Abstract
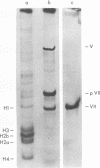
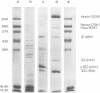
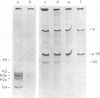
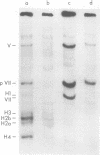
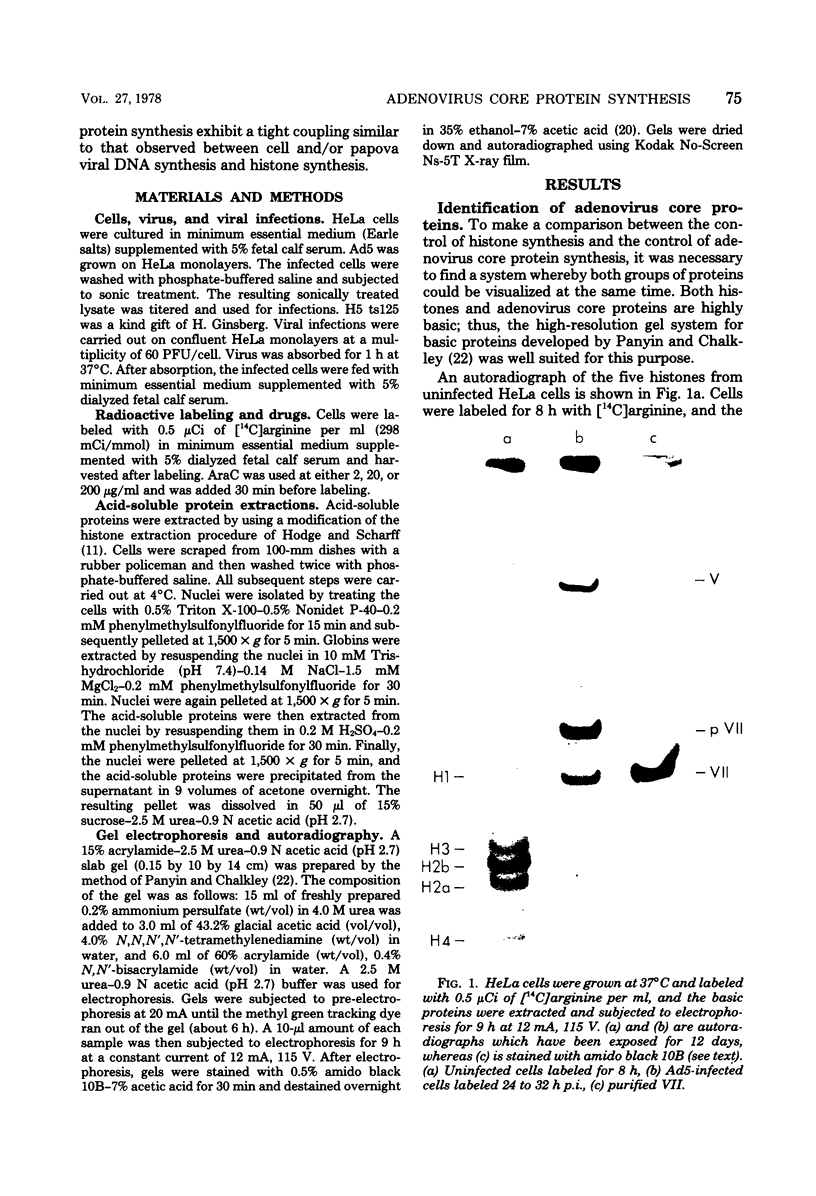
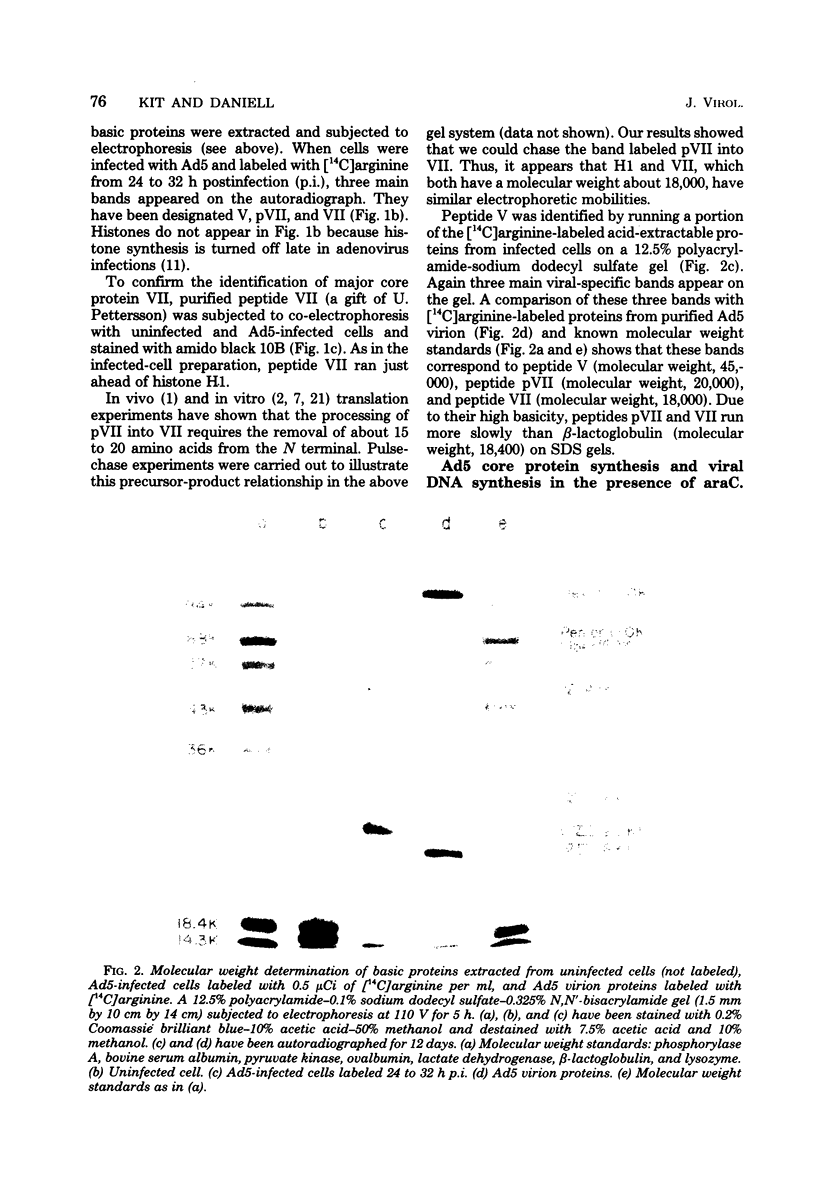
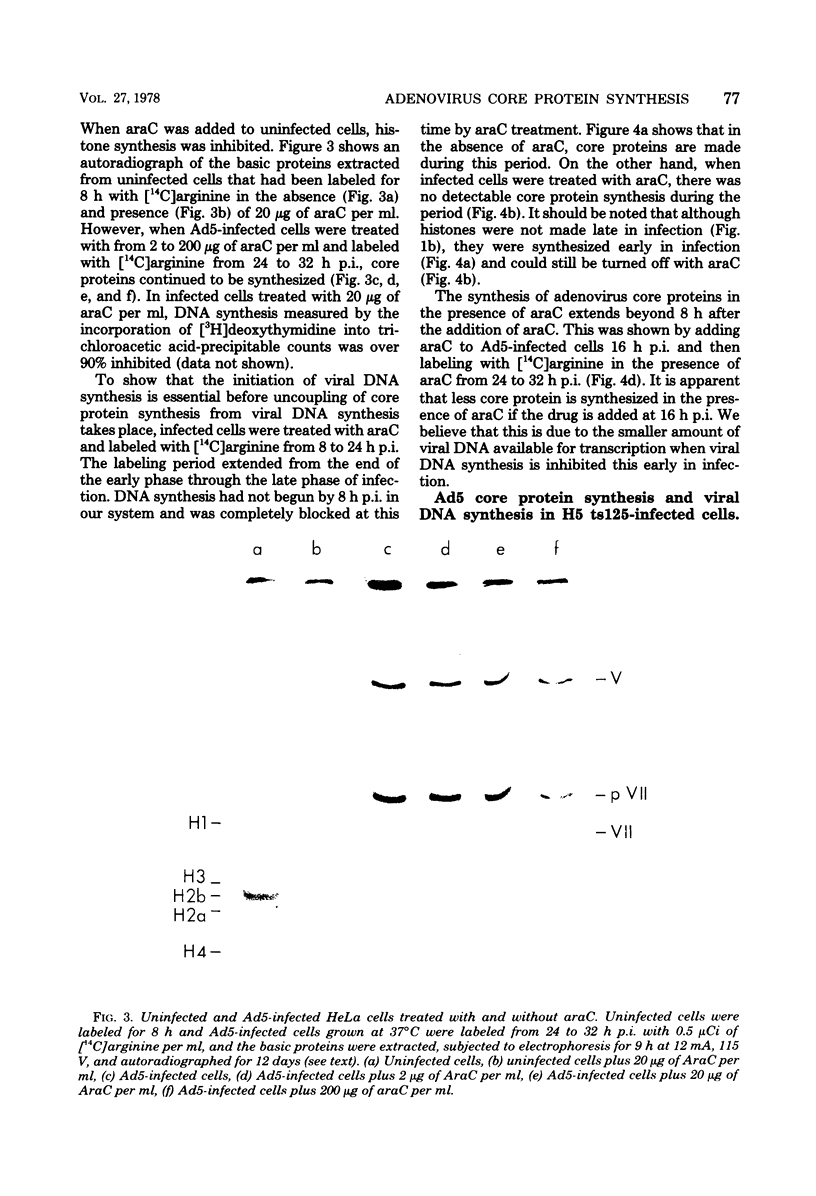
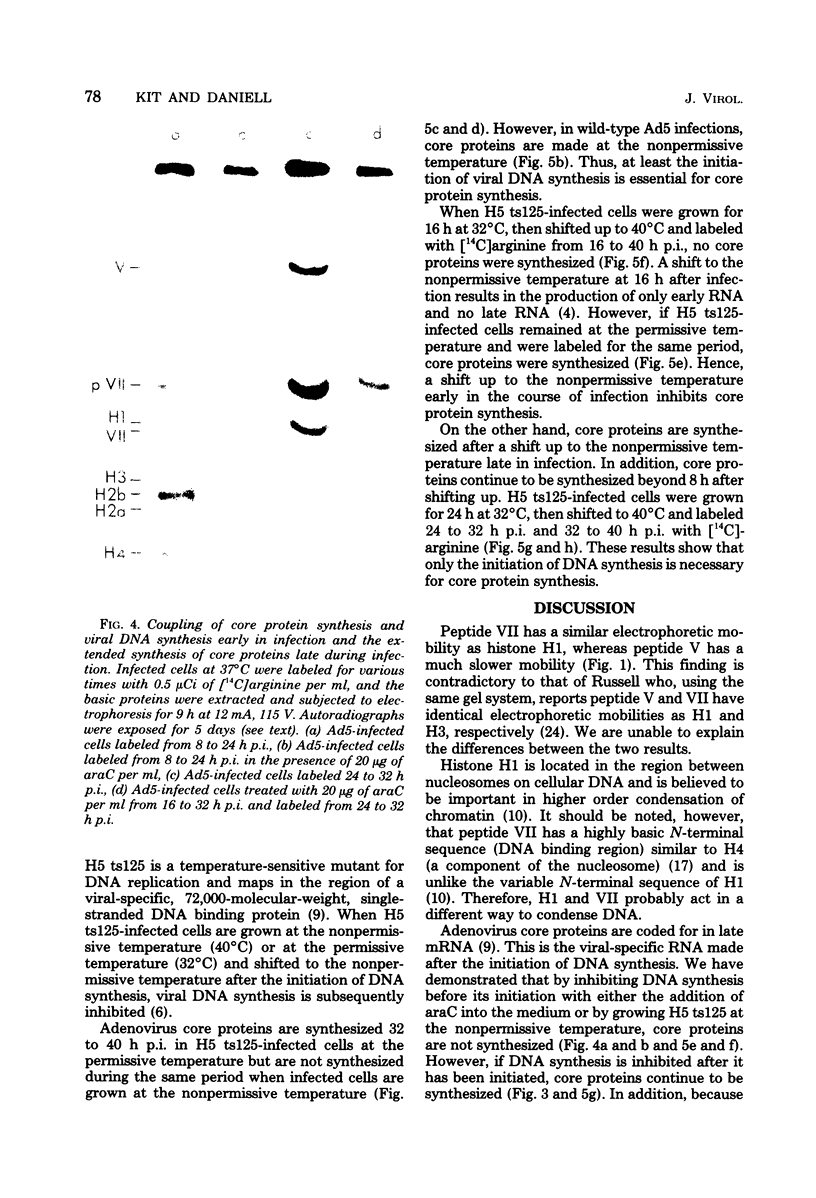
The acid extraction of the adenovirus type 5 core proteins V, VII, and pVII (the precursor to VII) from infected cells and the subsequent electrophoresis on a 15% acrylamide-2.5 M urea-0.9 N acetic acid (pH 2.7) gel, revealed that peptide VII has a similar electrophoretic mobility to that of histone H1. The core proteins, which are coded by late adenovirus mRNA, continued to be synthesized late in infection when viral DNA synthesis was inhibited either by cytosine arabinoside in wild-type infections or by shifting adenovirus H5 ts 125-infected cells to the nonpermissive temperature (40 degree C). Only the initiation, not the continuation, of viral DNA replication was essential for core protein synthesis. The synthesis of viral core proteins continued for over 8 h after the cassation of DNA synthesis. This was in contrast to the rapid shutdown of cellular histone synthesis in the absence of cellular DNA synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W., Lewis J. B., Atkins J. F., Gesteland R. F. Cell-free synthesis of adenovirus 2 proteins programmed by fractionated messenger RNA: a comparison of polypeptide products and messenger RNA lengths. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2756–2760. doi: 10.1073/pnas.71.7.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT L. L., Jr, SMITHERS D., WARD C. T. INHIBITION OF DNA SYNTHESIS IN MAMMALIAN CELLS BY ACTIDIONE. Biochim Biophys Acta. 1964 May 18;87:60–69. doi: 10.1016/0926-6550(64)90047-7. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Flint S. J., Williams J. F., Sharp P. A. Adenovirus transcription. IV. Synthesis of viral-specific RNA in human cells infected with temperature-sensitive mutants of adenovirus 5. J Virol. 1976 Sep;19(3):879–889. doi: 10.1128/jvi.19.3.879-889.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Ginsberg H. S. Viral transcription in KB cells infected by temperature-sensitive "early" mutants of adenovirus type 5. J Virol. 1976 Apr;18(1):156–166. doi: 10.1128/jvi.18.1.156-166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Wesphal H., Callahan R. In vitro synthesis of adenovirus core proteins. J Virol. 1974 Aug;14(2):375–383. doi: 10.1128/jvi.14.2.375-383.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Davoli D. Molecular biology of papovaviruses. Annu Rev Biochem. 1977;46:471–522. doi: 10.1146/annurev.bi.46.070177.002351. [DOI] [PubMed] [Google Scholar]
- Flint J. The topography and transcription of the adenovirus genome. Cell. 1977 Feb;10(2):153–166. doi: 10.1016/0092-8674(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Hartman P. G., Chapman G. E., Moss T., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur J Biochem. 1977 Jul 1;77(1):45–51. doi: 10.1111/j.1432-1033.1977.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Hodge L. D., Scharff M. D. Effect of adenovirus on host cell DNA synthesis in synchronized cells. Virology. 1969 Apr;37(4):554–564. doi: 10.1016/0042-6822(69)90273-6. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S., Brayton C., Baum S. G. Synthesis of type 2 adenovirus DNA in the presence of cycloheximide. J Virol. 1973 Apr;11(4):544–551. doi: 10.1128/jvi.11.4.544-551.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. S., Eshbach T. B., White D. A., Levine A. J. Deoxyribonucleic acid replication in simian virus 40-infected cells. IV. Two different requirements for protein synthesis during simian virus 40 deoxyribonucleic acid replication. J Virol. 1971 Jan;7(1):112–120. doi: 10.1128/jvi.7.1.112-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H. Histone messengers and histone genes. Cell. 1976 Jul;8(3):321–331. doi: 10.1016/0092-8674(76)90144-6. [DOI] [PubMed] [Google Scholar]
- Kit S., Kurimura T., De Torres R. A., Dubbs D. R. Simian virus 40 deoxyribonucleic acid replication. I. Effect of cycloheximide on the replication of SV40 deoxyribonucleic acid in monkey kidney cells and in heterokaryons of SV40-transformed and susceptible cells. J Virol. 1969 Jan;3(1):25–32. doi: 10.1128/jvi.3.1.25-32.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Sung M. T. A histone-like protein from adenovirus chromatin. Nature. 1977 Jun 9;267(5611):552–554. doi: 10.1038/267552a0. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Moll R., Wintersberger E. Synthesis of yeast histones in the cell cycle. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1863–1867. doi: 10.1073/pnas.73.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B., Saborio J., Persson T., Everitt E., Philipson L. Identification of the in vitro translation products of adenovirus mRNA by immunoprecipitation. J Virol. 1975 Jan;15(1):199–207. doi: 10.1128/jvi.15.1.199-207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- Russell W. C. The acid-soluble components of adenovirus and of adenovirus-infected cells. J Gen Virol. 1971 Apr;11(1):65–69. doi: 10.1099/0022-1317-11-1-65. [DOI] [PubMed] [Google Scholar]
- Seehafer J. G., Weil R. Synthesis of polyoma virus structural polypeptides in mouse kidney cells. Virology. 1974 Mar;58(1):75–85. doi: 10.1016/0042-6822(74)90142-1. [DOI] [PubMed] [Google Scholar]
- Stein G., Stein J., Shephard E., Park W., Phillips I. Evidence that the coupling of histone gene expression and DNA synthesis in HeLa S3 cells is not mediated at the transscriptional level. Biochem Biophys Res Commun. 1977 Jul 11;77(1):245–252. doi: 10.1016/s0006-291x(77)80189-7. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Robbins E. Histone synthesis in polyoma- and SV40-infected cells. Virology. 1970 Feb;40(2):307–315. doi: 10.1016/0042-6822(70)90406-x. [DOI] [PubMed] [Google Scholar]
- Young C. W. Inhibitory effects of acetoxycycloheximide, puromycin, and pactamycin upon synthesis of protein and DNA in asynchronous populations of HeLa cells. Mol Pharmacol. 1966 Jan;2(1):50–55. [PubMed] [Google Scholar]