Abstract
Promising phase II clinical results have been reported recently for several oncolytic viral therapeutics, including strains based on vaccinia virus. One reason for this has been an increased appreciation of the critical therapeutic importance of the immune response raised by these viruses. However, the most commonly used approaches to enhance these immunotherapeutic effects in oncolytic viruses, typically though expression of cytokine transgenes, often also result in a reduction in oncolytic activity and premature clearance of the virotherapy from the tumor. Approaches that enhance the immunotherapeutic effects while maintaining oncolytic activity would therefore be beneficial. Here, it is demonstrated that the expression of the chemokine CCL19 (ELC) from an oncolytic vaccinia virus (vvCCL19) results in increased antitumor effects in syngeneic mouse tumor models. This corresponded with increased t cell and dendritic cell infiltration into the tumor. However, vvCCL19 persisted in the tumor at equivalent levels to a control virus without CCL19, demonstrating that oncolytic activity was not curtailed. Instead, vvCCL19 was cleared rapidly and selectively from normal tissues and organs, indicating a potentially increased safety profile. The therapeutic activity of vvCCL19 could be further significantly increased through combination with adoptive transfer of therapeutic immune cells expressing CCR7, the receptor for CCL19. This approach therefore represents a means to increase the safety and therapeutic benefit of oncolytic viruses, used alone or in combination with immune cell therapies.
Introduction
There has been a resurgence of interest in oncolytic viral cancer therapies due to the exciting phase II clinical results reported with several different viral vectors [1–3] and the entry of these into randomized testing [4]. However, despite reports of therapeutic responses, complete responses remain elusive and approaches that enhance the activity of these vectors are still needed.
One factor driving the advances in this next generation of oncolytic viruses has been the realization that their immunotherapeutic activity is critical for their success [5,6]. However, this is a double-edged sword, as most approaches that increase the immunotherapeutic activity of the oncolytic vectors also result in reduced oncolytic activity and early immune-mediated clearance of the agents from the tumor. In particular, although expression of cytokines from oncolytic viruses frequently results in increased antitumor effects, a closer examination reveals that this increased overall activity comes despite a significant loss of viral replicative capacity within the tumor [7]. It is therefore apparent that approaches that enhance the interactions of oncolytic viruses with the immune response while maintaining oncolytic activity would result in the greatest therapeutic benefits. As such, the effects of chemokine expression from the viral vectors were explored. It was hypothesized that chemokine expression may simultaneously help overcome one of the major limitations of immunotherapy, an inability of effector cells to efficiently traffic to the tumor, while also leading to a more subtle targeting of the immune response, with less direct immune activation than seen with commonly used cytokine transgenes. In addition, chemokine expression may lead to synergistic combinations with immune cell therapies expressing the appropriate receptors.
We have previously reported on a strain of vaccinia virus expressing the chemokine CCL5 (RANTES) [8]. This strain (vvCCL5) displayed some unexpected properties, including the capacity to persist for extended periods in the tumors of mouse models of cancer. Here, the effects of expression of the chemokine CCL19 from an oncolytic vaccinia strain were examined. Whereas the receptors for CCL5 (CCR1, CCR3, and CCR5) are known to attract a wide range of immune cells, including immature dendritic cells (DCs) and Th1 and Th2 T cells to sites of inflammation, the lymph node targeting receptor for CCL19 (CCR7) is expressed on a more restricted set of immune cells, including mature DCs and T cells [9]. CCL19 was chosen for two reasons, first, to interact directly with cytokine induced killer (CIK) cells (that express the cognate receptor CCR7) and, second, to attract DCs and naïve T cells into the tumor in the context of viral infection and the associated transient overcoming of immune suppression, as this may enhance the previously demonstrated in situ vaccination effect produced by oncolytic vaccinia strains.
Initial in vivo testing in mouse tumor models determined that CCL19 expression enhanced therapeutic effects but had no significant effect on the level or persistence of the virus in the tumor and instead led to more rapid clearance of the virus from elsewhere within the host. The mechanisms behind this enhanced safety and the enhanced antitumor effects of oncolytic vaccinia expressing CCL19 used alone or in combination with adoptive immune cell therapy are examined.
Materials and Methods
Cell Lines and Viruses
MC38 murine colon adenocarcinoma cell line was originally induced with oral dimethylhydrazine in C57BL/6 mice and has been used extensively by ourselves and others. 6780 Cells were a kind gift from Dr Dean Felsher (Stanford University); these cells are a primary lymphoma cell line isolated from a transgenic mouse model of spontaneous lymphoma. The CV-1 cell line used for plaque assay was purchased from ATCC (Manassas, VA).
All recombinant vaccinia strains were in a Western Reserve background. The vaccinia virus double-deleted (vvDD) strain, a viral thymidine kinase and vaccinia growth factor double deletion, has been reported previously [10]. vvCCL19 (a version of vvDD expressing mCCL19 from the p7.5 promoter) was constructed for this work (Figure W1).
In Vitro Viral Replication Assay
MC38 cells infected at multiplicity of infection (MOI) of 0.1 were harvested at indicated times, subjected to three cycles of freeze-thaw, and the cell lysate was homogenized with a FastPrep Cell Disrupter (Model FP120; Qbiogene, Inc, Carlsbad, CA) to release virions. Virus was then titered by standard plaque assay on CV-1 cells.
Enzyme-Linked Immunosorbent Assay
MC38 cells were infected with either vvCCL19 or vvDD at an MOI = 1.0. Twenty-four hours later, supernatants were collected and run on ELISA assay for detection of CCL19 production with DuoSet ELISA Development Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's guidelines.
Immune Cell Preparation
Activated NKT (CIK) cells were expanded from spleens of Luc+ Tg FVB mice as previously described [11]. Briefly, splenocytes were cultured with interferon-gamma (IFN-γ) and anti-CD3e antibody for 24 hours, before being cultured for 10 to 15 days with interleukin-2 (IL-2) added. Bulk splenocytes represent whole-spleen populations after RBC lysis only.
In Vitro Transwell Chemotaxis Assay
Supernatant was added into the lower wells of 3.0-µm pore-size 96-well Transwell (Corning, NY) plates, while 50,000 immune cells were added into each of the upper wells. These were incubated at 37°C for 3 hours, and the numbers of migrated immune cells were counted by flow cytometry.
Mouse Models
Female FVB and C57BL/6 mice were obtained from Taconic (Germantown, NY) at 6 weeks old. All animal studies were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
For biodistribution, Luminex, and immunohistochemistry assays, C57BL/6 mice were injected subcutaneously with 200,000 MC38 cells. When the tumors reached 50 to 100 mm3, animals were treated with intraperitoneal (IP) or intravenous (IV) injection of phosphate buffered saline (PBS), virus, or immune cells as indicated. For viral biodistribution, mice were sacrificed, tissues were frozen and homogenized, and the viral plaque forming units recovered were titered; for immune cell biodistribution, cells expressing luciferase were analyzed by bioluminescence imaging on an IVIS200 (Xenogen, part of Caliper Life Sciences) after injection with luciferin (Caliper Life Sciences, Perkin Elmer, Waltham, MA) and anesthesia with 2% isoflurane; for flow cytometry, tumors were harvested and dissociated with enzyme lysis buffer; for immunohistochemistry, tissues were harvested and frozen for subsequent sectioning and staining; for Luminex array assays, tissues were collected, weighed, and ground in PBS buffer; BCA protein assays were performed, and the samples were normalized. For tumor response assays, tumor sizes were measured every other day by caliper measurement.
Ex Vivo Assays
Antibodies used in flow cytometry included labeled anti-CD4, anti-CD8, anti-NK1.1, and anti-CD11c (BD Pharmingen, San Jose, CA) with Mouse Regulatory T cell Staining Kit (eBioscience, San Diego, CA); samples were analyzed on a Beckman Coulter XL32 EXPO or a DAKO Cyan flow cytometer.
Immunohistochemistry and immunofluorescence sections were incubated with anti-CD4 or CD8 followed by appropriate secondary antibodies (eBioscience). Epifluorescence images were taken using an Olympus Provis light microscope (Olympus America, Center Valley, PA), and MetaMorph (Molecular Devices, Downington, PA) was used for image analysis.
Luminex array assays were performed with Milipore Mouse 32-Plex in the University of Pittsburgh Cancer Institute facility and were normalized to the level of overall protein in samples after homogenization and lysis of the tumor samples.
Statistical Methods
Unpaired and paired Student's t tests were used to determine statistical significance (defined as P < .05).
Results
vvCCL19 Selectively Attracts Lymphocytes Expressing CCR7 In Vitro
The oncolytic vaccinia strain vvDD has been studied extensively in preclinical models and is currently undergoing phase I clinical testing. This vector contains tumor-targeting deletions in the viral thymidine kinase and viral growth factor genes [10,12]. A version of vvDD was constructed for this work expressing murine CCL19 under control of the pSE/L promoter and expressed from within the thymidine kinase locus, vvCCL19 (Figure W1). This virus was confirmed to secrete CCL19 after infection of MC38 (mouse colorectal tumor) cells in vitro and viral replication was found to be unaffected by CCL19 expression (Figure W1).
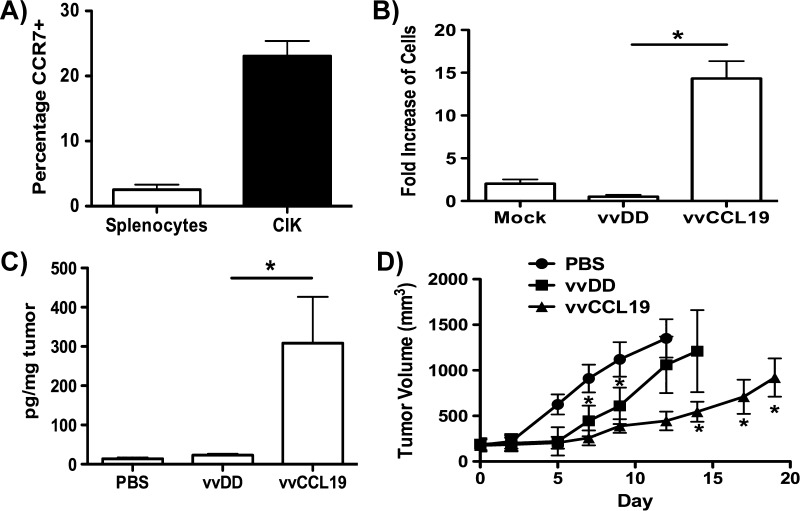
Expression profiles for CCR7 (the receptor for CCL19) were examined on both a naïve splenocyte population and activated natural killer (NK)-T cell therapeutic cells (CIK cells [11,13]). It was confirmed that whereas CCR7 was expressed at very low levels on the bulk splenocyte population, CCR7 was expressed on a significant subset (approximately 25%) of the CIK cells (Figure 1A). It is unclear why CCR7 is expressed on CIK cells, as these represent an activated T cell population that would not normally be expected to express this receptor. This was reflected in the capacity for the CIK cells to traffic to media collected from infected MC38 cells in an in vitro chemotaxis assay (Figure 1B). Only the CIK cells trafficked in significant numbers and only to media from vvCCL19-infected cells. This confirmed that vvCCL19 produced functionally active CCL19 that was capable of attracting cells expressing CCR7. It is also notable that vvDD apparently reduced trafficking of CIK cells (although not significantly); this may be indicative of expression of viral virulence genes known to block the action of chemokines.
Figure 1.
(A) CCR7 is expressed on CIK cells but not on bulk splenocytes. Populations of bulk inactivated mouse splenocytes and CIK cells were labeled with anti-CCL19 antibody and analyzed by flow cytometry. The percentage of cells positive for CCL19 (above an isotype control) is shown, average of three independent experiments (P < .001). (B) vvCCL19-infected MC38 cells secrete functional CCL19. Media from MC38 tumor cells infected with vvDD or vvCCL19 or mock infected for 24 hours (MOI = 1.0) were collected and filter sterilized before levels of CIK attraction were determined in a chemotaxis assay. Results are average of three experiments (*P < .01; note that vvDD shows a trend toward reduced attraction of CIK cells, but this is not significant; P =.06). (C) Systemic treatment with vvCCL19 leads to increased CCL19 levels in the tumors. Mice (C57/BL6 bearing MC38 subcutaneous tumors, n = 3 per group) were treated via IP injection with indicated viruses (1 x 108 PFU/mouse) and the levels of CCL19 were determined in recovered tumors by ELISA after 3 days (P < .05). (D) Mice (C57/BL6 bearing MC38 subcutaneous tumors, n= 8 per group) were treated as before, subsequent tumor volume was determined by caliper measurement. vvCCL19 treatment was significantly better (P < .05) than other treatments from day 14 onward.
vvCCL19 Displayed Enhanced Antitumor Effect In Vivo
Mice (C57/BL6) were implanted with subcutaneous MC38 tumors, and when these became palpable (50–100 mm3), they were treated via IP injection with 1 x 108 plaque forming units (PFU) of vvDD or vvCCL19. It was confirmed that CCL19 was expressed in the tumor only after vvCCL19 therapy (Figure 1C). Subsequent caliper measurements of tumor growth determined that vvCCL19 displayed significantly greater therapeutic benefit than vvDD (Figure 1D). This therapeutic benefit was seen despite the fact vvDD and vvCCL19 replicated to identical levels in the same MC38 cell line in vitro (Figure W1), implying that CCL19 expression is likely mediating an immune response that enhances the antitumor effects in vivo. It is notable that vvDD and vvCCL19 both produced significant therapeutic benefit relative to PBS controls over the first 7 to 10 days after treatment, but that only vvCCL19 treatment was capable of extending these significantly enhanced therapeutic effects (relative to vvDD) from day 14 onward. However, despite these significantly enhanced therapeutic effects no complete responses were produced.
vvCCL19 Selectively Attracts DCs and CD4+ T Cells into the Tumor
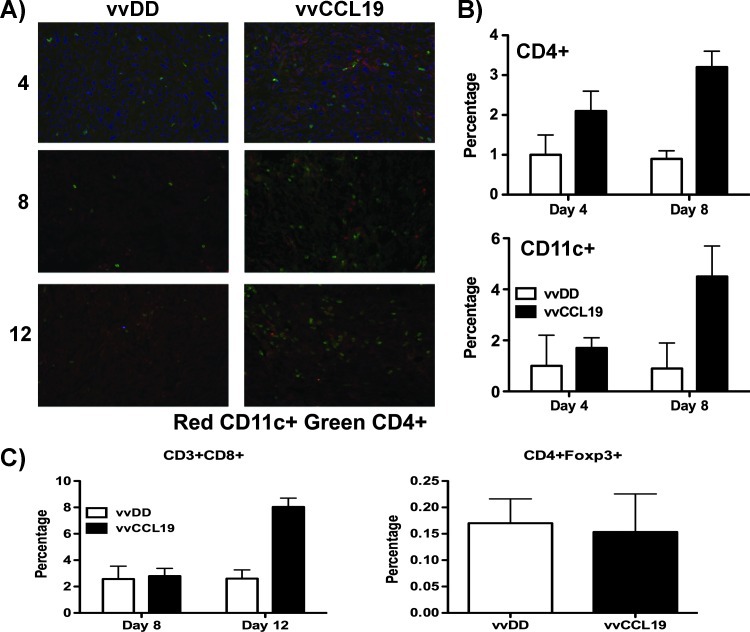
To explore the hypothesis that an altered immune response may be mediating the increased therapeutic benefit seen with vvCCL19, MC38 tumors were recovered from mice at different times after treatment with PBS, vvDD, or vvCCL19. Tumor sections were then stained with antibodies to CD11c, CD4, and CD8, while tumors were also dissociated into single-cell suspensions before staining with different antibodies (Figure 2A) and quantification of tumor-infiltrating cell types by flow cytometry. It was determined (Figure 2) that both CD4+ and CD11c+ cell populations were significantly increased in tumors from mice treated with vvCCL19 relative to any other treatment but not until 8 days after treatment (at a similar time to when the therapeutic advantage of vvCCL19 over vvDD also appears to become significant; Figure 1D and 2B). The level of CD8+ T cells in the tumor did not show any significant differences until even later after initial treatment, with increased levels appearing after vvCCL19 treatment (relative to vvDD) at day 12 only (Figure 2C). This is in line with the delayed induction of an immune response at later times after viral therapy. Other cell types, including NK cells displayed no significant differences between vvDD- and vvCCL19-treated animals at any time. Further, the CD4+ cells selectively attracted to the vvCCL19-treated tumors did not represent regulatory T cells, as there were no differences in the levels of CD4+Foxp3+ cells in the tumors with or without vvCCL19 treatment (Figure 2C). Treatment with vvCCL19 therefore appeared to enhance the infiltration of immune cells into the tumor but not until later times after treatment.
Figure 2.
Immune effects of CCL19 expression. (A) Sections from MC38 tumors treated systemically with the indicated viruses and recovered at the indicated time points (days after treatment). Sections were stained with anti-CD11c antibody (red), anti-CD4+ antibody (green), and 4′6-diamidino-2-phenylindole (DAPI) (nuclei) (original magnification, x200). (B) Tumors recovered as before were also dissociated into single-cell suspensions and stained with either anti-CD4 antibody or anti-CD11c. Percentages of different immune infiltrates were then determined by flow cytometry (n = 4 per group). At day 8, vvCCL19-treated tumors contained significantly more CD3+CD4+ (P = .003) and CD11c+ (P = .017) cells. (C) Samples from day 8 were also stained for regulatory T cells (CD4+FoxP3+) and CD3+CD8+ t cells; neither showed any difference between groups.
vvCCL19 Is Cleared Selectively from Nontumor Tissues but Persists in the Tumor
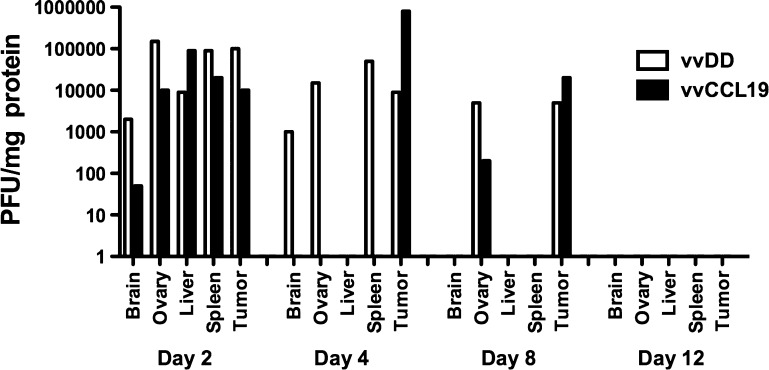
Previously, an oncolytic vaccinia strain expressing the chemokine CCL5 was found to display increased persistence within the tumor after IV delivery [8], while no significant differences were seen in viral load in any other organs. The levels of virus within different tissues were therefore examined after treatment of MC38 tumor-bearing mice with vvDD or vvCCL19. In contrast to vvCCL5, vvCCL19 did not display any significant differences in the viral load within the tumor at any times after systemic delivery relative to vvDD (Figure 3), meaning that CCL19 expression did not result in premature clearance from the tumor, like that seen with expression of many cytokines, or extended persistence like vvCCL5. However, it was noted that vvCCL19 was instead more rapidly cleared selectively from nontumor tissues, with no detectable virus recovered from brain, ovary, liver, or spleen as soon as 4 days after systemic delivery. The tumor was the only tissue from which vvCCL19 could be recovered at this point. In contrast, vvDD was still detectable in multiple nontumor tissues at this time. It therefore appears that expression of CCL19 may confer a safety benefit on oncolytic vaccinia strains, whereas tumor replication of vvCCL19 was apparently unaffected by the increased influx of DCs and CD4+ T cells (presumably as the increased immune cell infiltration did not become significant until 8 days after treatment, at which point virus is being cleared from the tumor anyway).
Figure 3.
Biodistribution and persistence of different viruses after treatment in tumor-bearing mice. C57/BL6 mice bearing subcutaneous MC38 tumors as before were again treated with 1 x 108 PFU of vvDD or vvCCL19 and sacrificed at the indicated times after treatment (n = 3 per group per time). The indicated organs were recovered and tissues were homogenized. Viral titers were determined by plaque assay and normalized to the concentration of protein in the samples.
Increased Cytokine Production in Nontumor Tissues Correlates with Rapid Clearance of vvCCL19
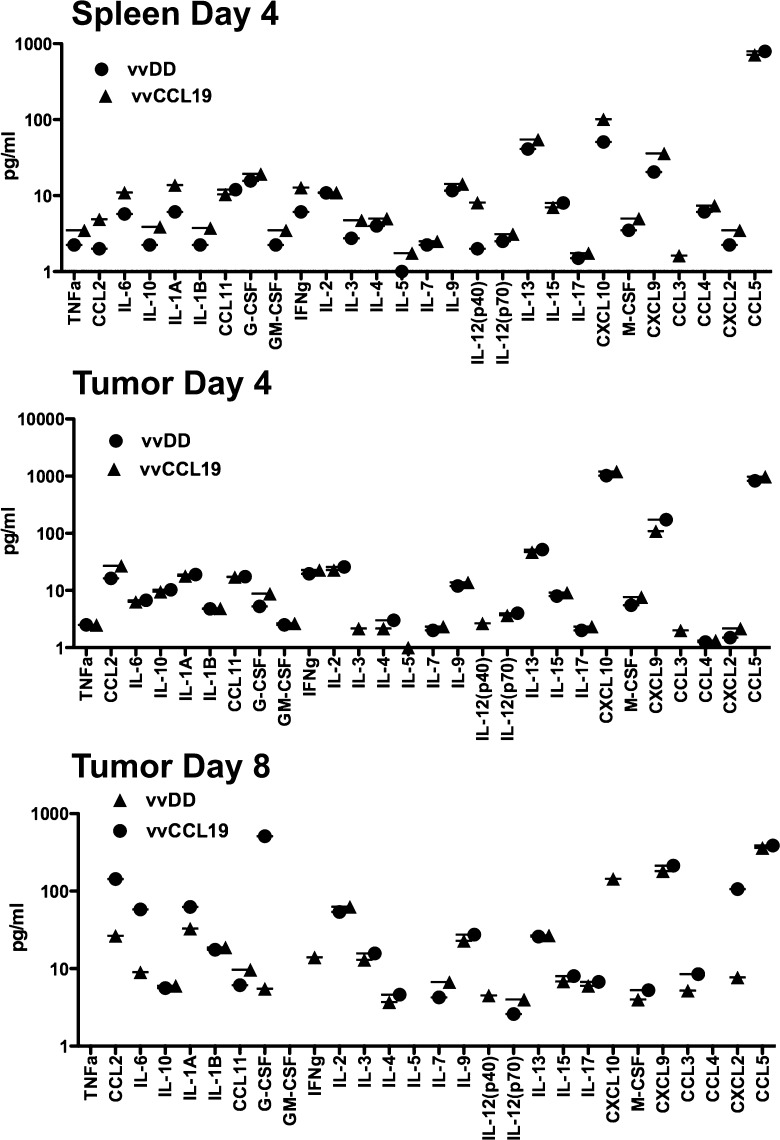
Luminex assay was used to determine the level of different cytokines within the spleen and the tumor at different times after systemic treatment with vvDD or vvCCL19 (Figure 4). It was noted that several cytokines, including IL-12, IFN-γ, and tumor necrosis factor alpha (TNF-α), and IL-1, and several chemokines, including CXCL9 (Mig), CCL2 (MCP-1), and CXCL10 (IP-10), were increased in the spleens from vvCCL19-treated animals relative to vvDD treatment within 4 days of treatment, while minimal differences were observed in the levels of any cytokines in the tumor at this time after treatment with either virus. It therefore appears that CCL19 expression selectively enhances immune activation in nontumor tissues at the earlier time points. Enhanced cytokine production was seen in the tumors of vvCCL19-treated animals at 8 days after treatment however (relative to vvDD treatment), again indicating that the increased immunotherapeutic effect is delayed within the tumor microenvironment and supporting the observation of enhanced immune cell infiltration only at the later times (Figure 2).
Figure 4.
Cytokine production in spleen and tumor after treatment with vvDD or vvCCL19. Mice (C57/BL6 bearing subcutaneous MC38 tumors, n = 5 per group) were treated systemically (IP) with 1 x 108 PFU of the indicated viruses. Mice were sacrificed after 4 and 8 days, and tumor and spleens were recovered for analysis of cytokine production by Luminex assay. Many cytokines are increased in the tumor after vvCCL19 production (relative to vvDD) at day 4, whereas there is little difference in the tumor until day 8.
Combining vvCCL19 with CIK Cell Therapy Significantly Enhances Therapeutic Benefit
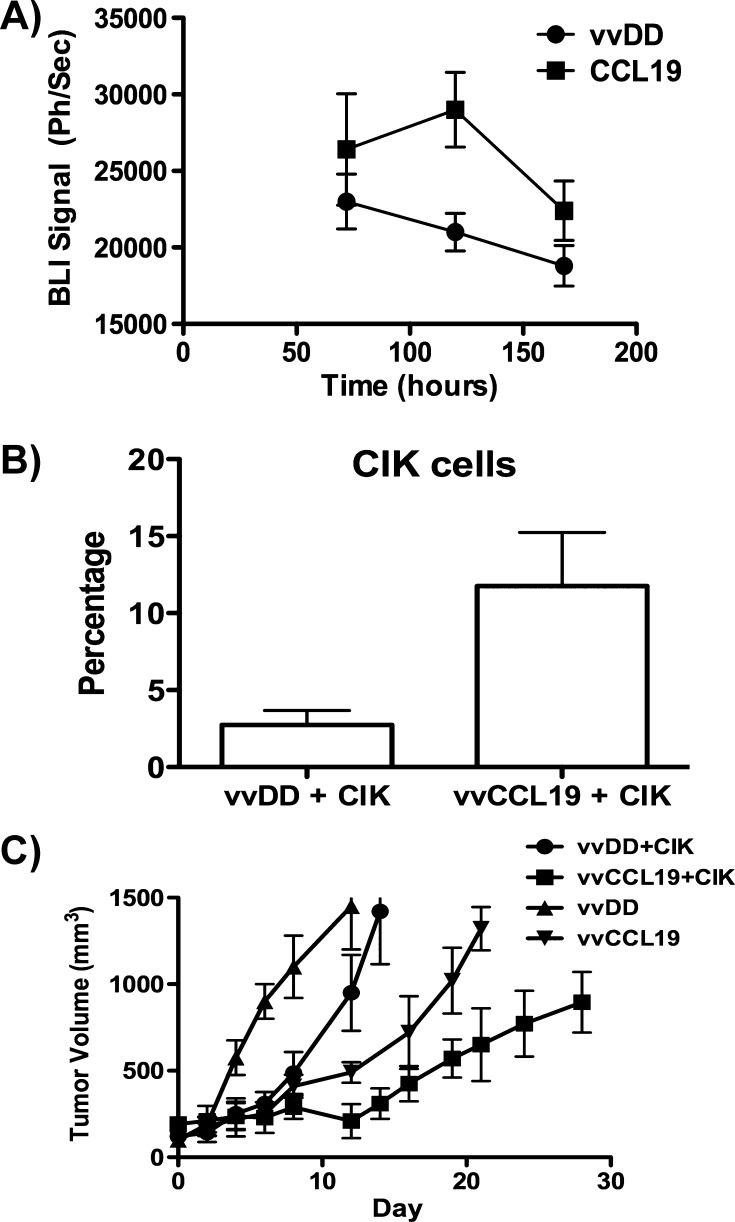
Because vvCCL19 is capable of selectively attracting CIK cells in culture (Figure 1B), it was hypothesized that a combination of vvCCL19 and CIK therapy would represent a potent combination in vivo. In initial experiments, mice were implanted with bilateral tumors, with one flank tumor receiving vvCCL19 via direct intratumoral injection and vvDD injected into the tumor on the opposite flank. IV delivery of CIK cells expressing luciferase combined with bioluminescence imaging determined that the CIK cells trafficked to the tumor treated with virus expressing CCL19 in significantly greater numbers (Figure 5A). In this experiment, the tumor model was switched to the lymphoma cell line 6780 implanted into FVB mice to take advantage of FVB-Luc transgenic mice for expansion of the CIK cells and also to extend the system into a second model.
Figure 5.
(A) Adoptively transferred immune cells preferentially traffic to tumors treated with vaccinia expressing CCL19. Mice (FVB bearing bilateral subcutaneous 6780 tumors on either flank, n = 5) received virus (1 x 108 PFU) via intratumoral injection, with vvDD on one flank and vvCCL19 on the opposite flank 24 hours after injection with CIK cells expressing luciferase (expanded from a transgenic luciferase expressing mouse) via tail vein injection. Subsequent trafficking of CIK cells to each flank tumor was quantified at times after injection by bioluminescence imaging (P < .05 at 72 and 120 hours). (B) vvCCL19 preferentially attracts CIK cells into the tumor. Mice (C57/BL6) with subcutaneous MC38 tumors received IP injection of vvDD or vvCCL19 (1 x 108 PFU, n = 8 per group), followed by 1 x 108 CIK cells prelabeled with cell tracker 72 hours later. After a further 72 hours, mice were sacrifice and tumors were recovered and dissociated, and the percentage of transferred CIK cells in the tumor was determined by flow cytometry (P > .01). (C) vvCCL19 in combination with CIK cells displayed enhanced therapeutic benefit. Mice (C57/BL6) with subcutaneous MC38 tumors received IP injection of vvDD or vvCCL19 (1 x 108 PFU, n = 8 per group), followed by 1 x 108 CIK cells or PBS 72 hours later. Antitumor effect was followed by caliper measurement (P < .05 at all times after day 10 for vvCCL19 and CIK group compared to all other groups.)
Furthermore, a comparison of treatment with vvDD (IP) followed by CIK therapy (IV) 3 days later relative to equivalent treatment with vvCCL19 followed by CIK therapy again determined a greater infiltration of CIK cells into the tumor in combination with vvCCL19 (Figure 5B) and a very significant benefit for the use of CCL19-expressing virus (Figure 5C). vvCCL19 therefore confers a therapeutic advantage both when used alone and when used in combination with adoptive immune cell transfer therapies expressing CCR7.
Discussion
Despite the promising clinical data reported with different oncolytic vaccinia strains, further advances to increase safety, specificity, and efficacy are still needed before regular complete responses are seen. One area that has gained much attention recently has involved approaches that enhance the immunotherapeutic potential of the viruses. This is most commonly achieved through expression of cytokines as transgenes from the viral vectors, and both the herpes simplex virus (HSV) and vaccinia oncolytic strains currently undergoing randomized clinical testing express the cytokine granulocyte macrophage-colony stimulating factor (GM-CSF) [4]. Other strains expressing cytokines including IFN [14] and TNF [7] have also displayed promising results in a preclinical setting. However, it is notable that although cytokine expression frequently increases the overall therapeutic effects of the viral vectors, this often comes at the cost of reduced oncolytic activity, with less viral replication within the tumor and earlier immune-mediated clearance of the therapy [7]. In addition, although cytokine expression can increase the safety of the viral vectors (through rapid clearance from nontumor tissues) [14], there have also been reports of toxicity resulting from systemic cytokine release, with some systemic cytokine expression from oncolytic viruses resulting in acute toxicity early after IV delivery (Thorne, in press, Molecular Therapy 2012).
As an alternative but complimentary approach, we have explored the effects of expressing chemokines from the oncolytic viruses. Because chemokines act to direct trafficking of immune cells, they have the potential to overcome one of the major limitations of immunotherapy of cancer, an inability to target effector cells into the tumor. Further, because chemokines primarily direct rather than activate the immune response, their effects will likely be more subtle, meaning that their expression may not act to directly inhibit viral replication and oncolytic effects and may result in improved safety. We previously reported on an oncolytic vaccinia virus expressing the chemokine CCL5 [8] that displayed an unexpected capacity to persist within the tumor for extended periods of time.
It was therefore decided to investigate the therapeutic potential of expression of the chemokine CCL19, whose interaction with its cognate receptor (CCR7) is more typically associated with recirculation of mature DCs and T cells into lymph nodes. The receptor for CCL19 (CCR7) is also expressed on many immune cell therapies (including CIK cells) and on T cells produced as a result of vaccination [15,16], meaning that it is likely to synergize with these immunotherapy approaches. Vaccinia oncolytic strain vvDD was therefore constructed expressing murine CCL19 (vvCCL19). Initial testing confirmed that functional CCL19 was secreted from infected cells and that this attracted cells expressing CCR7. In addition, CCL19 expression did not affect viral replication in vitro. It was also determined that CCL19 expression was sufficient to impart a therapeutic advantage on the oncolytic virus when applied in a mouse tumor model.
Analysis of the effects of CCL19 expression on immune cell infiltrate into the tumor and on viral persistence and biodistribution confirmed key differences in the use of chemokine expression relative to what is typically seen with most cytokine expression; CCL19 expression both enhanced the immunotherapeutic effects and the safety of the vector, yet did not result in a reduction in viral oncolytic potential. In particular, CCL19 expression resulted in increased infiltration of a variety of immune cells into the tumor, including DC and CD4+ and CD8+ T cells (but not NK cells). However, CCL19 expression did not result in increased viral persistence in the tumor or premature viral clearance from the tumor; instead, virus was selectively cleared more rapidly from nontumor tissues (including spleen, brain, and ovary). It therefore appears that CCL19 expression may 1) act to increase immunotherapeutic effects without decreasing oncolytic activity and 2) may enhance the safety of the viral therapy through rapid clearance from nontumor tissues. However, further investigation is still needed, as other factors in addition to the increased immune infiltrate may mediate the enhanced therapeutic effects. In addition, at the doses tested, vvCCL19 displayed only a slight but not significant benefit in reducing weight loss (a surrogate marker for viral pathogenicity) after treatment relative to vvDD (data not shown). It is likely however that more significant safety benefits would be seen at higher doses, meaning that it may be possible to apply vvCCL19 at higher doses in the clinic without encountering toxicity in other organs.
Although insignificant differences in immune cell infiltrate and cytokine levels in the tumors of vvCCL19-treated animals were seen as soon as 4 days after treatment (compared to vvDD-treated animals), large and significant differences were not seen in the tumor until 8 days after the treatments. This is also the last time point at which virus (vvDD or vvCCL19) was detected in the tumor environment. It therefore appears that the immunotherapeutic effects of CCL19 expression are not significantly felt in the tumor until around 8 days after treatment. This also coincided with the time at which vvCCL19 began to demonstrate significant therapeutic advantages over vvDD. This raises the possibility that over an initial “oncolytic phase” (the first 8 days after treatment), vvDD and vvCCL19 act in a similar way within the tumor. However, by 8 days after treatment, when the viral therapies are being cleared, CCL19 expression acts to enhance the immune infiltrate arriving within the tumor and so imparts an immunotherapeutic advantage on the mice treated with vvCCL19 that continues after the virus has been cleared.
In contrast to the situation in the tumor, in the spleen many cytokines were increased within 4 days of treatment with vvCCL19 relative to vvDD. This correlated with the more rapid clearance of vvCCL19 from nontumor tissues at this time. It therefore appears that vvCCL19 may be capable of inducing a more rapid immune response in non-tumor tissues, leading to its early clearance from these organs, while the immune response in the tumor may be delayed.
It is unclear why the effects of CCL19 expression are not felt in the tumor until 8 days after treatment (whereas they are clear by 4 days in other organs), but this may be indicative of the immune suppressive microenvironment in the tumor, meaning that either longer term or higher expression is needed for effect or that the first cells attracted into the tumor become subverted and that other adjuvant immune signals (such as those released after viral lysis of infected cells) are needed before immune activation is achieved.
It is also of note that after 8 days both vvDD and vvCL19 are cleared from the tumor, meaning that the period of oncolytic activity is limited. However, the additional immune activation of vvCCL19 may allow for long-term immune activation and targeting of tumor antigens, so allowing an ongoing therapeutic benefit. Although several strategies have been incorporated to increase the period of active viral replication in the tumor, these typically involve immune suppression and have little overall therapeutic benefit.
Although significant, the therapeutic advantage of vvCCL19 is not dramatic relative to vvDD. However, another advantage of chemokine expression from oncolytic viral therapies is the potential for synergy during combination treatment with vaccine or immune cell therapies. This was explored through combination with a model of adoptive T cell therapy, using CIK cells. CIK cells were found to express the CCR7 receptor on at least a subset of the population and displayed increased trafficking to vvCCL19-infected tumor cells both in vitro and in vivo. Further, when vvCCL19 was used in combination with CIK cells in mouse colorectal cancer models, it produced a far more significant and durable therapeutic response (relative to treatment with a vvDD and CIK combination).
It therefore appears that CCL19 expression from an oncolytic vaccinia vector imparts multiple advantages, including enhanced safety (more rapid clearance from nontumor tissues), enhanced immunotherapeutic effect (increased immune infiltration into the tumor), and maintenance of oncolytic activity (no premature clearance of the virus), as well as the capacity to synergize with immune cell or vaccine-based therapies. This resulted in enhanced therapeutic benefit, both for virus used alone and particularly when used in combination with adoptive transfer of CCR7 expressing therapeutic immune cells. These promising preclinical data warrant clinical testing of this vector.
Supplementary Material
Footnotes
This work was supported directly by the National Institutes of Health (grant Nos. R01 CA140215 and P01 CA132714; in addition, core facilities used in this work were supported by P30 CA047904-21S3).
This article refers to supplementary material, which is designated by Figure W1 and is available online at www.neoplasia.com.
References
- 1.Kaufman HL, Kim DW, DeRaffele G, Mitcham J, Coffin RS, Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 2.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 3.Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, Oh SY, Han SY, Yoon JH, Hong SH, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt C. Amgen spikes interest in live virus vaccines for hard-to-treat cancers. Nat Biotechnol. 2011;29:295–296. doi: 10.1038/nbt0411-295. [DOI] [PubMed] [Google Scholar]
- 5.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 6.Thorne SH. Immunotherapeutic potential of oncolytic vaccinia virus. Immunol Res. 2011;50:286–293. doi: 10.1007/s12026-011-8211-4. [DOI] [PubMed] [Google Scholar]
- 7.Banaszynski LA, Sellmyer MA, Contag CH, Wandless TJ, Thorne SH. Chemical control of protein stability and function in living mice. Nat Med. 2008;14:1123–1127. doi: 10.1038/nm.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, O'Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, Thorne SH, Bartlett DL. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther. 2011;19:650–657. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of t cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 10.McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, Moss B, Bartlett DL. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 11.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 12.Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, Brown C, Werier J, Cho JH, Lee DE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 14.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao X, Bose A, Komita H, Taylor JL, Kawabe M, Chi N, Spokas L, Lowe DB, Goldbach C, Alber S, et al. Intratumoral IL-12 gene therapy results in the crosspriming of Tc1 cells reactive against tumor-associated stromal antigens. Mol Ther. 2011;19:805–814. doi: 10.1038/mt.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.