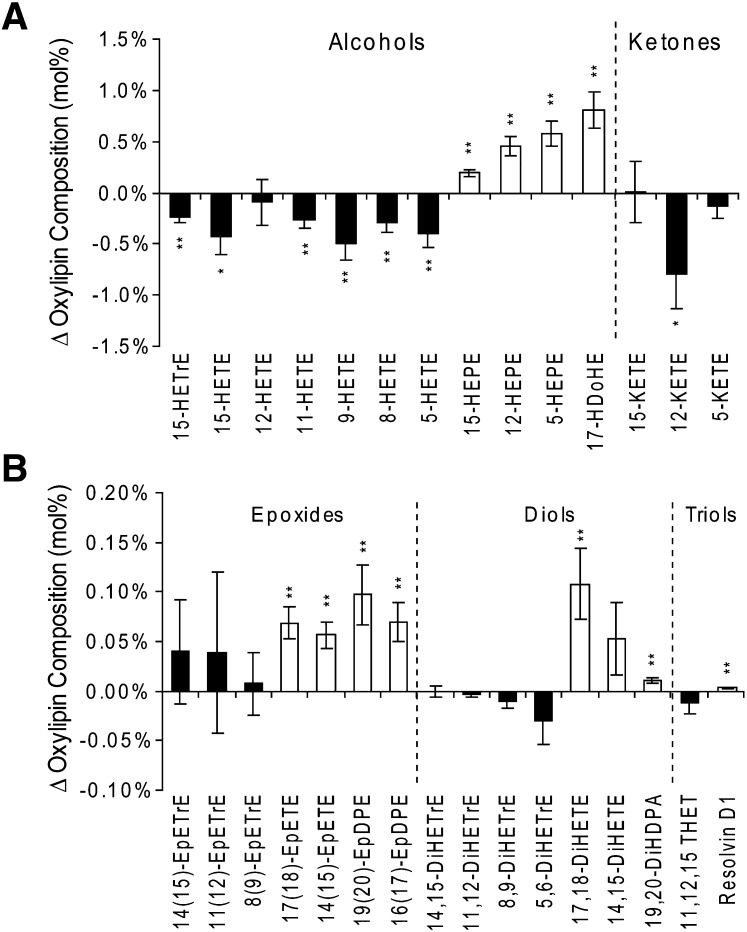
Quantitative measurements of 51 18- to 22-carbon oxylipins passed quality assurance criteria and are reported in Supplementary Tables VII and VIII. The relative abundances of esterified plasma oxylipins were altered by P-OM3 treatment (Fig. 2A). As observed in the n6 and n3-HUFAs (Fig. 1), the high abundance oxylipins derived from n6- and n3-HUFAs were decreased and increased, respectively (Fig. 2A). This included changes in the measured 20:4n6-, 20:5n3-, and 22:6n3- mid-chain alcohols and ketones. The P-OM3 challenge also increased the relative abundance of n3-HUFA epoxides, diols, and triols (i.e., Resolvin D1), whereas those derived from other parent FAs were not affected (Fig. 2B). Moreover, the concentrations of the sum of the measured 20:5n3 (P < 0.001) and 22:6n3 (P < 0.07) epoxides were increased (Table 1), as was true for some but not all of the measured n3-diols (see Supplementary Table VIII). Concentrations of the 18:2n6-derived oxylipins were not significantly changed by P-OM3 treatment (supplementary Table IX).
Fig. 2.
Changes in the mol% composition of the plasma esterified n6-HUFA (black) and n3-HUFA (white) oxylipins after ingestions of 4 g/day (47 ± 9 mg/kg) n3 FA ethyl esters. The alcohols and ketones (A) of n6-HUFAs were reduced by the P-OM3 challenge, whereas the n3-HUFAs of these oxylipins, as well as the epoxides, diols, and triols (B), were increased. Changes in composition are presented as mean ± SEM. Significant changes in composition were assessed with paired two-tailed t-tests and are indicated at *P < 0.05) and **P < 0.001.
Concentration changes are inversely proportional to baseline concentrations
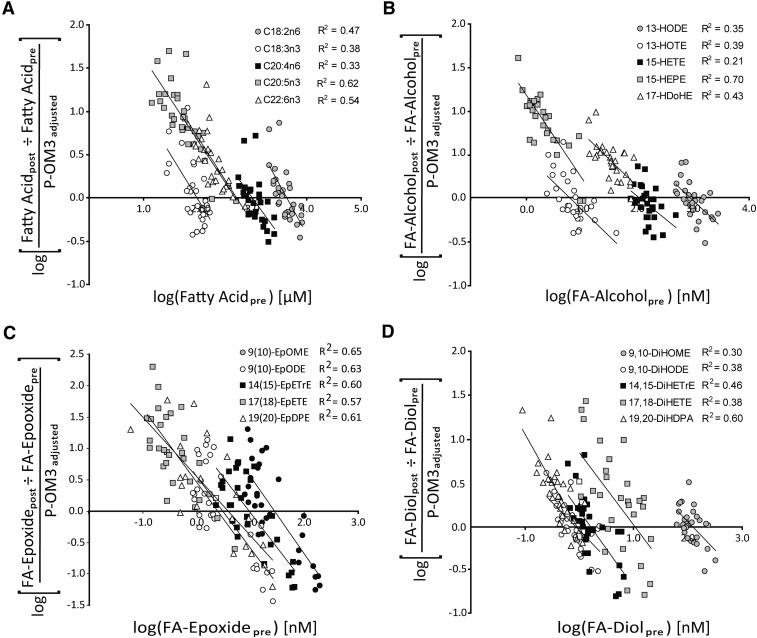
Despite high compliance, substantial variability was observed in the magnitude of plasma FA changes detected after the P-OM3 challenge. The 4 g P-OM3 daily intake is expressed as mg/kg dose. Before evaluating metabolite dose response behavior, each subject's P-OM3 dose was transformed to a percentage of the average cohort dose (47 mg/kg dose). In Fig. 3, the adjusted dose-dependent fold changes in esterified lipid concentrations are plotted against their baseline concentrations. A subject entering the study with a baseline concentration equal to the x-intercept would not be expected to change their metabolite concentrations if they received the average cohort dose. Therefore, we infer that the x-intercept defines a change thre shold for the population studied. As the difference between the subjects’ baseline concentrations and the change threshold increased, so did the magnitudes of changes observed.
Fig. 3.
Supplementation-dependent changes in representative unsaturated FAs (A), FA alcohols (B), FA epoxides (C), and FA diol (D) expressed as a function of pretreatment concentrations. The magnitudes of concentration changes with treatment decrease as baseline concentrations increase, indicating the presence of a finite and saturable lipid pool. The x-intercepts indicate the population's change threshold (i.e., the apparent baseline concentration of each FA), which would be most likely associated with change in concentration.
The effect of P-OM3 on the distribution of subjects above the change threshold was tested by chi-square test (supplementary Tables X–XII). Metabolites unaffected by lipid supplementation would have a random probability of increasing or decreasing during the 4 week treatment period, with high and low measurements tending to regress toward the population mean upon the repeated measurement (28). Therefore, if truly random, an equal number of subjects would be expected above and below the x-intercept. Although the 18 carbon FAs and oxylipins were evenly distributed about the threshold, the 20:4n6 and many of its metabolites showed >60% of subjects below this line. Thus, although P-OM3 did not produce significant decreases in the mean 20:4n6 concentrations (Table 1), 19 of 29 (∼65%) subjects were distributed below the 20:4n6 change threshold (χ2 p = 0.2), suggesting a subtle shift in the population distribution of 20:4n6. On the other hand, 20:5n3 and 22:6n3 showed a 100% distribution above the threshold, as did the n3 alcohols. Distributions of observed n3 epoxide and diol ranged from 90% to 60% above their respective change thresholds.
Omega-3 index response to P-OM3 is influenced by initial values and dose
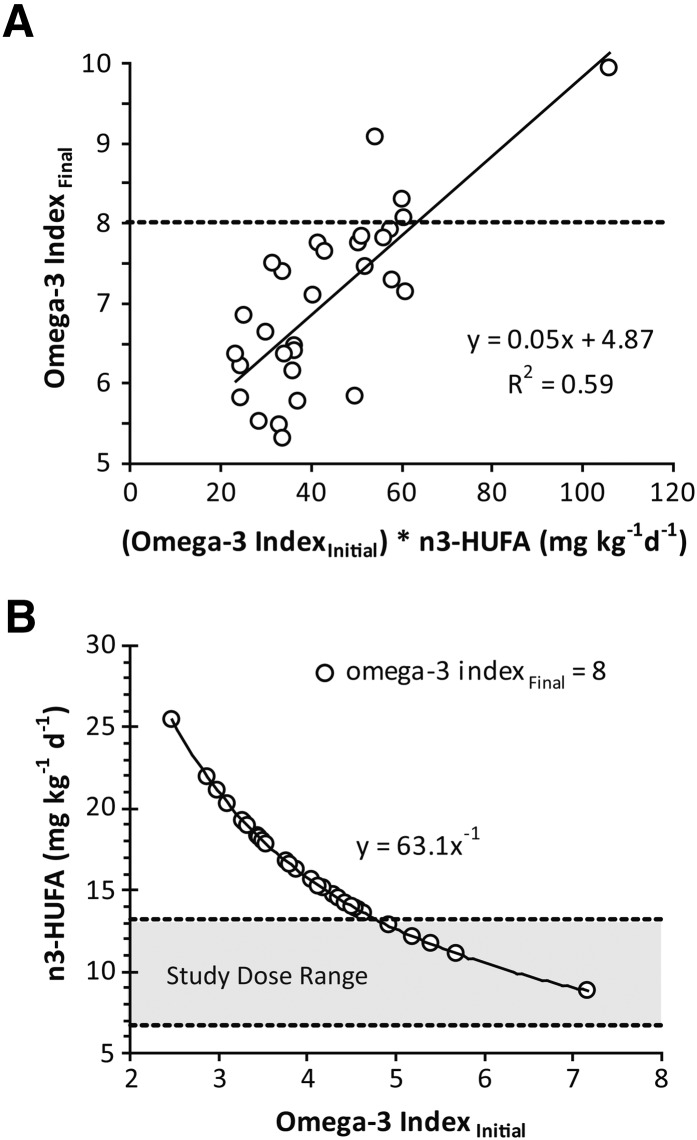
The initial and final n3 index values were strongly correlated (R2 = 0.82; P < 0.01). In addition, the dose-dependent change in the n3 index was negatively correlated with the baseline n3 index (R2 = −0.36; P = 0.05). In other words, all subjects increased n3-HUFA abundance in RBCs, but those with higher baseline values changed less. These observations led us to explore the potential use of an individual's baseline n3 index to estimate a dosing regimen required to achieve a desired n3 index after a 4 week period (Fig. 4). Based on the analysis of the current data set, an efficacious dosing regimen can be seen to follow a power curve, with higher doses being required to effect rapid change in individuals with lower initial n3 index values. Similarly, a dose of 35 mg/kg/day P-OM3 (4 mg/kg/day 20:5n3 + 3 mg/kg/day 22:6n3) is estimated to maintain an n3 index of 8 and thus reduce cardiovascular disease risk.
Fig. 4.
Changes in the n3 index are influenced by basal n3 status and dose. A: The n3 index achieved after 4 weeks of P-OM3 intake was positively correlated with the product of the initial n3 index and the dose. B: The theoretical dose required to cross an n3 index of 8% given an initial n3 index is estimated from this relationship. The study dose range (35–65 mg/kg/day) is shown in gray.
DISCUSSION
n3 Fatty acids have broad health-promoting effects (29, 30), with multifactorial impacts on genes, metabolism, and regulatory systems (31). Although n3 FAs are thought to have direct actions, they are also precursors to bioactive metabolites (32–34), and n3 intake increases many of these metabolites in plasma (22). However, variability in responses to n3 FA intake has been reported. In an earlier n3 FA feeding study, subjects with the lowest baseline n3-oxylipin concentrations showed the greatest increase after treatment. However, the cohort size was too small to report this observation with appropriate statistical rigor (22). To validate the earlier observation and to place it in a context of overall FA status, we have investigated the impact of a P-OM3 challenge on plasma and blood cell esterified FAs and plasma esterified oxylipins using a dose associated with the protective effects of n3 FA ingestion, higher doses having been associated with oxidative stress (24, 25). Although n3 FAs ingested as ethyl esters are incorporated into circulating triglycerides more slowly than ethyl ester forms (35), the absorption of ethyl esters and triglycerides and their incorporation into chylomicron are equivalent when dietary fats are held constant (36). Therefore, given the duration of the current study, the findings reported here should be relevant to other forms of ingested n3-HUFAs.
P-OM3 impact on cell and plasma FAs
Increasing n3-HUFA intake leads to their enrichment in platelet, RBC, and plasma phospholipid pools (26, 37–39), resulting in unique compositional shifts in each compartment (Fig. 1). Decreases in the relative abundance of 20:4n6 and other long-chain n6-HUFAs were expected with the P-OM3 challenge. For instance, reductions in platelet plasmalogen 22:4n6 and phosphatidylcholine 20:4n6 have been linked to its replacement with 20:5n3 (40). In the current study, P-OM3 reductions in platelet 20- and 22-carbon n6-HUFAs were balanced by increasing 20- and 22-carbon n3-HUFAs, consistent with competition between these FAs with similar structures. Similar effects were observed in RBCs and plasma, with the total plasma-HUFA concentrations being increased ∼2-fold. However, RBCs and plasma lipids also showed minor reductions in MUFA and PUFA pools. In a similar study of men on a Mediterranean diet fed 4 g/day n3-HUFA ethyl esters for 2 months, the magnitude of HUFA changes were similar to those observed in the current study; however, changes in MUFA and PUFA pools were not reported (38). These shifts in cellular HUFA composition may alter the generation of bioactive lipids from activated platelets (37) and RBCs (41).
P-OM3 impact on plasma oxylipins
The plasma esterified oxylipin pool reflects a time-integrated snapshot of oxygenated metabolites derived from multiple processes and physiological sources. As with the FAs, oxylipins were enriched in n3 species during the P-OM3 challenge. However, the sum of the oxylipin pools suggests that concentrations of these lipid classes as a whole were not increased despite the 2-fold increase in the sum plasma-HUFAs. Although n3-HUFA feeding has been reported to reduce systemic inflammation (42), levels of F2-isoprostanes, markers of oxidative stress, were not changed after the P-OM3 challenge (Supplementary Table VIII), suggesting little to no difference in oxidative stress in this cohort. The differences in change thresholds of different metabolites depicted in Fig. 3 show that oxylipin and FA profiles are not mirror images. Although the x-intercepts of FA alcohols and their precursor FAs showed similar rank orders (18:3n3 < 20:5n3 ≤ 22:6n3 ≤ 20:4n6 < 18:2n6), those of the other oxylipin profiles were distinct.
Although the sizes of the plasma 18- and 20-carbon oxylipin pools were unchanged, modest reductions in 20:4n6-derived alcohols and substantial increases in n3-HUFA alcohols and epoxides were observed (Table 1). In addition, the average fold change in the sums of these compound classes roughly matched that of their precursor FAs. Reductions in 20:4n6 metabolites are larger than measured n3-HUFA increases. However, the n3 oxylipins are underrepresented in the current assay (e.g., 3 of 7 20:5n3 alcohols and 1 of 9 22:6n3 alcohols). Therefore, the relative enrichment in 20:5n3 and 22:6n3 metabolites may be substantially larger than the 20:4n6 metabolites reductions. Because the membrane pools are of a static size, the n3-HUFA enrichment most likely produces significant reductions in oxylipins generated from other n6-HUFA, including 22:4n6 and 22:5n6. Although the biological effects of the n3 oxylipins are poorly described, these bioactive metabolites have been argued to underlie some of the health benefits associated with n3 FA intake. For instance, the DHA alcohol 17-HDoHE is a potent anti-inflammatory agent, inhibiting 5-lipoxygenase gene expression and TNF-α production in macrophages (43). Similarly, n3 epoxides have reported activity as potent analgesics (34), inhibitors of platelet aggregation (44), and pulmonary smooth muscle relaxants (45). Although the literature pertaining to the production and function of n3 epoxides is limited, the similarity of their effects to the well-described AA-derived epoxides (46–48) suggest that future research focusing on the relative potency of these n3 and n6 metabolites is needed to fully appreciate the potential impact and mechanisms of P-OM3 treatments and the association to inflammation and CVD risk in the population.
Magnitudes of POM-3-induced changes are a function of baseline concentration
Baseline adjustment is a common practice used to reduce variance in collected data where treatment-dependent changes occur on a substantial natural background. However, when the baseline concentration influences response, a static baseline adjustment does not optimally correct the dataset. In the current study, we see that constructing linear regressions for the fold change in concentrations as a function of baseline levels can effectively visualize baseline-dependent effects. Moreover, the presence of a population-wide “change threshold” is suggested.
A dynamic system that ceases to change despite continued input can be considered to have reached a saturation point or dynamic equilibrium (49). The circulating pool of esterified plasma FA and oxylipins can be viewed in this way. This is supported by our observation that an individual with high circulating 20:5n3 at baseline shows a greater resistance to a change in 20:5n3 after P-OM3 treatment than an individual starting with a substantially lower concentration. Similarly, high basal arachidonate concentrations are associated with significantly greater reductions in circulating 20:4n6 and its metabolites with P-OM3 feeding, and subjects with very low 20:4n6 levels actually saw increases in it and its metabolites despite increases in n3 FAs. A recent population-wide study reported that n3- and n6-HUFA levels in the RBCs are in such a homeostatic balance (50). Although the scope of the current study is limited by the discrete nature of the lipid challenge, the cohort size (n = 30) is sufficient to draw conclusions regarding the overall behavior of the sampled population (51). Given the high correlation between baseline concentrations and the magnitudes of change achieved and assuming that shifts in the background diet between samplings do not significantly affect these changes, each individual can be used as an independent gauge of the equilibrium within a population. Therefore, we would argue that regression of the random changes in lipid concentrations within a population can be used to establish the change threshold (i.e., the concentrations about which the population levels oscillate for any given FA despite changes in dietary lipid intake).
Additionally, although n3-HUFA supplementation might be expected to decrease circulating n6-PUFAs, such a finding may be masked by natural variance in the population. Changes in the FA and oxylipin levels can be considered to have two sources of variance: the response to the lipid challenge and a random oscillation about the population's equilibrium point that changes as a function of dietary variance combined with the regression to the mean effect in repeated measures (28). As a result, when the dietary pressure is high relative to the random oscillation, as is the case for the n3-HUFAs in the current study, uniform changes are observed. However, where pressures are low, such changes can be masked. Therefore, the cohort's 20:4n6 status and variability in dietary 20:4n6 are important factors explaining why some individuals showed increases in 20:4n6- and 20:4n6-derived n6-alcohols despite P-OM3 supplementation.
n3 index response to P-OM3 treatment
The n3 index (i.e., the percentage of 20:5n3 + 22:6n3 in RBC membrane FAs) provides a useful tool for quantifying CVD risk. An n3 index of 8–10% is recommended to reduce CVD risk (52). In this study, although the P-OM3 regimen increased the n3 index in all subjects, only four achieved the 8% level by the end of the 4 week study. As with the FAs and FA metabolites, the n3 index showed a P-OM3 dose response that was influenced by the subjects’ basal status. Therefore, n3 index status appears to be an important predictor of therapeutic efficacy. If this relationship is stable across multiple populations, the basal n3 status may provide an empirically rational means to establish and evaluate dosing regimens in the clinic. Using the current dataset, an estimate of this relationship was developed. This assessment yielded two interesting findings. It appears that to elicit a predetermined change in the n3 index, the dose required depends on the initial n3 status (Fig. 4). Additionally, the daily dose for a 70 kg individual to maintain an n3 index of 8 is ∼35 mg/kg/day P-OM3, or 7 mg/kg/day total n3-HUFA ethyl ester. The United States Department of Agriculture has recommended a daily intake of 250 mg of dietary n3-HUFAs for the prevention of heart disease (1), which is equivalent to 3.6 mg/kg/day for a 70 kg individual.
CONCLUSION
We assessed changes in plasma, platelet, and RBC FAs and in bound plasma oxylipins in 30 subjects after a 28 day challenge of 11 ± 2 mg/kg/day n3-HUFA ethyl esters. Both dose and initial n3 status influenced the impact of P-OM3 on circulating esterified lipid pools. The baseline-dependent changes in FA and oxylipin concentrations revealed the presence of change thresholds in affected pools. The resistance of blood esterified FA and oxylipin concentrations to change increased as those concentrations approached the population change threshold. This behavior is also reflected in the n3 index response, suggesting that the basal n3 index may have clinical utility to establish efficacious therapeutic regimens and experimental feeding regimens and to evaluate the USDA Dietary Guidelines. To refine and generalize these findings, future efforts should expand the dose and duration of n3-HUFA challenges in distinct cohorts and compare the results with those reported here. By using crossover study designs, such studies will also provide a means of identifying individuals based on their metabolic responsiveness to various lipids, improving our ability to predict individual responses to n3-HUFA ingestion. This approach is a step toward a new way of thinking about individual responses to dietary lipids.
Supplementary Material
Acknowledgments
The authors thank William S. Harris of Sanford Research Institute for his assistance in the design and execution of the clinical trial and Dr. Bruce D. Hammock of the University of California Davis, for the kind gift of 1-phenylurea-3-hexanoic acid.
Footnotes
Abbreviations:
- CVD
- cardiovascular disease
- HUFA
- highly unsaturated fatty acid
- P-OM3
- prescription omega-3 acid ethyl esters
- RBC
- red blood cell
This study was supported in part by USDA Agricultural Research Service grants 5306-51000-002-00D (J.W.N.) and 5306-51530-019-00D (J.W.N.), National Institute of Food and Agriculture National Needs Fellowship grant 2008-38420-04759 (A.H.K.), and by grant LVZ112860 from GlaxoSmithKline (G.C.S.). All opinions expressed in this manuscript represent the views of the researchers and not necessarily those of the USDA. The USDA is an equal opportunity provider and employer.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of supplemental methods and references, 12 tables, and two figures.
REFERENCES
- 1.McGuire S.2011. US Department of Agriculture and US Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: US Government Printing Office, January 2011. Adv Nutr2: 293–294.
- 2.Brunton S., Collins N. 2007. Differentiating prescription omega-3-acid ethyl esters (P-OM3) from dietary-supplement omega-3 fatty acids. Curr. Med. Res. Opin. 23: 1139–1145 [DOI] [PubMed] [Google Scholar]
- 3.Breslow J. L. 2006. n-3 fatty acids and cardiovascular disease. Am. J. Clin. Nutr. 83: 1477S–1482S [DOI] [PubMed] [Google Scholar]
- 4.Ebrahimi M., Ghayour-Mobarhan M., Rezaiean S., Hoseini M., Parizade S. M., Farhoudi F., Hosseininezhad S. J., Tavallaei S., Vejdani A., Azimi-Nezhad M., et al. 2009. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 64: 321–327 [DOI] [PubMed] [Google Scholar]
- 5.Harris W. S., Miller M., Tighe A. P., Davidson M. H., Schaefer E. J. 2008. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 197: 12–24 [DOI] [PubMed] [Google Scholar]
- 6.Harris W. S., Ginsberg H. N., Arunakul N., Shachter N. S., Windsor S. L., Adams M., Berglund L., Osmundsen K. 1997. Safety and efficacy of Omacor in severe hypertriglyceridemia. J. Cardiovasc. Risk. 4: 385–391 [PubMed] [Google Scholar]
- 7.Davidson M. H., Stein E. A., Bays H. E., Maki K. C., Doyle R. T., Shalwitz R. A., Ballantyne C. M., Ginsberg H. N. 2007. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 29: 1354–1367 [DOI] [PubMed] [Google Scholar]
- 8.Calder P. C. 2006. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 75: 197–202 [DOI] [PubMed] [Google Scholar]
- 9.Lankinen M., Schwab U., Erkkila A., Seppanen-Laakso T., Hannila M. L., Mussalo H., Lehto S., Uusitupa M., Gylling H., Oresic M. 2009. Fatty fish intake decreases lipids related to inflammation and insulin signaling–a lipidomics approach. PLoS ONE. 4: e5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weylandt K. H., Nadolny A., Kahlke L., Kohnke T., Schmocker C., Wang J., Lauwers G. Y., Glickman J. N., Kang J. X. 2008. Reduction of inflammation and chronic tissue damage by omega-3 fatty acids in fat-1 transgenic mice with pancreatitis. Biochim. Biophys. Acta. 1782: 634–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leaf A., Weber P. C. 1988. Cardiovascular effects of n-3 fatty acids. N. Engl. J. Med. 318: 549–557 [DOI] [PubMed] [Google Scholar]
- 12.Larson M. K., Shearer G. C., Ashmore J. H., Anderson-Daniels J. M., Graslie E. L., Tholen J. T., Vogelaar J. L., Korth A. J., Nareddy V., Sprehe M., et al. 2011. Omega-3 fatty acids modulate collagen signaling in human platelets. Prostaglandins Leukot. Essent. Fatty Acids. 84: 93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubnov G., Berry E. M. 2004. Omega-6 fatty acids and coronary artery disease: the pros and cons. Curr. Atheroscler. Rep. 6: 441–446 [DOI] [PubMed] [Google Scholar]
- 14.Harris W. S., Mozaffarian D., Rimm E., Kris-Etherton P., Rudel L. L., Appel L. J., Engler M. M., Engler M. B., Sacks F. 2009. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 119: 902–907 [DOI] [PubMed] [Google Scholar]
- 15.Toledo L., Masgrau L., Marechal J. D., Lluch J. M., Gonzalez-Lafont A. 2010. Insights into the mechanism of binding of arachidonic acid to mammalian 15-lipoxygenases. J. Phys. Chem. B. 114: 7037–7046 [DOI] [PubMed] [Google Scholar]
- 16.Harris W. S. 2008. The omega-3 index as a risk factor for coronary heart disease. Am. J. Clin. Nutr. 87: 1997S–2002S [DOI] [PubMed] [Google Scholar]
- 17.von Schacky C., Harris W. S. 2007. Cardiovascular risk and the omega-3 index. J. Cardiovasc. Med. (Hagerstown). 8(Suppl 1): S46–S49 [DOI] [PubMed] [Google Scholar]
- 18.Burrows T., Collins C. E., Garg M. L. 2011. Omega-3 index, obesity and insulin resistance in children. Int. J. Pediatr. Obes. 6: e532–e539 [DOI] [PubMed] [Google Scholar]
- 19.Baghai T. C., Varallo-Bedarida G., Born C., Hafner S., Schule C., Eser D., Rupprecht R., Bondy B., von Schacky C. 2011. Major depressive disorder is associated with cardiovascular risk factors and low Omega-3 index. J. Clin. Psychiatry. 72: 1242–1247 [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan T. A., Ambrosini G. L., Mori T. A., Beilin L. J., Oddy W. H. 2011. Omega-3 index correlates with healthier food consumption in adolescents and with reduced cardiovascular disease risk factors in adolescent boys. Lipids. 46: 59–67 [DOI] [PubMed] [Google Scholar]
- 21.Harris W. S. 2010. The omega-3 index: clinical utility for therapeutic intervention. Curr. Cardiol. Rep. 12: 503–508 [DOI] [PubMed] [Google Scholar]
- 22.Shearer G. C., Harris W. S., Pedersen T. L., Newman J. W. 2010. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J. Lipid Res. 51: 2074–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson M. K., Ashmore J. H., Harris K. A., Vogelaar J. L., Pottala J. V., Sprehe M., Harris W. S. 2008. Effects of omega-3 acid ethyl esters and aspirin, alone and in combination, on platelet function in healthy subjects. Thromb. Haemost. 100: 634–641 [PubMed] [Google Scholar]
- 24.Guillot N., Caillet E., Laville M., Calzada C., Lagarde M., Vericel E. 2009. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: effects on platelet functions and redox status in healthy men. FASEB J. 23: 2909–2916 [DOI] [PubMed] [Google Scholar]
- 25.Calzada C., Colas R., Guillot N., Guichardant M., Laville M., Vericel E., Lagarde M. 2010. Subgram daily supplementation with docosahexaenoic acid protects low-density lipoproteins from oxidation in healthy men. Atherosclerosis. 208: 467–472 [DOI] [PubMed] [Google Scholar]
- 26.Block R. C., Harris W. S., Reid K. J., Sands S. A., Spertus J. A. 2008. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 197: 821–828 [DOI] [PubMed] [Google Scholar]
- 27.Hochberg Y., Benjamini Y. 1990. More powerful procedures for multiple significance testing. Stat. Med. 9: 811–818 [DOI] [PubMed] [Google Scholar]
- 28.Barnett A. G., van der Pols J. C., Dobson A. J. 2005. Regression to the mean: what it is and how to deal with it. Int. J. Epidemiol. 34: 215–220 [DOI] [PubMed] [Google Scholar]
- 29.Saleem T. 2009. Role of omega-3 fatty acids in improving health. J. Pak. Med. Assoc. 59: 864–865 [PubMed] [Google Scholar]
- 30.Calder P. C., Yaqoob P. 2009. Omega-3 polyunsaturated fatty acids and human health outcomes. Biofactors. 35: 266–272 [DOI] [PubMed] [Google Scholar]
- 31.Deckelbaum R. J., Worgall T. S., Seo T. 2006. n-3 fatty acids and gene expression. Am. J. Clin. Nutr. 83: 1520S–1525S [DOI] [PubMed] [Google Scholar]
- 32.Powell W. S., Gravel S., Gravelle F. 1995. Formation of a 5-oxo metabolite of 5,8,11,14,17-eicosapentaenoic acid and its effects on human neutrophils and eosinophils. J. Lipid Res. 36: 2590–2598 [PubMed] [Google Scholar]
- 33.Serhan C. N. 2005. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr. Opin. Clin. Nutr. Metab. Care. 8: 115–121 [DOI] [PubMed] [Google Scholar]
- 34.Morisseau C., Inceoglu B., Schmelzer K., Tsai H. J., Jinks S. L., Hegedus C. M., Hammock B. D. 2010. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J. Lipid Res. 51: 3481–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.el Boustani S., Colette C., Monnier L., Descomps B., Crastes de Paulet A., Mendy F. 1987. Enteral absorption in man of eicosapentaenoic acid in different chemical forms. Lipids. 22: 711–714 [DOI] [PubMed] [Google Scholar]
- 36.Nordoy A., Barstad L., Connor W. E., Hatcher L. 1991. Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am. J. Clin. Nutr. 53: 1185–1190 [DOI] [PubMed] [Google Scholar]
- 37.Goodnight S. H., Jr, Harris W. S., Connor W. E. 1981. The effects of dietary omega 3 fatty acids on platelet composition and function in man: a prospective, controlled study. Blood. 58: 880–885 [PubMed] [Google Scholar]
- 38.Prisco D., Filippini M., Francalanci I., Paniccia R., Gensini G. F., Abbate K., Neri Serneri G. G. 1996. Effect of n-3 polyunsaturated fatty acid intake on phospholipid fatty acid composition in plasma and erythrocytes. Am. J. Clin. Nutr. 63: 925–932 [DOI] [PubMed] [Google Scholar]
- 39.Cartwright I. J., Pockley A. G., Galloway J. H., Greaves M., Preston F. E. 1985. The effects of dietary omega-3 polyunsaturated fatty acids on erythrocyte membrane phospholipids, erythrocyte deformability and blood viscosity in healthy volunteers. Atherosclerosis. 55: 267–281 [DOI] [PubMed] [Google Scholar]
- 40.Aukema H. M., Holub B. J. 1989. Effect of dietary supplementation with a fish oil concentrate on the alkenylacyl class of ethanolamine phospholipid in human platelets. J. Lipid Res. 30: 59–64 [PubMed] [Google Scholar]
- 41.Jiang H., Anderson G. D., McGiff J. C. 2010. Red blood cells (RBCs), epoxyeicosatrienoic acids (EETs) and adenosine triphosphate (ATP). Pharmacol. Rep. 62: 468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saraswathi V., Gao L., Morrow J. D., Chait A., Niswender K. D., Hasty A. H. 2007. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J. Nutr. 137: 1776–1782 [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Periz A., Planaguma A., Gronert K., Miquel R., Lopez-Parra M., Titos E., Horrillo R., Ferre N., Deulofeu R., Arroyo V., et al. 2006. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 20: 2537–2539 [DOI] [PubMed] [Google Scholar]
- 44.VanRollins M. 1995. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J. Pharmacol. Exp. Ther. 274: 798–804 [PubMed] [Google Scholar]
- 45.Morin C., Sirois M., Echave V., Rizcallah E., Rousseau E. 2009. Relaxing effects of 17(18)-EpETE on arterial and airway smooth muscles in human lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 296: L130–L139 [DOI] [PubMed] [Google Scholar]
- 46.Zou A. P., Fleming J. T., Falck J. R., Jacobs E. R., Gebremedhin D., Harder D. R., Roman R. J. 1996. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am. J. Physiol. 270: F822–F832 [DOI] [PubMed] [Google Scholar]
- 47.Behm D. J., Ogbonna A., Wu C., Burns-Kurtis C. L., Douglas S. A. 2009. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J. Pharmacol. Exp. Ther. 328: 231–239 [DOI] [PubMed] [Google Scholar]
- 48.Xiang L., Naik J. S., Hester R. L. 2008. Functional vasodilation in the rat spinotrapezius muscle: role of nitric oxide, prostanoids and epoxyeicosatrienoic acids. Clin. Exp. Pharmacol. Physiol. 35: 617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkins P. W., De Paula J.2006. Atkins’ physical chemistry. 8th ed. Oxford University Press, Oxford; New York.
- 50.Luxwolda M. F., Kuipers R. S., Smit E. N., Velzing-Aarts F. V., Janneke Dijck-Brouwer D. A., Muskiet F. A. 2011. The relation between the omega-3 index and arachidonic acid is bell shaped: synergistic at low EPA+DHA status and antagonistic at high EPA+DHA status. Prostaglandins Leukot. Essent. Fatty Acids. 85: 171–178 [DOI] [PubMed] [Google Scholar]
- 51.Pearson E. S. 1967. Studies in the history of probability and statistics. XVII. Some reflexions on continuity in the development of mathematical statistics, 1885–1920. Biometrika. 54: 341–355 [PubMed] [Google Scholar]
- 52.Harris W. S., Von Schacky C. 2004. The omega-3 index: a new risk factor for death from coronary heart disease? Prev. Med. 39: 212–220 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.