Abstract
Recombinant expression systems have become powerful tools for understanding the structure and function of proteins, including the apolipoproteins that comprise human HDL. However, human apolipoprotein (apo)A-II has proven difficult to produce by recombinant techniques, likely contributing to our lack of knowledge about its structure, specific biological function, and role in cardiovascular disease. Here we present a novel Escherichia coli-based recombinant expression system that produces highly pure mature human apoA-II at substantial yields. A Mxe GyrA intein containing a chitin binding domain was fused at the C terminus of apoA-II. A 6× histidine-tag was also added at the fusion protein's C terminus. After rapid purification on a chitin column, intein auto-cleavage was induced under reducing conditions, releasing a peptide with only one extra N-terminal Met compared with the sequence of human mature apoA-II. A pass through a nickel chelating column removed any histidine-tagged residual fusion protein, leaving highly pure apoA-II. A variety of electrophoretic, mass spectrometric, and spectrophotometric analyses demonstrated that the recombinant form is comparable in structure to human plasma apoA-II. Similarly, recombinant apoA-II is comparable to the plasma form in its ability to bind and reorganize lipid and promote cholesterol efflux from macrophages via the ATP binding cassette transporter A1. This system is ideal for producing large quantities of recombinant wild-type or mutant apoA-II for structural or functional studies.
Keywords: intein, fusion protein, affinity tag, bacteria, inexpensive, low temperature cleavage, high density lipoprotein
Numerous human and animal studies have established an inverse correlation between HDL plasma levels and incidence of cardiovascular disease (CVD) (1–3). Apolipoprotein (apo)A-I, the main protein component of HDL, plays a direct role in HDL's cardioprotective effects (4–6). However, much less is known about apoA-II, the second most abundant protein in HDL (≈20% in mass). Synthesized primarily in the liver, apoA-II is a 77 amino acid protein with a single cysteine at position 6. In healthy humans, apoA-II exists almost entirely as a disulfide-linked homodimer (molecular mass, 17.4 kDa), though it can be occasionally found as a heterodimer with other Cys-containing proteins (7). It is primarily found in mature, spherical HDL particles with a small fraction associated with VLDLs and chylomicrons (8). ApoA-II has been associated with numerous aspects of HDL metabolism (9–15), but, despite many years of study, its overall physiological function is still enigmatic (16).
The impact of apoA-II on cardiovascular disease also remains unclear. In humans, recent case-control studies have pointed to an inverse correlation between plasma apoA-II levels and CVD (17–19). However, the only known case of human apoA-II deficiency has shown no evidence of CVD (20, 21). In mice, overexpression of murine apoA-II consistently increased aortic fatty streak lesions (22–24). In this animal model, HDL particles with high apoA-II content have reduced binding and selective lipid uptake by the class B scavenger receptors (15, 25) and are poor activators of hepatic lipase (11, 14, 26). Furthermore, they are proinflammatory and inefficient antioxidants relative to those containing primarily apoA-I (14, 23). The combination of these properties may partly explain the proatherogenic phenotype produced by apoA-II overexpression. This effect may be mediated directly or, as suggested by recent studies by Julve et al. (27), apoA-II may alter the proteome of discrete HDL particle subfractions to indirectly modulate factors such as lipoprotein lipase.
Overexpression of human apoA-II in mice yielded different, and in many cases opposite, results in terms of plasma lipoprotein profile, cholesterol efflux efficiency, and atherosclerosis development (reviewed in References 16, 28–30). The applicability of these studies to human disease is significantly complicated by the fundamentally different lipid metabolism of rodents versus humans and by major differences in regulation and structure of their apoA-II.
Another factor that has impeded our understanding of human apoA-II structure and function is the lack of an efficient recombinant apoA-II expression system capable of producing large quantities of pure wild-type and variant proteins. Such a tool would greatly facilitate the in vitro study of human apoA-II structure and its interaction with human enzymes, lipoproteins, and cell lines. Similarly, recombinant murine apoA-II variants could be useful for in vivo infusion studies in mice. An Escherichia coli expression system that used a glutathione-S-transferase fusion protein and thrombin cleavage to liberate the final apoA-II peptide was reported in the 1990s; however, its low efficiency necessitated large-scale fermentation for reasonable protein yields (31). Here, we report an E. coli expression system capable of producing highly pure human apoA-II in standard research lab-scale culture conditions. This system features a self-cleaving intein-apoA-II fusion protein and redundant affinity purification tags yielding mature human apoA-II. This recombinant apoA-II is shown to be structurally and functionally comparable to human plasma isolated apoA-II.
MATERIALS AND METHODS
Isopropyl β-D-1-thiogalactopyranoside (IPTG) was obtained from Fisher. BL21(DE3) competent E. coli cells were purchased from Agilent Technologies. Dimyristoyl-phosphatidylcholine (DMPC) was obtained from Avanti Polar Lipids. Chitin resin and His binding resin were purchased from New England Biolabs and Novagen, respectively. The RAW264.7 macrophages used for the cholesterol efflux assays were purchased from the American Type Culture Collection and were maintained in DMEM supplemented with 10% FBS and 10 μg/ml gentamicin (all from Invitrogen). cAMP and gentamicin were purchased from Sigma. [1,2-3H(N)]cholesterol (3H-cholesterol) was obtained from Amersham Biosciences. All other reagents were of the highest quality available. Human plasma apoA-II was purified as previously described (32).
Circular dichroism measurements were made on a Jasco J-715 spectropolarimeter. The fluorescence measurements were performed on a Photon Technology International Quantamaster spectrometer. A 1900CA Packard liquid scintillation analyzer was used to measure the radioactive cholesterol counts associated with the cholesterol efflux assay. All absorbance measurements were made on an Amersham Biosciences Ultraspec 4000 UV/visible spectrophotometer.
Expression of recombinant human apoA-II
The apo-AII expression plasmid (Fig. 1) was transformed into BL21(DE3) host cells. Cells from an individual clone were grown in 2× YTA medium (16 g of tryptone, 10 g of yeast extract, 5 g of NaCl per liter) at 37°C with shaking. When the OD600 was about 0.6, protein expression was induced by the addition of IPTG at a final concentration of 0.5 mM. After induction, cell cultures were incubated for 3 h at 37°C with shaking. Typical expression volumes were 500 ml per 1 liter flask. Cells were pelleted and stored at −80°C until protein purification.
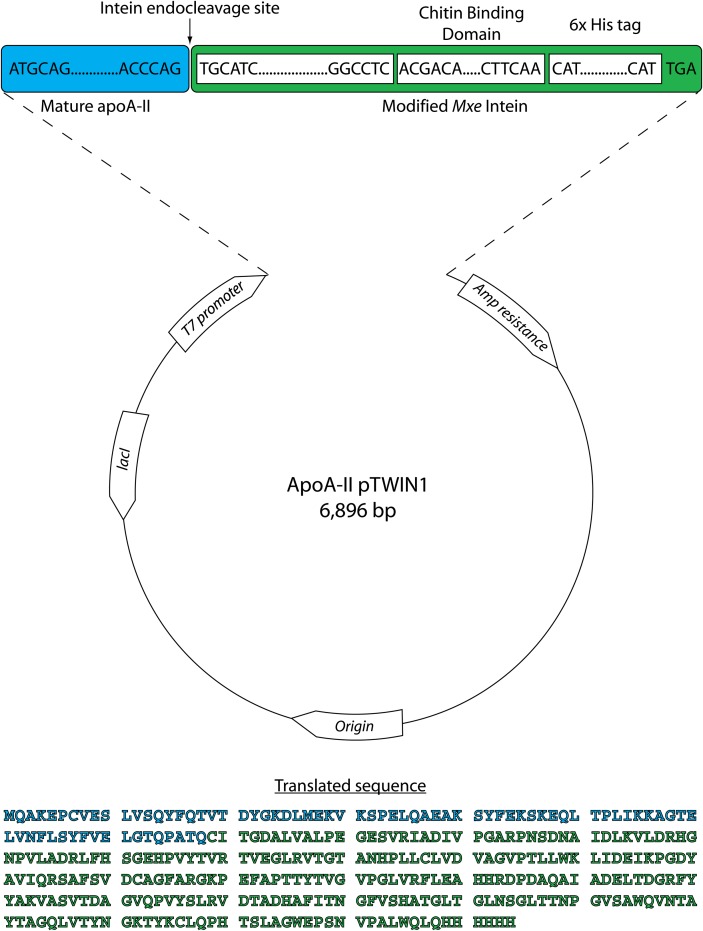
Fig. 1.
pTWIN1 vector map showing the design of the apoA-II expression construct. Mature human apoA-II (blue) was subcloned into the pTWIN1 expression vector upstream of the T7 promoter with Mxe intein, a chitin binding domain, and a 6× histidine tag on its C terminus (green). To initiate transcription, a ATG codon was added to the N-terminus of the protein sequence. The site of intein-mediated endo-cleavage is indicated by a black arrow. The amino acid sequence of the translated apoA-II-intein fusion protein is reported: mature apoA-II (blue), modified Mxe intein (green).
Purification of recombinant human apoA-II
The cell pellets were suspended in chitin binding buffer (B2: 20 mM Na-HEPES [pH 8.5], 500 mM NaCl, 1 mM EDTA) and subjected to three 1 min cycles of probe sonication (Fisher model 550 sonic dismembrator) at maximum output on ice. Soluble and insoluble fractions were separated by centrifugation at 10,000 g for 30 min at 4°C. A chitin bead column (1 × 25 cm) was packed with chitin beads for a total bead volume of about 15 ml and prewashed with buffer B2. Thirty milliliters of the lysed cell supernatant was passed through the column by gravity, followed by a wash with 5 column volumes of buffer B2. On-column cleavage of the chitin-bound protein was initiated by exchanging the column buffer with buffer B3 (20 mM Na-HEPES [pH 8.5], 500 mM NaCl, 40 mM DTT, 1 mM EDTA). After overnight incubation at 4°C, the liquid phase was eluted from the column and dialyzed against 1× His binding buffer (20 mM Tris-HCl [pH 7.9], 5 mM imidazole, 500 mM NaCl). The final volume was typically 15 ml. To remove residual uncleaved fusion protein and free intein-CBD fragments (which are his-tagged), the column eluate was passed through a nickel chelating column. The flow-through fraction containing purified apoA-II was dialyzed against stardard tris buffer (STB) (10 mM Tris [pH 8.2], 150 mM NaCl, 1 mM EDTA, 0.2% NaN3) for further use.
Mass spectrometry analysis
Protein mass detection was carried out on an Applied Biosystems-Sciex QStar® XL mass spectrometer equipped with an ion spray source and a quadrapole time-of-flight dual analyzer. Intact proteins were separated on a capillary HPLC system (Agilent 1100) connected in-line with the mass spectrometer to remove any contaminants in the samples before the mass detection. A capillary C18 column (0.5 mm i.d. × 5 cm length, 300 Å particle size) (Grace Deerfield, IL) was used in HPLC separations with a solvent gradient of acetonitrile from 2% to 60% at a flow rate of 8 µl/min over 40 min. The gradient was generated using a solvent A (0.1% formic acid and 2% acetonitrile in water) and a solvent B (0.075% formic acid and 2% water in acetonitrile). The protein samples were solubilized in 0.1% TFA, and 30 pmol were injected in a 1 µl volume. Protein mass detection was determined using the Baysian protein reconstruct tool in the BioAnalyst suite of the Analyst QS software (Applied Biosystems).
Circular dichroism analysis
Proteins were freshly dialyzed against 20 mM phosphate buffer (pH 7.4), and relative concentration was determined by the Markwell-Lowry method (33). Proteins were diluted to 150 μg/ml, and spectra were collected in a 1 mm cell as an average of three accumulations. The scans were from 250 to 190 nm at 50 nm/min with a 0.5 nm step size and 0.5 s response. The bandwidth was set to 1 mm, and slit width was 500 μm. To confirm the accuracy of dilution, protein concentration was verified by A280, and mean residual ellipticity was calculated based on this value as described by Woody (34) using 113.0 as the mean residual weight for apoA-II. Fractional helical content was calculated using the formula of Chen et al. (35) and the mean residual ellipticity at 222 nm. Each experiment was repeated on two independent protein preparations. The guanidine-HCl (Gnd-HCl) denaturation experiments were performed as in (36). Midpoints of denaturation (D1/2) were calculated by fitting the experimental data points with sigmoidal curves generated by a four-parameter logistic equation. Native and fully denatured state ellipticities were measured at 0 and 6 M Gdn-HCl, respectively (37).
Liposome clearance assay
A turbid solution of DMPC multilamellar liposomes was generated on the day of the assay by combining N2-dried lipid with STB at 5 mg/ml using brief probe sonication. The liposomes and apolipoproteins, also in STB, were quickly mixed, yielding a final DMPC to apoA-II mass ratio of 2.5:1, with final apoA-II concentration of 0.17 mg/ml. A decrease in solution turbidity over time was monitored as a reduction in absorbance at 325 nm with measurements every 30 s for 10 min. The solution was maintained at 24.5°C, the gel/liquid crystalline transition temperature for DMPC, throughout the experiment with a thermostated water circulator. The data are expressed as a normalized optical density (OD) calculated by dividing the sample OD by the initial OD (OD0). Two trials were performed on different days with independent preparations of protein.
Cholesterol efflux assay
RAW264.7 cells were grown to 70% confluency, washed twice with DMEM with 0.22% BSA (minimal media), and labeled with DMEM, 0.22% BSA, and 1.0 μCi/ml 3H-cholesterol with or without 0.3 mM cAMP for 18 h. After labeling, cells were washed twice with minimal media and incubated with efflux media (DMEM, 0.22% BSA, with or without 0.3 mM cAMP, and 1–10 μg/ml apolipoprotein) for 6 h. At the start of the experiment, a control plate of labeled cells was washed with 3× PBS and incubated overnight in isopropanol to extract cellular lipids to determine the total radioactivity in the cells at T = 0 h. The isopropanol extract was dried under air, resolubilized in toluene, and counted. At the end of the 6 h incubation, a sample from each experimental well was filtered and counted. Each apolipoprotein was tested in triplicate wells, and the calculated percent efflux was the average of these three wells. The reported percent efflux is the media counts at the end of the 6 h incubation divided by the total internalized counts per well calculated using the control plate, with background efflux to STB subtracted.
RESULTS
Summary of previous attempts
Our laboratory has developed bacterial expression protocols for numerous apolipoproteins using the PET system under control of the T7 promoter (38). Our initial attempts at apoA-II expression involved the simple insertion of the apoA-II cDNA into our previous apoA-I construct (38). Although we were able to detect expressed apoA-II in this system, the yield was unacceptably low. We then tested various combinations that included the addition of the apoA-I and apoA-II pro-segments, fusions with apoA-I, thioredoxin or glutathione S-transferase, and alternate expression systems using other promoters or host cell systems. Overall, these strategies met with little success, producing poor yields or no protein at all. However, attempts to express apoA-II in the pTWIN1 vector as a fusion with intein and the chitin binding domain (Fig. 1) offered acceptable protein yields for the first time. Furthermore, intein-mediated auto-cleavage allowed for efficient release of the target human mature apoA-II from the fusion construct. Finally, the chitin binding domain (CBD), as well as our added C-terminal histidine-tag (His-tag), allowed for a simple two-step purification strategy that yielded highly pure protein.
Expression of human apoA-II
The final expression construct is shown in Fig. 1. pTWIN1 is an E. coli expression vector derived from pBR322 with a polylinker that allows the in-frame insertion of a target gene between two intein fusion proteins and a CBD. In our case, we elected to clone apoA-II upstream from the Mxe GyrA intein cassette, placing the intein and CBD at the apolipoprotein's C terminus. For added flexibility in purification, we added a 6× His-tag C-terminal to the CBD. Fig. 2A shows that activation of the T7 promoter by addition of IPTG stimulated expression of a ∼40 kDa protein (lane 3) that was consistent with the anticipated mass of 37 kDa for the fusion protein (27 kDa for the intein/CBD [manufacturer's documentation, New England Biolabs], 9 kDa for apoA-II, and 1 kDa for the His-tag). This band reacted with a polyclonal antibody for human apoA-II as determined by Western blot (not shown).
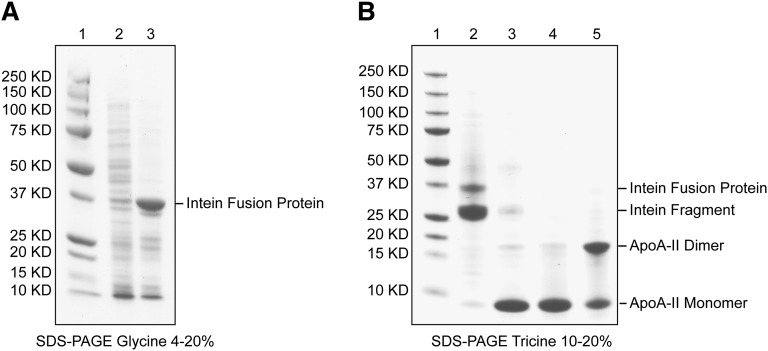
Fig. 2.
Purification summary. A: SDS-glycine PAGE gel (4–20%) stained with Coomassie blue. Lane 1: Precision Plus protein standards from Bio-Rad with indicated molecular mass; lane 2: uninduced bacterial lysate; lane 3: IPTG-induced bacterial lysate. Amplified band of intein fusion protein is marked. B: SDS-tricine PAGE gel (10–20%) stained with Coomassie blue. Lane 1: Precision Plus protein standards from Bio-Rad; lane 2: chitin beads after activation of intein auto-cleavage reaction and column elution; lane 3: eluate from the chitin column after activation of intein auto-cleavage reaction; lanes 4 and 5: flow through fraction from a Ni-chelating affinity column in which residual fusion protein has been removed; reducing and nonreducing conditions, respectively. The final purity of recombinant apoA-II is >95% by combined SDS-PAGE and mass spectrometric analyses.
The apoA-II-intein/CBD fusion protein was purified on a chitin column and auto-cleavage was induced on-column by addition of a thiol-reagent (DTT, 40 mM). After overnight incubation at 4°C, the liquid phase was eluted from the column and analyzed on a reducing SDS-PAGE gel. Fig. 2B, lane 3 shows a prominent band at about 9 kDa corresponding to mature, monomeric apoA-II (8708 Da) and a faint ∼30 kDa band indicating leakage from the column of the residual intein/CBD fragment after cleavage of the fusion protein (Fig. 2B, lane 2). Further purification of the eluate from the chitin column on a His-binding resin guaranteed complete removal of the cleaved fusion protein fragment, leaving highly pure (>95% by SDS-PAGE and mass spectrometric analyses) apoA-II (Fig. 2B, lanes 4 and 5). Overall, the yield of purified apoA-II was approximately 12 mg per liter of bacterial culture.
Mass spectrometry
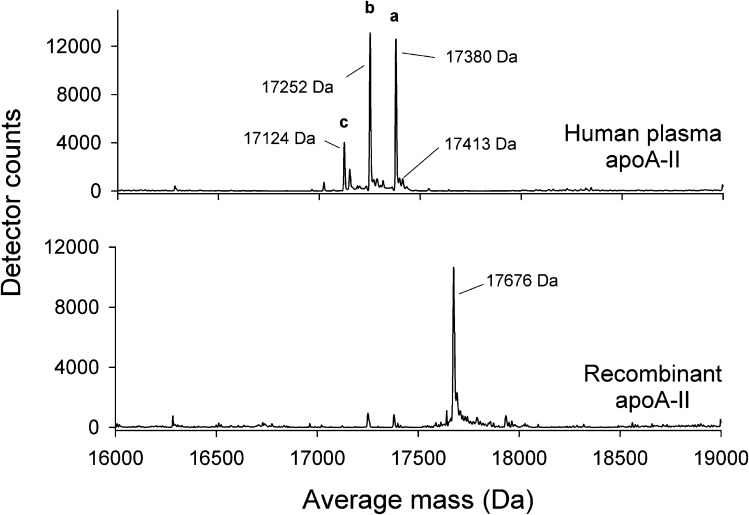
To verify the sequence of the expressed protein, we performed electrospray mass spectrometry on the recombinant protein and compared it with apoA-II purified from human plasma. Fig. 3 (top) shows the mass spectrum of human plasma apoA-II eluted from a capillary reverse phase HPLC column at 18 min (see Experimental Procedures). Three prominent mass peaks were apparent: Peak A: 17,380; Peak B: 17,252; and Peak C: 17,124 Da. Human plasma apoA-II exists in solution primarily as a homodimer due to a disulfide linkage mediated through Cys 6. The expected molecular mass is 17,414 Da. Although a very small peak near this value could be found by zooming in on this region in the mass spectrum, the most prominent peak (Peak A) exhibited a mass that was 33 Da lower. Peaks B and C were 128 and 256 Da, respectively, lower in mass than Peak A. This pattern was remarkably reproducible between independent plasma apoA-II isolations from our laboratory as well as others.
Fig. 3.
Top-down mass spectrometry analysis of human plasma purified and recombinant apoA-II. Protein samples were injected into a HPLC system equipped with a C18 column. The column flow-through was electrosprayed into a QStar XL mass spectrometer. Mass spectra at 18 min retention time are shown. Top: Human plasma apoA-II showing three major peaks. Bottom: Recombinant apoA-II showing a single major peak. Observed average masses resulting from the proteins are reported in Da. The mass accuracy of the instrumentation in this molecular mass range is ±2 Da.
By contrast, the mass spectrum of recombinant apoA-II at the same HPLC retention time (18 min) exhibited a single major peak at 17,676 Da. Our recombinant form contains an additional Met residue at the N-terminus (required to initiate translation), followed by the Gln normally at position 1 in mature plasma apoA-II after cleavage of its pre and pro sequence. With two extra Met residues per protein dimer, the predicted molecular mass of the recombinant form is 17,676 Da, in excellent agreement with the experimental value. This indicates that our recombinant apoA-II is of the correct sequence and is highly pure. In repeated SDS-PAGE analyses, we saw no evidence of monomeric apoA-II under the conditions of the experiment, indicating nearly 100% dimerization.
A comparison of the two mass spectra in Fig. 3 allowed for speculation on the complexity of the plasma apoA-II spectrum. When glutamine is present at the N-terminus, as in human plasma apoA-II, it can undergo cyclization to pyroglutamate with the release of NH3 under acidic conditions (−17 Da) (39). Dimeric apoA-II, containing two N-terminal Gln residues, would then be expected to lose 34 Da if both residues cyclize, explaining the near absence of the expected 17,414 Da mass for dimeric plasma apoA-II and the prevalence of Peak A (17,380 Da). Early attempts to sequence apoA-II by Edman degradation were unsuccessful, but treatment with pyrrolidonecarboxyl peptidase revealed the presence of an N-terminal pyroglutamic acid, which impedes the sequencing reaction (40). This is supported by the fact that recombinant apoA-II was measured at its expected mass; the presence of the N-terminal Met precludes cyclization of the Gln at position 2. The explanations for Peaks B and C in plasma apoA-II are less straightforward but may involve the loss of the C-terminal Gln residues from each apoA-II strand (∼128 Da each), likely as a result of nonspecific exoproteases acting either in vivo or during the purification.
Circular dichroism
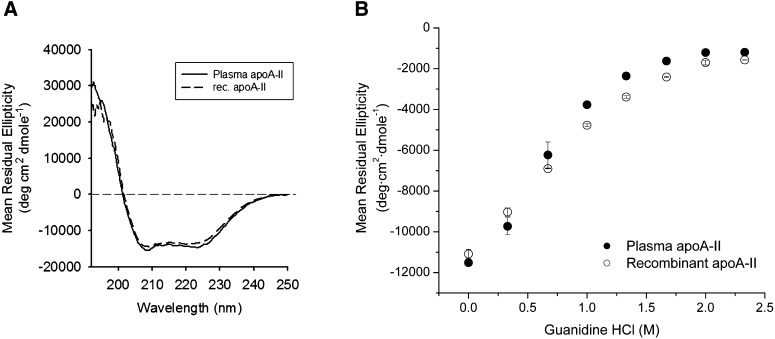
To determine if the primary structure differences between human plasma and recombinant apoA-II noted above affected their secondary structures, we characterized them by far UV circular dichroism in the lipid-free state. Plasma apoA-II exhibited a characteristic spectrum for a helical protein with minima at 208 and 222 nm (Fig. 4A). The average fractional helicity was calculated at about 40% in agreement with previous measurements (41). Recombinant apoA-II exhibited a highly similar spectrum with a fractional helicity of about 38%. The denaturant-induced unfolding curves of plasma and recombinant apoA-II are shown in Fig. 4B. Midpoints of denaturation (D1/2) for the two proteins (0.74 ± 0.05 M and 0.88 ± 0.03 M, respectively) were not significantly different. These data indicate that the overall secondary structural content and stability of the two proteins are quite similar despite the different the N-terminal amino acid.
Fig. 4.
Far UV circular dichroism spectra and Gnd-HCl denaturation. A: Far UV CD spectra of human plasma (solid) and recombinant (dashed) apoA-II. Samples were run at room temperature in triplicate for three accumulations each and reported as the overall mean. B: Isothermal Gnd-HCl denaturation of lipid-free human plasma (closed circles) and recombinant (open circles) apoA-II. Samples at 100 µg/ml were incubated at 4°C with the indicated concentration of Gnd-HCl for 72 h to ensure equilibrium. Average values and error bars from at least two separate experiments are reported.
Functional characterizations of recombinant apoA-II
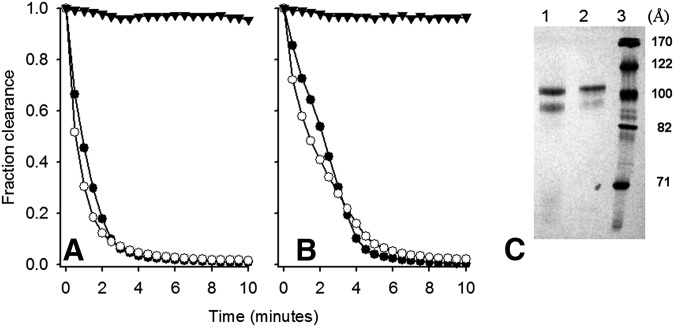
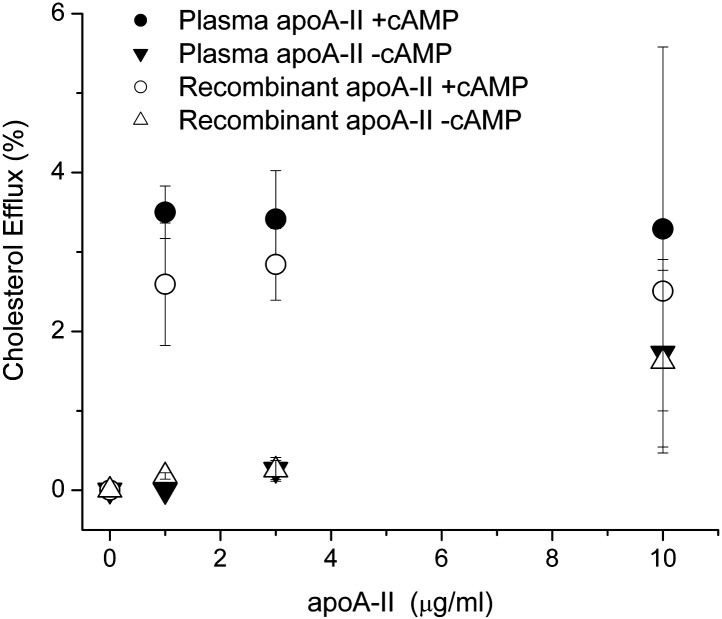
We performed DMPC clearance assays, which determine the ability of a given protein to bind and reorganize multilamellar vesicles. Fig. 5 shows that DMPC liposomes incubated at 24.5°C in the absence of protein exhibited a high degree of light scattering due to the persistence of large multilamellar vesicles. Upon addition of human plasma apoA-II, the liposomes were rapidly cleared within 2–4 min. Under these conditions, apoA-II cleared lipid faster than apoA-I and apoA-IV (38) consistent with its well-known high hydrophobicity (42). Recombinant apoA-II was similarly efficient and even slightly more so across individual experiments, indicating that the sequence differences had little impact on the ability to bind and rearrange lipid bilayers. No significant differences between human plasma and recombinant apoA-II liposome clearance kinetics were observed for different DMPC:protein ratios. After lipid clearance, the reaction mixtures were analyzed by nondenaturing gradient gel electrophoresis. Fig. 5c shows that both plasma and recombinant apoA-II generated two populations of lipoprotein particles, a major one at around 105 Å and a minor one at 96 Å, indicating that both forms can reorganize DMPC into similarly sized lipoproteins. We next assessed the ability of the two forms of apoA-II to promote cholesterol efflux via the ATP-binding cassette transporter A1 (ABCA1) cell membrane transporter. We incubated the apoA-II proteins with RAW macrophages that had been stimulated to produce ABCA1 with exogenous cAMP (43). In the absence of cAMP-stimulated ABCA1 expression, both recombinant and plasma apoA-II promoted little cholesterol release in the cell culture medium in 6 h (Fig. 6). Upon addition of cAMP, both proteins promoted robust cholesterol efflux with no statistically significant differences between the two protein forms at all protein concentrations tested. We found no evidence that recombinant apoA-II produced in our system is structurally or functionally different from plasma purified apoA-II, despite the differences in sequence at the N-terminus.
Fig. 5.
Ability of human plasma and recombinant apoA-II to bind and reorganize DMPC liposomes. A and B show two separate experiments with independent preparations of protein (n = 1 each). All samples were run at 24.5°C, the transition temperature of DMPC, for 10 min with readings every 30 s. Closed triangles: DMPC liposomes only; closed circles: human plasma apoA-II; open circles: recombinant apoA-II. Panel C shows a 8–25% native polyacrylamide gel analysis of the end products of the reaction. Lane 1: Human plasma apoA-II; lane 2: recombinant apoA-II; lane 3: Amersham HMW molecular markers with hydrodynamic diameters expressed in Å. The gel was stained with Coomassie blue.
Fig. 6.
Ability of human plasma and recombinant apoA-II to promote cellular cholesterol efflux via ABCA1. The apoA-II samples were used as cholesterol acceptors from cholesterol-labeled RAW264.7 macrophages. The proteins were added to the media at concentrations in the 1–10 μg/ml range for 6 h. Percent efflux was calculated by dividing the efflux media counts by the total counts (n = 3; error bars represent 1 SD). Closed and open circles: Human plasma and recombinant apoA-II in the presence of cAMP, respectively. Closed and open triangles: Human plasma and recombinant apoA-II without cAMP, respectively.
DISCUSSION
The secretion of apoA-II from mammalian cells appears to be complex. Initially translated as a prepro form, the presegment is removed intracellularly, followed by possible O-linked glycosylation containing two or four sialic acid residues (44). Upon cellular release, the pro-segment is quickly cleaved, and the protein is desialyated concomitant with cyclization of the N-terminal Gln (likely by an enzymatic process [39]), producing the mature plasma form. Our mass spectrometry work suggests that additional clipping of the C-terminal Gln residues may also occur along this pathway. The reason for such a complicated secretion process is not clear but may be related to the efficient dimerization of the protein. Although Remaley et al. (45) have previously reported that O-linked glycosylation modifies the lipid binding properties of apoA-II, further studies are needed to understand what, if any, significance these posttranslational modifications have on apoA-II functionality or distribution in HDL particles. Despite this complexity during secretion, our mass spectrometric data on isolated plasma apoA-II provide no evidence that the circulating form is glycosylated or contains other mass additions, indicating that it is a reasonable candidate for bacterial expression.
One recombinant expression system for human apoA-II has been developed previously in bacteria (31). Unfortunately, this protocol had several disadvantages. First, as far as we can determine, this construct is no longer available for use. Second, cleavage from the glutathione-S-transferase fusion protein required a long incubation with thrombin, which adds laborious steps and cost to the purification. Third, the expression conditions required careful monitoring to prevent spontaneous degradation of the product after expression. Fourth, the produced apoA-II had three nonnative residues on its N-terminus. Finally, the expression yield was relatively low, requiring large-scale fermentation techniques to produce reasonable amounts of protein. Despite these issues, the expressed apoA-II exhibited a similar structure and ability to generate HDL-like particles as the human plasma form (31). By contrast, the system described here is capable of producing milligram quantities of highly pure apoA-II that contains only an N-terminal Met residue. The purification protocol is rapid, does not depend on expensive and temperamental proteases, and features two redundant affinity chromatography options that result in highly pure protein. As demonstrated by circular dichroism spectroscopy, the secondary structure and stability of this recombinant apoA-II are similar to plasma isolated human apoA-II, as is its ability to reorganize phospholipid bilayers and promote ABCA1-mediated cholesterol efflux.
Although the price of chemically synthesized peptides is dropping consistently, our recombinant system is still highly cost effective because labor is the only expensive variable. Currently, the cost of 10 mg of recombinant protein can be estimated at about 200 US$. Thus, such a recombinant system is ideal for use in structural or functional studies requiring large quantities of apoA-II that can be mutated as needed. Potential applications include studies of the lipid-bound structure of apoA-II; the basis of its potential interactions with other HDL proteins, such as apoA-I; and exploration of putative functions, such as modulation of triglyceride lipase activity (27, 46).
Acknowledgments
The authors thank Drs. Vasanthy Narayanaswami, Robert O. Ryan, and Henry Pownall for providing reagents and Drs. Paul Hauser and Ayako Kamei for useful discussions and technical assistance.
Footnotes
Abbreviations:
- CBD
- chitin binding domain
- CD
- circular dichroism
- CVD
- cardiovascular disease
- DMPC
- dimyristoyl phospatidylcholine
- His-tag
- histidine-tag
- IPTG
- isopropyl β-D-1-thiogalactopyranoside
- OD
- optical density
- STB
- standard tris buffer
This work was supported by New Investigator Award no. 8KT-0021 from the Tobacco-Related Disease Research Program of California and grant HL113059 from the National Heart Lung and Blood Institute (NHLBI) (G.C.); by grants HL62542, HL67093, and HL082734 from NHLBI (W.S.D.); and by K99/R00 grant HL087561 from NHLBI (R.A.G.D.S.).
REFERENCES
- 1.Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62: 707–714 [DOI] [PubMed] [Google Scholar]
- 2.Assmann G., Schulte H., von Eckardstein A., Huang Y. 1996. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 124(Suppl): S11–S20 [DOI] [PubMed] [Google Scholar]
- 3.Rader D. J. 2006. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 116: 3090–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paszty C., Maeda N., Verstuyft J., Rubin E. M. 1994. Apolipoprotein AI transgene corrects apolipoprotein E deficiency-induced atherosclerosis in mice. J. Clin. Invest. 94: 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plump A. S., Scott C. J., Breslow J. L. 1994. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc. Natl. Acad. Sci. USA. 91: 9607–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawashiri M. A., Zhang Y., Pure E., Rader D. J. 2002. Combined effects of cholesterol reduction and apolipoprotein A-I expression on atherosclerosis in LDL receptor deficient mice. Atherosclerosis. 165: 15–22 [DOI] [PubMed] [Google Scholar]
- 7.Gillard B. K., Lin H. Y., Massey J. B., Pownall H. J. 2009. Apolipoproteins A-I, A-II and E are independently distributed among intracellular and newly secreted HDL of human hepatoma cells. Biochim. Biophys. Acta. 1791: 1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alaupovic P., Knight-Gibson C., Wang C. S., Downs D., Koren E., Brewer H. B., Jr, Gregg R. E. 1991. Isolation and characterization of an apoA-II-containing lipoprotein (LP-A-II:B complex) from plasma very low density lipoproteins of patients with Tangier disease and type V hyperlipoproteinemia. J. Lipid Res. 32: 9–19 [PubMed] [Google Scholar]
- 9.Labeur C., Lambert G., Van Cauteren T., Duverger N., Vanloo B., Chambaz J., Vandekerckhove J., Castro G., Rosseneu M. 1998. Displacement of apo A-I from HDL by apo A-II or its C-terminal helix promotes the formation of pre-beta1 migrating particles and decreases LCAT activation. Atherosclerosis. 139: 351–362 [DOI] [PubMed] [Google Scholar]
- 10.Durbin D. M., Jonas A. 1999. Lipid-free apolipoproteins A-I and A-II promote remodeling of reconstituted high density lipoproteins and alter their reactivity with lecithin:cholesterol acyltransferase. J. Lipid Res. 40: 2293–2302 [PubMed] [Google Scholar]
- 11.Zhong S., Goldberg I. J., Bruce C., Rubin E., Breslow J. L., Tall A. 1994. Human ApoA-II inhibits the hydrolysis of HDL triglyceride and the decrease of HDL size induced by hypertriglyceridemia and cholesteryl ester transfer protein in transgenic mice. J. Clin. Invest. 94: 2457–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brousseau M. E., Millar J. S., Diffenderfer M. R., Nartsupha C., Asztalos B. F., Wolfe M. L., Mancuso J. P., Digenio A. G., Rader D. J., Schaefer E. J. 2009. Effects of cholesteryl ester transfer protein inhibition on apolipoprotein A-II-containing HDL subspecies and apolipoprotein A-II metabolism. J. Lipid Res. 50: 1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pussinen P. J., Jauhiainen M., Ehnholm C. 1997. ApoA-II/apoA-I molar ratio in the HDL particle influences phospholipid transfer protein-mediated HDL interconversion. J. Lipid Res. 38: 12–21 [PubMed] [Google Scholar]
- 14.Hedrick C. C., Castellani L. W., Wong H., Lusis A. J. 2001. In vivo interactions of apoA-II, apoA-I, and hepatic lipase contributing to HDL structure and antiatherogenic functions. J. Lipid Res. 42: 563–570 [PubMed] [Google Scholar]
- 15.de Beer M. C., Durbin D. M., Cai L., Mirocha N., Jonas A., Webb N. R., de Beer F. C., van Der Westhuyzen D. R. 2001. Apolipoprotein A-II modulates the binding and selective lipid uptake of reconstituted high density lipoprotein by scavenger receptor BI. J. Biol. Chem. 276: 15832–15839 [DOI] [PubMed] [Google Scholar]
- 16.Blanco-Vaca F., Escola-Gil J. C., Martin-Campos J. M., Julve J. 2001. Role of apoA-II in lipid metabolism and atherosclerosis: advances in the study of an enigmatic protein. J. Lipid Res. 42: 1727–1739 [PubMed] [Google Scholar]
- 17.Birjmohun R. S., Dallinga-Thie G. M., Kuivenhoven J. A., Stroes E. S., Otvos J. D., Wareham N. J., Luben R., Kastelein J. J., Khaw K. T., Boekholdt S. M. 2007. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation. 116: 2029–2035 [DOI] [PubMed] [Google Scholar]
- 18.Winkler K., Hoffmann M. M., Seelhorst U., Wellnitz B., Boehm B. O., Winkelmann B. R., Marz W., Scharnagl H. 2008. Apolipoprotein A-II Is a negative risk indicator for cardiovascular and total mortality: findings from the Ludwigshafen Risk and Cardiovascular Health Study. Clin. Chem. 54: 1405–1406 [DOI] [PubMed] [Google Scholar]
- 19.Buring J. E., O'Connor G. T., Goldhaber S. Z., Rosner B., Herbert P. N., Blum C. B., Breslow J. L., Hennekens C. H. 1992. Decreased HDL2 and HDL3 cholesterol, Apo A-I and Apo A-II, and increased risk of myocardial infarction. Circulation. 85: 22–29 [DOI] [PubMed] [Google Scholar]
- 20.Deeb S. S., Takata K., Peng R. L., Kajiyama G., Albers J. J. 1990. A splice-junction mutation responsible for familial apolipoprotein A-II deficiency. Am. J. Hum. Genet. 46: 822–827 [PMC free article] [PubMed] [Google Scholar]
- 21.Shadrina M. I., Slominskii P. A., Perova N. V., Limborskaia S. A. 1997. The apolipoprotein AII gene is not a risk factor for development ischemic heart disease in the Moscow population. Genetika. 33: 1316–1318 [PubMed] [Google Scholar]
- 22.Warden C. H., Hedrick C. C., Qiao J. H., Castellani L. W., Lusis A. J. 1993. Atherosclerosis in transgenic mice overexpressing apolipoprotein A-II. Science. 261: 469–472 [DOI] [PubMed] [Google Scholar]
- 23.Castellani L. W., Navab M., Van Lenten B. J., Hedrick C. C., Hama S. Y., Goto A. M., Fogelman A. M., Lusis A. J. 1997. Overexpression of apolipoprotein AII in transgenic mice converts high density lipoproteins to proinflammatory particles. J. Clin. Invest. 100: 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng W., Breslow J. L. 1996. Dramatically decreased high density lipoprotein cholesterol, increased remnant clearance, and insulin hypersensitivity in apolipoprotein A-II knockout mice suggest a complex role for apolipoprotein A-II in atherosclerosis susceptibility. Proc. Natl. Acad. Sci. USA. 93: 14788–14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Beer M. C., Castellani L. W., Cai L., Stromberg A. J., de Beer F. C., van der Westhuyzen D. R. 2004. ApoA-II modulates the association of HDL with class B scavenger receptors SR-BI and CD36. J. Lipid Res. 45: 706–715 [DOI] [PubMed] [Google Scholar]
- 26.Boucher J., Ramsamy T. A., Braschi S., Sahoo D., Neville T. A., Sparks D. L. 2004. Apolipoprotein A-II regulates HDL stability and affects hepatic lipase association and activity. J. Lipid Res. 45: 849–858 [DOI] [PubMed] [Google Scholar]
- 27.Julve J., Escola-Gil J. C., Rotllan N., Fievet C., Vallez E., de la Torre C., Ribas V., Sloan J. H., Blanco-Vaca F. 2010. Human apolipoprotein A-II determines plasma triglycerides by regulating lipoprotein lipase activity and high-density lipoprotein proteome. Arterioscler. Thromb. Vasc. Biol. 30: 232–238 [DOI] [PubMed] [Google Scholar]
- 28.Kalopissis A. D., Pastier D., Chambaz J. 2003. Apolipoprotein A-II: beyond genetic associations with lipid disorders and insulin resistance. Curr. Opin. Lipidol. 14: 165–172 [DOI] [PubMed] [Google Scholar]
- 29.Tailleux A., Duriez P., Fruchart J. C., Clavey V. 2002. Apolipoprotein A-II, HDL metabolism and atherosclerosis. Atherosclerosis. 164: 1–13 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Sawashita J., Qian J., Zhang B., Fu X., Tian G., Chen L., Mori M., Higuchi K. 2011. ApoA-I deficiency in mice is associated with redistribution of apoA-II and aggravated AApoAII amyloidosis. J. Lipid Res. 52: 1461–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez J., Latta M., Collet X., Vanloo B., Jung G., Denefle P., Rosseneu M., Chambaz J. 1994. Purification and characterization of recombinant human apolipoprotein A-II expressed in Escherichia coli. Eur. J. Biochem. 225: 1141–1150 [DOI] [PubMed] [Google Scholar]
- 32.Silva R. A., Schneeweis L. A., Krishnan S. C., Zhang X., Axelsen P. H., Davidson W. S. 2007. The structure of apolipoprotein A-II in discoidal high density lipoproteins. J. Biol. Chem. 282: 9713–9721 [DOI] [PubMed] [Google Scholar]
- 33.Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87: 206–210 [DOI] [PubMed] [Google Scholar]
- 34.Woody R. W. 1995. Circular dichroism. Methods Enzymol. 246: 34–71 [DOI] [PubMed] [Google Scholar]
- 35.Chen Y. H., Yang J. T., Martinez H. M. 1972. Determination of the secondary structures of proteins by circular dichroism and optical rotatory dispersion. Biochemistry. 11: 4120–4131 [DOI] [PubMed] [Google Scholar]
- 36.Sparks D. L., Davidson W. S., Lund-Katz S., Phillips M. C. 1993. Effect of cholesterol on the charge and structure of apolipoprotein A-I in recombinant high density lipoprotein particles. J. Biol. Chem. 268: 23250–23257 [PubMed] [Google Scholar]
- 37.Sparks D. L., Lund-Katz S., Phillips M. C. 1992. The charge and structural stability of apolipoprotein A-I in discoidal and spherical recombinant high density lipoprotein particles. J. Biol. Chem. 267: 25839–25847 [PubMed] [Google Scholar]
- 38.Tubb M. R., Smith L. E., Davidson W. S. 2009. Purification of recombinant apolipoproteins A-I and A-IV and efficient affinity tag cleavage by tobacco etch virus protease. J. Lipid Res. 50: 1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busby W. H., Jr, Quackenbush G. E., Humm J., Youngblood W. W., Kizer J. S. 1987. An enzyme(s) that converts glutaminyl-peptides into pyroglutamyl-peptides. Presence in pituitary, brain, adrenal medulla, and lymphocytes. J. Biol. Chem. 262: 8532–8536 [PubMed] [Google Scholar]
- 40.Brewer H. B., Jr, Lux S. E., Ronan R., John K. M. 1972. Amino acid sequence of human apoLp-Gln-II (apoA-II), an apolipoprotein isolated from the high-density lipoprotein complex. Proc. Natl. Acad. Sci. USA. 69: 1304–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anantharamaiah G. M., Hughes T. A., Iqbal M., Gawish A., Neame P. J., Medley M. F., Segrest J. P. 1988. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J. Lipid Res. 29: 309–318 [PubMed] [Google Scholar]
- 42.Weinberg R., Patton C., DaGue B. 1988. Analytic and preparative separation of apolipoproteins A-I, A-II, and A-IV by reverse phase high pressure liquid chromatography. J. Lipid Res. 29: 819–824 [PubMed] [Google Scholar]
- 43.Panagotopulos S. E., Witting S. R., Horace E. M., Hui D. Y., Maiorano J. N., Davidson W. S. 2002. The role of apolipoprotein A-I helix 10 in apolipoprotein-mediated cholesterol efflux via the ATP-binding cassette transporter ABCA1. J. Biol. Chem. 277: 39477–39484 [DOI] [PubMed] [Google Scholar]
- 44.Hussain M. M., Zannis V. I. 1990. Intracellular modification of human apolipoprotein AII (apoAII) and sites of apoAII mRNA synthesis: comparison of apoAII with apoCII and apoCIII isoproteins. Biochemistry. 29: 209–217 [DOI] [PubMed] [Google Scholar]
- 45.Remaley A. T., Wong A. W., Schumacher U. K., Meng M. S., Brewer H. B., Jr, Hoeg J. M. 1993. O-linked glycosylation modifies the association of apolipoprotein A-II to high density lipoproteins. J. Biol. Chem. 268: 6785–6790 [PubMed] [Google Scholar]
- 46.Jahn C. E., Osborne J. C., Jr, Schaefer E. J., Brewer H. B., Jr 1983. Activation of the enzymic activity of hepatic lipase by apolipoprotein A-II. Characterization of a major component of high density lipoprotein as the activating plasma component in vitro. Eur. J. Biochem. 131: 25–29 [DOI] [PubMed] [Google Scholar]