Abstract
Spine stabilization upon spinal cord injury (SCI) is a standard procedure in clinical practice, but rarely employed in experimental models. Moreover, the application of biodegradable biomaterials for this would come as an advantage as it would eliminate the presence of a nondegradable prosthesis within the vertebral bone. Therefore, in the present work, we propose the use of a new biodegradable device specifically developed for spine stabilization in a rat model of SCI. A 3D scaffold based on a blend of starch with polycaprolactone was implanted, replacing delaminated vertebra, in male Wistar rats with a T8-T9 spinal hemisection. The impact of spinal stabilization on the locomotor behavior was then evaluated for a period of 12 weeks. Locomotor evaluation—assessed by Basso, Beatie, and Bresnahan test; rotarod; and open field analysis—revealed that injured rats subjected to spine stabilization significantly improved their motor performance, including higher coordination and rearing activity when compared with SCI rats without stabilization. Histological analysis further revealed that the presence of the scaffolds not only stabilized the area, but also simultaneously prevented the infiltration of the injury site by connective tissue. Overall, these results reveal that SCI stabilization using a biodegradable scaffold at the vertebral bone level leads to an improvement of the motor deficits and is a relevant element for the successful treatment of SCI.
Introduction
Spinal cord injury (SCI) represents a significant health and social problem. It was estimated that approximately 500,000 people, in the United States and Europe alone, have to deal each day with the burden of having an SCI. Importantly, the incidence of SCI lies between 10.4 and 83 per million inhabitants each year, in western countries.1,2
Currently, medical practice for SCI patients is mainly based on three steps: stabilization of the spine, using metallic/vertebral spinal fusions3; decompression of the cord4; and administration of the anti-inflammatory drug methylprednisolone.5 Regarding spine stabilization, there is presently an open debate among physicians concerning the timing of surgery, even though there is strong evidence within the literature that early surgical stabilization consistently leads to shorter hospital stays, shorter intensive care unit stays, less days on mechanical ventilation, and less pulmonary complications.3 Nevertheless, the use of metallic devices, such as spinal fusions, have some disadvantages, namely, the potential need of a second surgery to remove it as well as interfering with magnetic resonance imaging during postoperative follow-up.6 Dynamic stabilization is a promising alternative to traditional spinal fusions; Cakir and colleagues,7 for instance, presented favorable short-term results when this system was applied. However, the longevity of a dynamic stabilization construct in an active adult, with constant motion, is an important consideration. Some devices have been abandoned because of failure over time.8 Moreover, Goldstein and colleagues demonstrated that the infection rate in patients undergoing dynamic stabilization is higher than that for instrumented fusion.9 We believe that biocompatible scaffolds that can act as stabilization devices and at same time promote bone regeneration are a very promising alternative to both traditional and dynamic devices. Biodegradable tools have been studied, namely, in the form of 3D scaffolds. However, to date, none of these have been specifically designed for spine stabilization after SCI.6 Surprisingly, in rat SCI models, spine stabilization is not performed at all. However, the majority of the surgical procedures used in rat SCI models comprise a laminectomy that may affect the locomotor behavior and trunk stability of the animals. In this sense, the absence of spine stabilization may compromise the regenerative process of the spinal cord tissue after the injury.
To specifically target this problem, we previously reported the development and characterization of a biodegradable scaffold composed of a blend of starch with polycaprolactone (SPCL) aimed for spine stabilization. SPCL scaffolds were fabricated by rapid prototyping and have shown to disclose appropriate mechanical performance, in vitro noncytotoxic behavior, and in vivo biocompatibility.10 Moreover, SPCL has been originally proposed by our group for a wide range of tissue engineering applications including bone regeneration.11–13 In this sense, the objective of the present study was to evaluate to what extent the stabilization of the vertebral column through the implantation of the referred scaffold is feasible and whether it promotes beneficial motor effects.
Materials and Methods
Processing of SPCL scaffolds
The SPCL scaffolds were processed as previously reported.10 Briefly, porous sheets featuring inter-filament orientations of 90° were produced by 3D plotting, a rapid prototyping/additive manufacturing technology (Bioplotter®; Envisiontec GmbH). Tubular scaffolds were obtained by rolling up porous sheets around a cylinder and subsequent heat treatment at 65°C during 30 min for inducing the adhesion between filaments. Scaffolds were then cut in semi-tubular structures. All the materials were sterilized with ethylene oxide.
Animals
Eight-week-old male Wistar rats (Charles River), housed in light and temperature controlled rooms and fed a standard diet, were used in this study. The Animal Care Committee of the Research Institute approved the animal protocols in accordance with standardized Animal Care Guidelines.14 After SCI, the animals were divided into two experimental groups: animals with spine stabilization through SPCL scaffold implantation (SPCL, n=9) and animals without spine stabilization (SCI, n=5). A third group of animals, subjected to laminectomy only, were used as controls (Sham, n=5). Handling was performed for 3 days before the surgery.
Surgery and postoperative care
All animals were anesthetized by intraperitoneal injection of a mixture (1.5:1) of ketamine (60 mg/mL, Imalgen; Merial) and medetomidine hydrochloride (0.4 mg/mL, Dorbene Vet; Laboratorios SYVA S.A.). Once anesthetized, fur was shaved from the surgical site and the skin was disinfected with chlorohexidine (AGB). Then, a dorsal midline incision was made from T6 to T11 and the paravertebral muscles were retracted. A laminectomy was performed at the junction T8–T9 in which the spinous processes were removed and the spinal cord was exposed. Two hemisections were performed, 3-mm apart on the left side, and the tissue in between was removed. SPCL scaffolds were implanted at the vertebral bone level, juxtaposed to the spinal cord, providing spine stabilization. Bone cement (Biomet) was used to fix the scaffold margins to bone. Paravertebral muscles and skin were then separately closed with Vicryl sutures (Johnson and Johnson). The incision of control animals was closed after SCI, without SPCL implantation. Following surgery, rats were kept under heat lamps and received vitamins (duphalyte and Farmoquil), analgesic (butorphanol tartrate, 1 mg/mL; Fort Dodge), and antibiotic (enrofloxacine, 1 mg/mL; Bayer). Bladder evacuation was done manually. Throughout the treatment and recovery period, animals were examined for symptoms of illness or potential reaction to the treatment.
Assessment of locomotor function by Basso, Beatie, and Bresnahan test
All rats were assessed with the Basso, Beattie, and Bresnahan Locomotor Rating Scale (BBB)15 on day 3 and 2, 5, 7, 9, and 12 weeks after injury. The BBB is a 21-point scale designed to assess hindlimb locomotor recovery following thoracic SCI. A BBB score of 0 indicates no hindlimb movement. A BBB score of 1 through 8 indicates joint movement, but no weight support. A BBB score of 9 through 20 indicates an ability to support weight and use the limb for locomotion but with some degree of abnormality. A BBB score of 21 corresponds to the locomotion of a normal rat.
Motor behavior analyses in an open field chamber
The open field (OF) is a versatile test that permits the assessment of motor behavior by measuring the amount of rearing activity and the total distance traveled by the rats.16 The OF was performed in a square (43.2 cm×43.2 cm) arena with transparent acrylic walls (Med Associates, Inc.) placed in a brightly illuminated room. Animals started the test at the arena's center and were given 5 min to explore it. Total distance traveled in the arena and number of rearings were automatically registered by equipment sensors.
Rotarod test
Motor coordination of the animals was evaluated in a rotarod equipment (TSE systems). Each animal was placed on a 10-cm diameter, 15-cm-long rod, rotating at constant speed. Impairment of motor coordination was defined as the inability of rats to remain on the rotating rod for a 60-s test period. Experimentally animals were pretrained on the rotating track 24 h before the proper test. The protocol consisted of 3 days of testing at 4, 8, and 12 rpm in, respectively, 1st, 2nd, and 3rd day for a maximum of 60 s in four trials, with a 10-min interval between each trial. The latency to fall (in seconds) was recorded by equipment sensors.
Tissue preparation
Twelve weeks after the scaffold implantation, the rats were deeply anesthetized by an intraperitoneal injection of sodium pentobarbital (Ceva Saude Animal). Then, animals were perfused through the ascending aorta with 4% paraformaldehyde in phosphate-buffered saline. A 2.5–3 cm length of spine and spinal cord, centered on the site of hemisection and scaffold placement, was carefully removed and fixed in neutral buffered formalin. After decalcification, spinal cord and spine were carefully split and then embedded in paraffin and processed for hematoxylin and eosin (H&E) staining. The tissue was sectioned on the coronal plane and apoptotic cells, vacuolated neurons, and tissue infiltration/organization were evaluated on three tissue slices of each subdivision (dorsal, median, and ventral cord). Apoptotic cells and vacuolated neurons were counted both rostral and caudal to the injury center and on the left side of the cord.
Statistical analysis
To assess whether the values come from a Gaussian distribution, the BBB, OF, Rotarod, and histological data were analyzed using the Kolmogorov–Smirnov test. Then, to evaluate statistical differences among groups, a “two way analysis of variance” test followed by a Bonferroni post-test was performed. Statistical significance was defined for p<0.05. All data are presented as mean±SEM.
Results
Locomotor function evaluation by BBB test
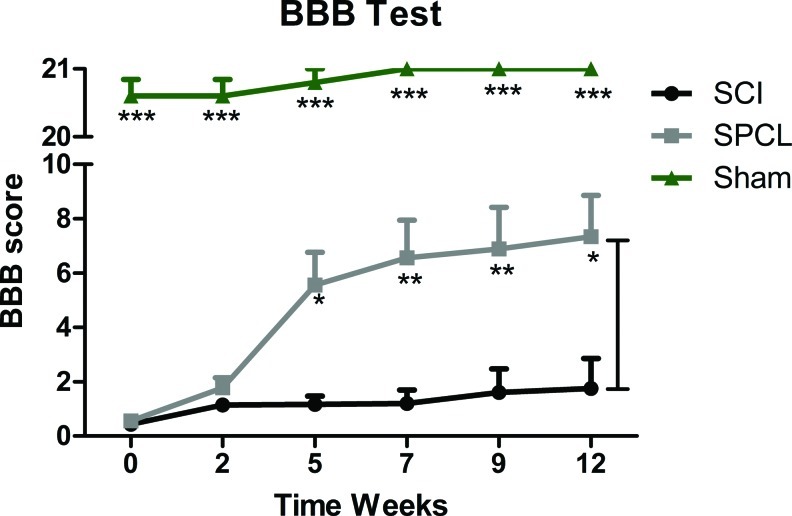
Left hindlimb function was evaluated 1 day after the hemisection injury. Only the animals that were completely paralyzed were selected for the study. From the initial 24 animals subjected to surgery, 2 died during the surgical procedure (from groups sham and SCI) and another 2 died during the experimental protocol (from groups SCI and SPCL). Motor evaluation began 3 days after surgery and continued each 2–3 weeks for 3 months. In all experiments the identity of the animals was kept blind to the observer. After SCI, SPCL scaffolds were implanted in the vertebral column of 9 animals (Fig. 1). Five weeks after, animals stabilized with SCPL scaffolds presented significant motor improvements when compared with the nonstabilized group (Fig. 2). Moreover, these differences persisted up to 12 weeks. Injured animals subjected to spine stabilization presented extensive movements of all three joints of the left hindlimb (SPCL, 7.3±1.5 in the BBB score), whereas rats without spine stabilization presented slight-to-extensive movements of just one joint (SCI, 1.8±1.1 in the BBB score) (Fig. 2). Animals from sham group scored 21 (maximum) in BBB scale, showing that laminectomy alone did not affect the animals' motor skills (Fig. 2).
FIG. 1.
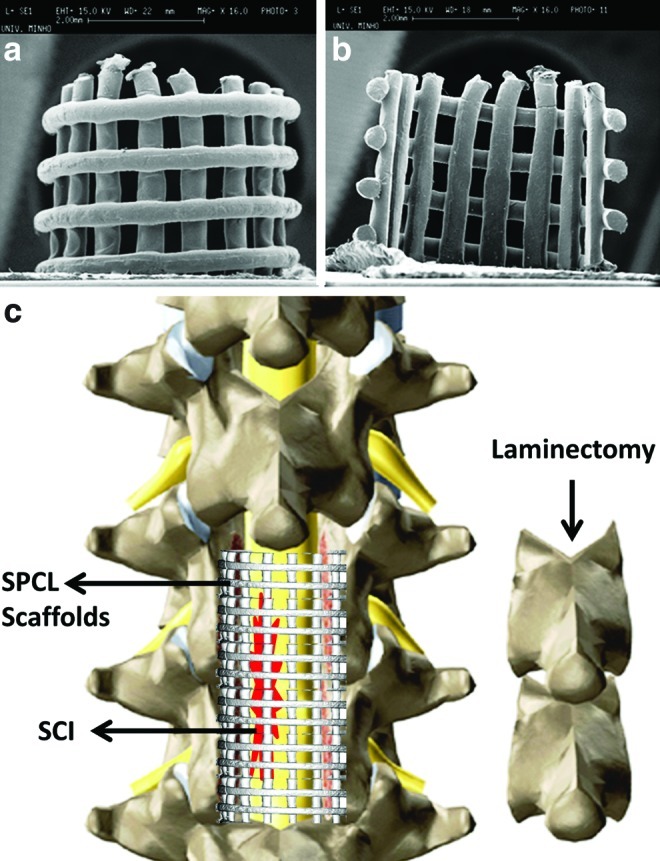
Scanning electron microscope pictures of starch with polycaprolactone (SPCL) scaffolds used for spine stabilization (a, b). Schematic representation of spine stabilization using SPCL scaffolds (c). The biodegradable scaffolds were implanted at vertebra level using bone cement. Color images available online at www.liebertpub.com/tec
FIG. 2.
Locomotor behavior evaluation of spinal cord injury (SCI) rats with spine stabilization (SPCL, n=9), without spine stabilization (SCI, n=5), and animals subjected only to a laminectomy (Sham, n=5). The BBB test showed significant motor skill improvement in rats with spine stabilization, comparing to those without stabilization. Sham animals did not present motor impairments. Values are shown as mean±SEM, *p<0.05; **p<0.01; ***p<0.001. BBB, Basso, Beattie, and Bresnahan Locomotor Rating Scale. Color images available online at www.liebertpub.com/tec
OF analyses
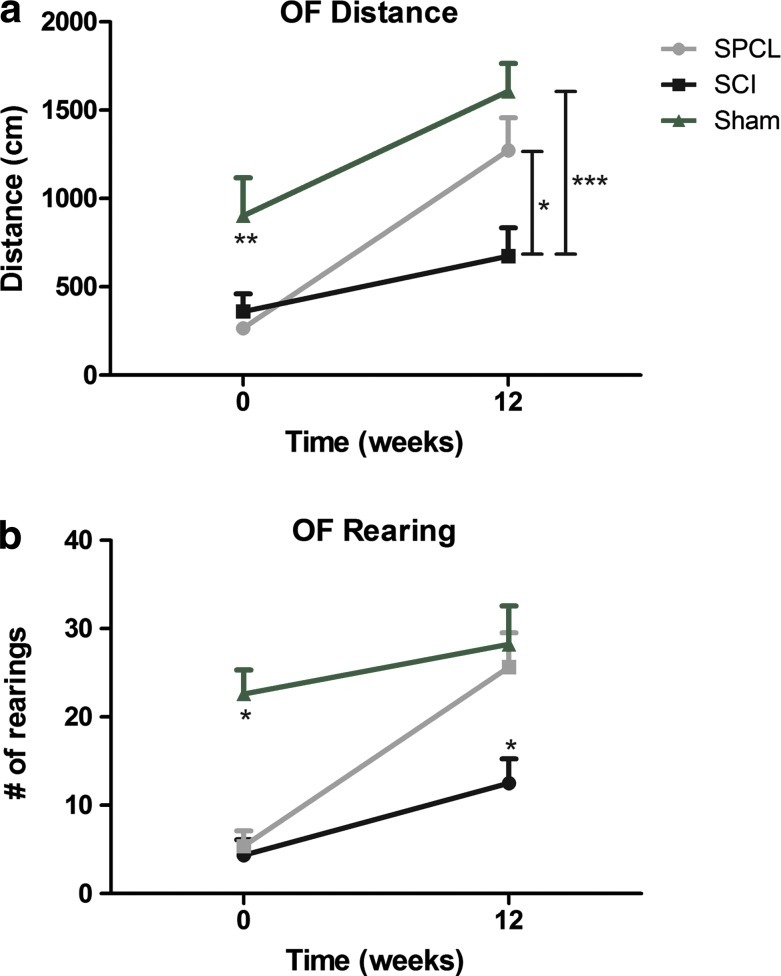
Unlike the BBB, the OF test is dependent on animal motivation to explore a new environment. Since the repetition of the same environment could lead to a decrease of the exploratory behavior, the OF test was not performed each 2 weeks. Instead, OF analyses were carried out in the first and last weeks of the experimental protocol and in different rooms. The total distance traveled in the OF arena just after the surgery was not significantly different between groups (266.6±19.3 cm for SPCL and 361.5±99.0 cm for SCI). However, at 12 weeks the total distance traveled by animals with a spine stabilized was significantly greater than that seen in the nonstabilized animals (1272.6±184.4 cm for SPCL and 673.5±161.0 cm for SCI) (Fig. 3a). Moreover, the number of rearings (exploratory behavior where animals stand up only in their hindpaws) was also assessed. Once again animals from both groups had a similar performance after surgery (5.4±1.7 for SPCL and 4.3±1.8 for SCI) while at 12 weeks, animals with SPCL implantation performed significantly more rearing activity than nonstabilized animals (25.7±3.8 for SPCL and 12.5±2.8 for SCI) (Fig. 3b). Importantly, despite a remarkable difference to sham animals in distance and rearings after surgery, 12 weeks after stabilization, animals did not show statistical differences (Fig. 3).
FIG. 3.
Distance and rearing behavior evaluation. When tested in an open field (OF) apparatus, injured rats with spine stabilization (SPCL) performed significantly better, both in distance traveled (a) and in number of rearings (b), comparing to nonstabilized animals (SCI). Comparing to the sham group, spine stabilized animals (SPCL) performed worse in the first week; however, at 12 weeks, both groups performed equally, showing motor improvements. Values are shown as mean±SEM, *p<0.05; ***p<0.001. Color images available online at www.liebertpub.com/tec
In addition to the just discussed results, we also performed the OF test in animals that did not suffer any surgery, which was used as baseline. Interestingly, these animals performed significantly more rearings (p<0.01) than the sham animals but covered approximately the same distance (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec). This data indicates that, although the hindlimb function of the sham animals was not affected (distance covered), they presented specific difficulties on rearing behavior. This difference on rearing behavior is most likely due to the absence of spine stabilization on sham animals.
Rotarod test
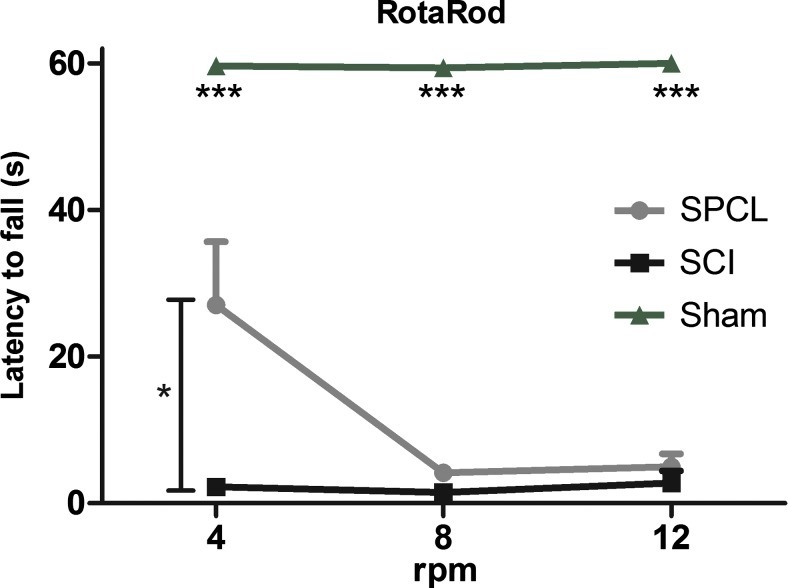
To assess motor coordination, a rotarod test was performed in the last week of the experiment. Once again, this test was not performed throughout all the experimental protocol because the falls from the rotating rod could lead to further injuries. The test was performed on 3 consecutive days. In first, at 4 rpm, spine-stabilized animal latency to fall from the rotating rod was significantly higher than nonstabilized animals (27.1±8.6 s for SPCL and 2.2±1.2 s for SCI). However, at 8 and 12 rpm (respectively, day 2 and 3), both stabilized and nonstabilized animals showed lack of motor coordination. The latency to fall happened only a few seconds from the start of the trial (8 rpm, 4.1±1.1 s; 12 rpm, 4.9±1.8 s for SPCL and 1.8±1.9; 2.7±1.6 for SCI). Once again, sham animals successfully executed the test, showing that their motor coordination was not affected by the laminectomy (Fig. 4).
FIG. 4.
Motor coordination assessment. In the rotarod test, rats with stabilization presented significantly higher motor coordination at 4 rpm; however, for more demanding task (8 rpm and 12 rpm), rats performed equally to nonstabilized animals. Sham animals successfully performed the test, showing no motor coordination impairment. Values are shown as mean±SEM, *p<0.05; ***p<0.001. Color images available online at www.liebertpub.com/tec
Histological characterization
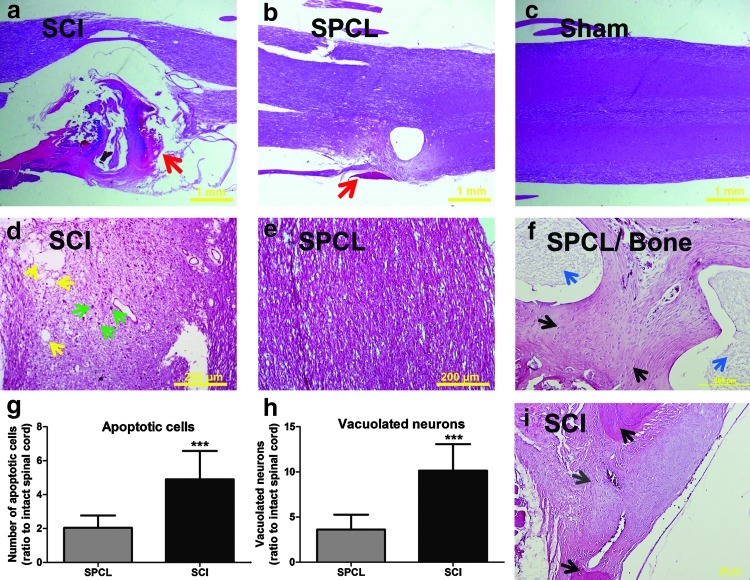
H&E staining was performed to assess the effects of spine stabilization on the spinal cord organization, surrounding tissue infiltration, and axon vacuolization after an SCI. A decrease of connective tissue infiltration into the spinal cord after the injury in animals subjected to spine stabilization was observed (Fig. 5a, b). As shown in Figure 5a, animals without scaffold implantation have a higher connective tissue infiltration. Less connective tissue infiltration was observed in treated animals after injury (Fig. 5b). When observed at higher magnification it was possible to detect less cell death and less vacuolated neurons in injured animals with spine stabilization than in nonstabilized animals (Fig. 5d, e, g, h). We observed that the number of apoptotic cells was significantly different between groups. There was a 4.9-fold increase (ratio to intact spinal cord) in the number of apoptotic cells found in the spinal cord tissue of nonstabilized animals and only a 2.0-fold increase (ratio to sham spinal cord) in the spinal cord of animals with spine stabilization (Fig. 5g). A similar trend was observed in the number of vacuolated neurons. Animals without spine stabilization presented significantly more vacuolated neurons (10.1-fold increase) than stabilized animals (3.6-fold increase; Fig. 5h). The histological analysis of the vertebral bone revealed that SPCL scaffolds were able to support new bone formation. The typical morphology of newly formed bone is characterized by a high number of cells and extracellular matrix more intensely stained for hematoxylin than eosin.17 Both of these features were found in the tissue between the SPCL fibers (Fig. 5f). Moreover, it was also possible to observe a few attributes typical in mature bone, such as some possible lamellar organization as well as spaces within the bone matrix, known as lacunae, which contain osteocytes. These results revealed that newly formed bone involving the SPCL fibers started maturation. This is contrary to what is seen in animals that did not receive SPCL implants. In those animals we only observe the formation of granulation tissue and apparently no bone formation yet (Fig. 5i). The granulation tissue (mainly formed by fibroblast and periosteal cells) is a standard response after bone fractures. This new loose connective tissue provides a temporary extracellular matrix that supports new bone formation.
FIG. 5.
Histological analyses of spinal cord tissue and vertebra bone stained with hematoxylin and eosin. (a) Injured rats without spine stabilization (SCI) presented higher vertebral canal tissue infiltration (red arrows) than the ones with stabilization (SPCL) (b). The laminectomy alone did not affect the spinal cord of sham animals (c). With higher magnification it was possible to observe more cell death (green arrows pointing to chromatin condensation) and more vacuolated neurons (yellow arrows pointing to cyst-like structures) in rats without stabilization (d) than in those with SPCL stabilization (e). In animals without stabilization, both apoptotic cells (g) and vacuolated neurons (h) were present in greater numbers than in animals with stabilization. (f) At the vertebral level it was possible to observe that new bone was able to grow between the SPCL fibers (blue arrows). Moreover, it was possible to observe some signals of bone maturation, as the presence of lacunas, small spaces on bone matrix filled by osteocytes (black arrows). (i) In animals without SPCL implantation, it was only possible to observe the formation of granulation tissue (gray arrow) and no bone formation. Scale bar of (a), (b), and (c): 1 mm; scale bar of (d), (e), (f), and (i): 200 mm; spinal cord figures are from dorsal region; values are shown as mean±SEM, ***p<0.001. Color images available online at www.liebertpub.com/tec
Discussion
Biodegradable materials have been successfully used in various clinical applications. These materials were first introduced more than 30 years ago by Kulkarni and colleagues18 for use as absorbable sutures. After that, absorbable polymers have been successfully employed for various methods of fixation for small bone fractures.19 Recently, these implants have been studied for spine surgery; nonetheless, the use of metallic spinal fixation is still the standard clinical practice.6 Moreover, until now none of the developed biodegradable implants were specifically designed for spine stabilization after an SCI.
We show that spine stabilization is an important element in the therapeutic intervention of SCI whenever there is a laminectomy. In fact, animals subjected to spinal stabilization presented significant motor recovery. These animals were able to recover from no apparent movement on the left paw, after surgery, to extensive movement of all three joints in the hindlimb (7 on the BBB scale), at 12 weeks. On the other hand, animals without spinal stabilization by the SPCL scaffolds, only recovered from no apparent movement to slight/extensive movements of one joint (2 on the BBB scale). The BBB results here presented for injured animals without treatment are in concordance with other previously published experiments.20,21 However, others also report a greater motor recovery from control animals (between 6 and 8 on the BBB scale).22,23 These variations may be explained by different protocols in the postoperative care performed by each lab. For instance, some authors use fibrin as a natural sealant or to stabilize tissue grafts within the spinal cord.24,25 This protocol may influence the motor recovery of control rats, given that, it was previously described that fibrin alone promotes tissue regeneration.26
Due to the subjectivity inherent to BBB test, the motor function of the animals was also assessed by the rotarod and OF test methods. Analysis of motor coordination, balance, motor control, and trunk stability was performed with these tests. Moreover, there is lower interference by the observer in rotarod and OF tests since the motor analysis is generated by software. The results from OF analyses showed that animals with spine stabilization were able to walk longer distances and execute a higher number of rearings than nonstabilized animals. Either distance or rearing performance is correlated with the motor improvements observed in the BBB test. Once again, the benefits of spine stabilization were confirmed. The greater amount of rearing activity may be correlated by an increase in trunk stability due to the implanted scaffolds. Moreover, the rearing behavior showed that the SPCL scaffolds are mechanically able to support the body weight of the animals when they stand up. Further, we previously reported that rapid prototyping allows us to adjust the mechanical properties of SPCL scaffolds.10 In this sense, the higher mechanical strength required in case of implantation in bigger animal models or in humans can be achieved. The mechanical properties of the SPCL scaffolds are also strongly linked to polymer degradation rate. Previous studies showed that SPCL presents a slow degradation rate27 and here we observed that they allow bone ingrowth; so, it is expectable that the vertebral bone is completely repaired after total reabsorption of the scaffold by the human body in order to avoid catastrophic failure. However, studies with longer implantation periods are still needed.
The results from rotarod revealed that motor coordination and balance are partially improved. To perform this test the rats had to walk over a constant moving rod in a coordinated way or they fall off the drum. The results showed that animals with the spine stabilized by the scaffolds, unlike the nonstabilized animals, were able to perform a coordinated motor behavior at 4 rpm. However, when the difficulty of the task increased to 8 and 12 rpm, all of the animals were not able to adequately perform the test. These results support the fact that stabilization provided by the implanted scaffold improves the motor function of SCI animals. Both the lack of coordination in more demanding tasks and the BBB score (just 7) corroborate the fact that spine stabilization should be combined with other strategies to promote nervous tissue repair.
The results presented here can be explained by the fact that the stabilization of the cord prevented further tissue damage derived from animal movements. Unlike humans, most SCI animal models perform body movements a few minutes after the surgery. However, these animals suffered a laminectomy that will provoke trunk instability and most of the times, trunk torsion. In this sense, prevention of vertebral column instability will avoid further mechanical impacts between bone and spinal cord. Moreover, SPCL scaffolds were implanted in the open wound, establishing a connection to the adjacent vertebral bone and decreasing the infiltration of connective tissue, as it was possible to observe by histological analyses. Thus, it is most likely that the conjugation of spine stabilization and reduction of connective tissue infiltration provided by SPCL scaffolds protects the spinal cord, leading to the decrease in cell death and a smaller amount of vacuolated neurons observed in the spinal cord tissue of the stabilized rats.
The locomotor behavior improvements herein described may be explained by spontaneous neural plasticity after SCI. Previous studies demonstrated that central nervous tissue is able to undergo anatomical rearrangement after an SCI. For instance, Weidner and colleagues28 verified that transection of the dorsally projecting corticospinal tract (CST) led to spontaneous sprouting of the ventral CST projection. Moreover, this plasticity was responsible for motor improvements in the forelimb. Additionally, Bareyre and co-workers29 demonstrated that some of the transected CST axons that would normally innervate lumbar segments sprouted into the cervical cord to innervate propriospinal neurons. This led to a novel, indirect motor pathway to lumbar motor circuits, which was responsible for motor recovery. In this sense, the protection against further damage to both damaged and intact fibers that the scaffold is able to provide after the SCI seems to create a more suitable environment for plasticity to take place.
These results show a correlation between spine stabilization and motor recovery in SCI rats. However, after an overview of the literature, it was almost impossible to find references to spine stabilization after an SCI in rat/mouse animal models. With exception of the Tator lab, which uses a metallic device,30 researchers rarely have tried to mimic the standard clinical practice after SCI. An interesting attempt to improve scaffold alignment after implantation in the spinal cord revealed that spine stabilization (using steel wires) prevents scoliosis and reduces kyphosis in SCI rats.31 Unfortunately, the authors did not assess motor recovery by the BBB test; nonetheless, this study reinforces our conception that the absence of spine stabilization may jeopardize the regenerative process. In this sense, current and future attempts to develop a successful treatment for SCI32 might have to take into account this process. It is important to refer that the choice of the biomaterial and 3D architecture of the stabilization device might be crucial, as the results herein reported suggest that a device that prevents surrounding connective tissue infiltration into the spinal cord and allows the vertebral bone to regenerate contributes to nervous tissue reorganization. Other tissue engineering approaches to promote spine regeneration are mainly focused on intervertebral disc regeneration.33 However, Dong and coworkers34 demonstrated that laminae of the vertebral arch can be successfully reconstructed using collagen scaffolds and bone marrow stromal cells. In an SCI situation, collagen scaffolds would have to be combined with metallic devices to promote spine stabilization, given that, collagen presents weak mechanical properties. In any case, this is an interesting work showing that tissue engineering can successfully regenerate vertebral bone.
The hemisection model employed here preserves the integrity and function of one side of the cord that usually is sufficient to maintain bladder and bowel function resulting in less postoperative care and reduced animal death. Nonetheless, in future studies it will be important to analyze the benefits of spine stabilization in other models, namely, the contusion model that reveals features similar to those seen in the clinical context. Moreover, future studies may also be focused on the use of mathematical models of human spine in order to study the applicability of SPCL scaffolds in patients. The finite element model (FEM) is the most common spine model and it incorporates realistic geometry of the vertebrae and physical properties of the soft tissue connecting the vertebrae.35 With FEM it is possible to study kinematics (intervertebral motions), kinetics (motions in response to applied loads), and internal strains and stresses of the human spine.35 In this sense, it would be possible to study the response of the SPCL scaffolds to mechanical stresses usually found in the human body. Additionally, the spine model may be combined with an SCI mathematical model, as the one described by Russell and colleagues,36 in order to further understand the neuroprotective effect of SPCL stabilization in an SCI situation.
In conclusion, in this work we present a biodegradable scaffold specifically designed for spine stabilization of SCI in the rat that can also provide support for bone ingrowth. More importantly, we also revealed that stabilization by SPCL scaffolds leads to an improvement of the motor skills of affected animals. Therefore, researchers currently testing treatments for SCI repair might have to take into account the use of spine stabilization in combination with their approaches. Further work will be focused on the combination of spine stabilization and regeneration comprising SPCL scaffolds in combination with a hydrogel loaded with cells for SCI repair.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the Portuguese Foundation for Science and Technology (Doctoral fellowship to Nuno Silva, SFRH/BD/40684/2007; Ciência 2007 Program to António Salgado; Grant N° PTDC/SAU-BMA/114059/2009) and the Foundation Calouste de Gulbenkian to funds attributed to A.J. Salgado under the scope of the The Gulbenkian Programme to Support Cutting Edge Research in the Life Sciences. This work was also partially supported by the European FP7 Project Find and Bind (NMP4-SL-2009-229292).
Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Wyndaele M. Wyndaele J.J. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44:523. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 2.Ouzký M. Towards concerted efforts for treating and curing spinal cord injury. Concil Eur Doc. 2002;9401:27. [Google Scholar]
- 3.Dimar J.R. Carreon L.Y. Riina J. Schwartz D.G. Harris M.B. Early versus late stabilization of the spine in the polytrauma patient. Spine. 2010;35:S187. doi: 10.1097/BRS.0b013e3181f32bcd. [DOI] [PubMed] [Google Scholar]
- 4.Fehlings M.G. Wilson J.R. Timing of surgical intervention in spinal trauma what does the evidence indicate? Spine. 2010;35:S159. doi: 10.1097/BRS.0b013e3181f330f4. [DOI] [PubMed] [Google Scholar]
- 5.Bracken M.B. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine. 2001;26:S47. doi: 10.1097/00007632-200112151-00010. [DOI] [PubMed] [Google Scholar]
- 6.Vaccaro A.R. Singh K. Haid R. Kitchel S. Wuisman P. Taylor W., et al. The use of bioabsorbable implants in the spine. Spine J. 2003;3:227. doi: 10.1016/s1529-9430(02)00412-6. [DOI] [PubMed] [Google Scholar]
- 7.Cakir B. Richter M. Huch K. Puhl W. Schmidt R. Dynamic stabilization of the lumbar spine. Orthopedics. 2006;29:716. doi: 10.3928/01477447-20060801-04. [DOI] [PubMed] [Google Scholar]
- 8.Molinari R.W. Dynamic stabilization of the lumbar spine. Curr Opin Orthop. 2007;18:215. [Google Scholar]
- 9.Goldstein I.M. Agarwal N. Mammis A. Barrese J. Christiano L.D. Rates of Infection with Dynamic Stabilization Compared to Posterior Instrumented Fusion. 28th Annual Meeting of the AANS/CNS Section on Disorders of the Spine and Peripheral Nerves Orlando; 2012. [Google Scholar]
- 10.Silva N.A. Salgado A.J. Sousa R.A. Oliveira J.T. Pedro A.J. Leite-Almeida H., et al. Development and characterization of a novel hybrid tissue engineering based scaffold for spinal cord injury repair. Tissue Eng Part A. 2010;16:45. doi: 10.1089/ten.TEA.2008.0559. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues M.T. Gomes M.E. Viegas C.A. Azevedo J.T. Dias I.R. Guzón F.M., et al. Tissue-engineered constructs based on SPCL scaffolds cultured with goat marrow cells: functionality in femoral defects. J Tissue Eng Regen Med. 2011;5:41. doi: 10.1002/term.287. [DOI] [PubMed] [Google Scholar]
- 12.Rada T. Santos T.C. Marques A.P. Correlo V.M. Frias A.M. Castro A.G., et al. Osteogenic differentiation of two distinct subpopulations of human adipose-derived stem cells: an in vitro and in vivo study. J Tissue Eng Regen Med. 2012;6:1. doi: 10.1002/term.388. [DOI] [PubMed] [Google Scholar]
- 13.Martins A. Chung S. Pedro A.J. Sousa R.A. Marques A.P. Reis R.L., et al. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J Tissue Eng Regen Med. 2009;3:37. doi: 10.1002/term.132. [DOI] [PubMed] [Google Scholar]
- 14.Van Zutphen LFM. Baumans V. Beynen AC. Amsterdam: Elsevier; 2001. Principles of Laboratory Animal Science. [Google Scholar]
- 15.Basso D.M. Beattie M.S. Bresnahan J.C. A Sensitive and reliable locomotor rating-scale for open-field testing in rats. J Neurotrauma. 1995;12:1. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 16.Sousa N. Almeida O.F.X. Wotjak C.T. A hitchhiker's guide to behavioral analysis in laboratory rodents. Genes Brain Behav. 2006;5:5. doi: 10.1111/j.1601-183X.2006.00228.x. [DOI] [PubMed] [Google Scholar]
- 17.Ross M. Kaye G.I. Pawlina W. Philadelphia: Lippincott Williams & Wilkins; 2002. Histology: A Text and Atlas. [Google Scholar]
- 18.Kulkarni R.K. Pani K.C. Neuman C. Leonard F. Polylactic acid for surgical implants. AMA Arch Surg. 1966;93:839. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 19.Bucholz R.W. Henry S. Henley M.B. Fixation with bioabsorbable screws for the treatment of fractures of the ankle. J Bone Joint Surg Am. 1994;76A:319. doi: 10.2106/00004623-199403000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hou T. Wu Y. Wang L. Liu Y. Zeng L. Li M., et al. Cellular prostheses fabricated with motor neurons seeded in self-assembling peptide promotes partial functional recovery after spinal cord injury in rats. Tissue Eng Part A. 2011;18:974. doi: 10.1089/ten.TEA.2011.0151. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J.F. Zhao F.S. Wu G. Kong Q.F. Sun B. Cao J., et al. Therapeutic effect of co-transplantation of neuregulin 1-transfected-Schwann cells and bone marrow stromal cells on spinal cord hemisection syndrome. Neuroscience Letters. 2011;497:128. doi: 10.1016/j.neulet.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 22.Teng Y.D. Lavik E.B. Qu X. Park K.I. Ourednik J. Zurakowski D., et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc of the Natl Acad Sci. 2002;99:3024. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S. Strittmatter S.M. Delayed systemic nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng H. Cao Y. Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard C.D. Slotkin J.R. Yu D. Dai H. Lawrence M.S. Bronson R.T., et al. Establishing a model spinal cord injury in the African green monkey for the preclinical evaluation of biodegradable polymer scaffolds seeded with human neural stem cells. J Neurosci Methods. 2010;188:258. doi: 10.1016/j.jneumeth.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp K.G. Dickson A.R. Marchenko S.A. Yee K.M. Emery P.N. Laidmåe I., et al. Salmon fibrin treatment of spinal cord injury promotes functional recovery and density of serotonergic innervation. Exp Neurol. 2012;235:345. doi: 10.1016/j.expneurol.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azevedo H.S. Gama F.M. Reis R.L. In vitro assessment of the enzymatic degradation of several starch based biomaterials. Biomacromolecules. 2003;4:1703. doi: 10.1021/bm0300397. [DOI] [PubMed] [Google Scholar]
- 28.Weidner N. Ner A. Salimi N. Tuszynski M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci. 2001;98:3513. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bareyre F.M. Kerschensteiner M. Raineteau O. Mettenleiter T.C. Weinmann O. Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 30.Nomura H. Katayama Y.M. Shoichet M.S. Tator C.H. Complete spinal cord transection treatedby implantation of a reinforced synthetic hydrogel channel results in syringomyelia and caudal migration of the rostral stump. Neurosurgery. 2006;59:183. doi: 10.1227/01.NEU.0000219859.35349.EF. [DOI] [PubMed] [Google Scholar]
- 31.Rooney G.E. Vaishya S. Ameenuddin S. Currier B.L. Schiefer T.K. Knight A., et al. Rigid fixation of the spinal column improves scaffold alignment and prevents scoliosis in the transected rat spinal cord. Spine. 2008;33:914. doi: 10.1097/BRS.0b013e318186b2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tohda C. Kuboyama T. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacol Ther. 2011;132:57. doi: 10.1016/j.pharmthera.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 33.O'Halloran D.M. Pandit A.S. Tissue-engineering approach to regenerating the intervertebral disc. Tissue Eng. 2007;13:1927. doi: 10.1089/ten.2005.0608. [DOI] [PubMed] [Google Scholar]
- 34.Dong Y. Chen X. Wang M. Hong Y. Construction of artificial laminae of the vertebral arch using bone marrow mesenchymal stem cells transplanted in collagen sponge. Spine. 2012;37:648. doi: 10.1097/BRS.0b013e31822ecebc. [DOI] [PubMed] [Google Scholar]
- 35.Panjabi M.M. Cervical spine models for biomechanical research. Spine. 1998;23:2684. doi: 10.1097/00007632-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 36.Russell C.M. Choo A.M. Tetzlaff W. Chung T-E. Oxland T.R. Maximum principal strain correlates with spinal cord tissue damage in contusion and dislocation injuries in the rat cervical spine. J Neurotrauma. 2012;29:1574. doi: 10.1089/neu.2011.2225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.