Abstract
Mindfulness meditation has been shown to promote emotional stability. Moreover, during the processing of aversive and self-referential stimuli, mindful awareness is associated with reduced medial prefrontal cortex (MPFC) activity, a central default mode network (DMN) component. However, it remains unclear whether mindfulness practice influences functional connectivity between DMN regions and, if so, whether such impact persists beyond a state of meditation. Consequently, this study examined the effect of extensive mindfulness training on functional connectivity within the DMN during a restful state. Resting-state data were collected from 13 experienced meditators (with over 1000 h of training) and 11 beginner meditators (with no prior experience, trained for 1 week before the study) using functional magnetic resonance imaging (fMRI). Pairwise correlations and partial correlations were computed between DMN seed regions’ time courses and were compared between groups utilizing a Bayesian sampling scheme. Relative to beginners, experienced meditators had weaker functional connectivity between DMN regions involved in self-referential processing and emotional appraisal. In addition, experienced meditators had increased connectivity between certain DMN regions (e.g. dorso-medial PFC and right inferior parietal lobule), compared to beginner meditators. These findings suggest that meditation training leads to functional connectivity changes between core DMN regions possibly reflecting strengthened present-moment awareness.
Keywords: mindfulness meditation, functional connectivity, default mode network, prefrontal cortex, resting state
INTRODUCTION
Originating from Ancient Eastern traditions, meditation has become increasingly studied with brain mapping methods. Particularly, there is evidence that mindfulness meditation is beneficial for the treatment of psychological disorders involving emotional dysregulation, such as major depressive disorder (MDD) and anxiety disorders (Baer, 2003). Mindfulness promotes an objective manner of interpreting thoughts, events and emotions, without elaborating or ‘ruminating’ on their potential implications for the self (Bishop, 2004).
Mindfulness has been shown to diminish activity in the medial prefrontal cortex (MPFC; Farb et al., 2007), a cortical area playing a pivotal role in self-referential processing and the ‘default mode network’ (DMN) (Gusnard et al., 2001). Indeed, Farb et al. (2007) recently showed that an experiential focus condition, involving the mindful monitoring of present-moment circumstances, was associated with decreased MPFC function (dorso-medial PFC [DMPFC], ventro-medial PFC [VMPFC]), compared with a narrative focus condition (i.e. monitoring self-descriptive traits). These researchers also found that the MPFC deactivations associated with experiential focus were more pronounced in participants having received an 8-week mindfulness-based stress-reduction (MBSR) program, relative to a wait-listed control group.
There is also evidence supporting the view that mindfulness training leads to MPFC deactivations during the processing of aversive stimuli, such as painful stimulations (Grant et al., 2011). Moreover, individuals with MDD fail to deactivate the MPFC (and other cerebral structures in the DMN) while passively looking at negative pictures or trying to reappraise them (Sheline et al., 2009). These findings may reflect a failure to down-regulate DMN regions involved in monitoring of internal emotional states, self-referential processing and rumination.
Other core regions in the DMN—which are consistently deactivated during goal-directed tasks and activated during a restful state—include the inferior parietal lobule (IPL), the precuneus (PC), the posterior cingulate cortex (PCC), as well as the inferolateral temporal cortex (ITC) (Gusnard et al., 2001; Raichle et al., 2001; Greicius et al., 2003; Buckner et al., 2008). These brain regions are thought to operate as a coherent network, as they exhibit synchronized low-frequency blood-oxygen-level-dependent (BOLD) signal fluctuations during ‘resting states’, i.e. when participants are scanned for several minutes and are instructed to rest without engaging in any specific mental activity or task (Biswal et al., 1995; Beckmann and Smith, 2004; Damoiseaux et al., 2006; De Luca et al., 2006; Fransson and Marrelec, 2008). DMN activity has been proposed to be associated with cognitive processes such as envisioning future scenarios, theory of mind, autobiographical memory, moral decision making and self-referential processing (Gusnard et al., 2001; Northoff et al., 2006; Buckner et al., 2008). It therefore appears that this network may underlie adaptive planning and reflection mechanisms when not engaged in any external activity (Buckner et al., 2008).
Examining the relationship between meditation training and functional connectivity within the DMN during rest is important to determine whether the effects of such training extend beyond a meditative state. Functional connectivity within the DMN during a state of rest has been examined between an inexperienced control group and experienced meditation practitioners of a specific form of meditation called ‘Brain-wave vibration meditation’ (a kind of moving meditation that is designed to help quiet the thinking mind and to release negative emotions through performing natural rhythmic movements and focusing on bodily sensations (Jang et al., 2011)) and an inexperienced control group. Their results revealed that meditation practitioners had increased functional connectivity within the DMN in the MPFC relative to controls (Jang et al., 2011). Nevertheless, the results from Jang et al. (2011) were obtained using seed-based functional connectivity analyses with anatomically pre-determined seed locations. To date, however, the relationship between mindfulness meditation training and connectivity within DMN regions has not been examined using data-driven independent component analysis techniques to identify intrinsic connectivity network maps at the group level, and then examining group differences in pairwise connections between regions of the identified network. In this context, the aim of this functional magnetic resonance imaging (fMRI) study was to investigate the impact of extensive mindfulness training on functional connectivity between regions of the DMN during a restful state. Spatial independent component analysis was used to identify a DMN map at the group level and determine seed regions. Pairwise correlations and partial correlations were then computed between the time courses of these DMN seed regions. The various correlations between all pairs of nodes were then compared between individuals highly experienced in meditation (with over 1000 h of experience in mindfulness-based meditation) relative to beginner meditators (with no prior exposure to mindfulness meditation, trained for one week before the completion of the study). Predicated on evidence that mindfulness is associated with deactivation of MPFC during self-referential processing (Farb et al., 2007), it was hypothesized that functional connectivity between medial prefrontal cortical areas and other DMN regions would be weaker in experienced relative to beginner meditators.
MATERIALS AND METHODS
Participants
The sample consisted of two groups. The first group was composed of 13 (six males) experienced meditators (age: M = 46 years, s.d. = 11) with over 1000 h of meditation experience (M = 6519, s.d. = 14 445). They were recruited from Zen meditation centres in the Montreal metropolitan area. One participant deviated from the group in terms of the number of hours of meditation (45 000 h of practice), and hence constituted an outlier from the rest of the group. Without the inclusion of this participant, the group of experienced meditators had on average 1709 h of meditation practice (s.d. = 694). Consequently, the analyses reported in the present study were also conducted with the exclusion of this participant. Since the results remained essentially unchanged, this participant was kept in the analyses to avoid losing any statistical power by decreasing the sample size. All experienced meditators reported that the core approach of their regular meditation practice consisted in the mindfulness practice technique.
The second group was composed of 11 beginner meditators (seven males), with an average of 37 years of age (s.d. = 13) recruited with the use of advertizement posters placed at the Université de Montréal and the Centre de Recherche de l'Institut Gériatrique de Montréal (CRIUGM). These individuals had no prior exposure to meditation (or other practices such as yoga) and were trained for one week before the completion of the study. Implemented by the experimenters, the mindfulness training was documented from several sources (Kabat-Zinn, 1994; Thich Nhat Hanh, 1994; Ricard, 2008) and consisted of a guided meditation session recorded on a compact disc (a written record was also provided). Participants were instructed to meditate for 20 min each day for 7 days. The experimenters followed-up throughout the training week to ensure that participants completed their practice, and they all confirmed to have understood and successfully completed their training (except for one participant who had 2 days of training due to scheduling constraints, but confirmed having practiced the compensating number of hours). There were no significant differences in the male-to-female ratio or age between the group of experienced meditators and the group of beginners. Participant characteristics are presented in Table 1.
Table 1.
Participant characteristics
| Experienced meditators | Beginners | |
|---|---|---|
| Age (years) | 46 ± 11 years | 37 ± 13 years |
| Gender | 6 M; 7 F | 7 M; 4 F |
| Education | 12 undergraduate university degree or higher; 1 high school education | 9 undergraduate university degree or higher; 2 high school education |
| Ethnicity | 11 Caucasian; 1 Asian; 1 multi-racial (African and European descent) | 9 Caucasian; 2 multi-racial (African and European descent) |
Before being selected to participate in the study, all participants underwent telephone screening, and were excluded if they had a current or past mental health illness, took any psychotropic drugs, were suffering from major physical health problems or were not eligible to undergo an MRI exam (pregnancy, metal parts in body, pacemaker, etc.). After the completion of the study, participants were compensated 50$ for their time. This research project was approved by the Ethics Research Committee of the CRIUGM.
fMRI data acquisition
Whole-brain T2*-weighted functional images were acquired from a 3 T scanner (Siemens, Erlanger, Germany) using a two-dimensional echo-planar imaging pulse sequence (TR = 2500 ms, TE = 40 ms, voxel size = 3 × 3 × 3.5 mm, 35 contiguous axial slices, no gap between slice acquisition, matrix size = 64 × 64, flip angle = 90°). A high-resolution T1-weighted anatomical scan was also acquired for each subject (three-dimensional, spoiled gradient echo sequence, TR = 19 ms, TE = 4.92 ms, flip angle = 25°, matrix size = 256 × 256 voxels voxel size = 1 × 1 × 1 mm, 176 contiguous axial slices).
Experimental protocol
Participants first gave informed consent and underwent screening and questionnaires related to MRI safety and eligibility. Then, to obtain sufficient power for the functional connectivity analyses given the relatively small sample size, each participant completed two functional 6-min runs (144 volumes) in a state of rest (except for two participants from each group who completed only one run, due to time or testing constraints). Throughout these runs, participants observed a cross fixated centrally on the screen inside the scanner, and were instructed to rest, without engaging in any specific task or mental activity. In order not to induce boredom or other carry-over effects by performing both resting state sessions consecutively, and given that connectivity within the default mode network has been shown to be consistently reproducible across time (Shehzad et al., 2009; Meindl et al., 2010; Zuo et al., 2010), resting state sessions were performed at the beginning and at the end of the experimental paradigm. In between these two sessions, participants also completed sessions consisting of different experimental paradigms; these data are not reported here. At the end of the experiment, participants were compensated for their time.
Data analysis
The fMRI data were pre-processed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK). For each subject, functional images were slice-time corrected, realigned and spatially smoothed using a Gaussian kernel (8 mm at full-width at half-maximum). Next, functional connectivity analyses were conducted using the NetBrainWork software (https://sites.google.com/site/netbrainwork/, Laboratoire d'Imagerie Fonctionnelle, Paris, France). To identify functional network maps across participants, the NEDICA (for Network Detection using Independent Component Analysis; Perlbarg et al., 2008) approach was employed. First, as previously validated (Esposito et al., 2005) the data for each run were reduced to 40 temporal dimensions using principal component analysis (PCA). Next, 40 spatially independent components were extracted from each run using the infomax algorithm (Bell and Sejnowski, 1995). Independent component (IC) maps were then converted into z-maps and normalized into MNI standard stereotaxic space.
After the ICs were extracted at the individual level for each run, a hierarchical clustering algorithm (Marrelec et al., 2008) was used to gather all ICs from all runs across both groups of participants into clusters or ‘classes’ based on their spatial similarity, the distance between two ICs being taken as their spatial correlation. The number of classes was calculated automatically by NetBrainWork as a way to optimize both the degree of representativity (DR; number of runs contributing to the class divided by the total number of runs) and the degree of Unicity (DU; number of runs contributing to class with only one IC, divided by the total number of runs). As these scores should ideally be equal to 1, only classes with DR > 0.5 and DU > 0.75 were retained (Perlbarg et al., 2008).
Then, fixed effect analyses were conducted to compute t-maps for each class: at each voxel, the mean value of each IC contributing to the class was divided by the variance of each IC contributing to the class. The resulting t-maps were thresholded at P < 0.05 corrected at the false discovery rate (FDR). Finally, a bootstrap procedure was conducted to assess the confidence interval of each class retained. To do this, NEDICA was reapplied on half of the runs (randomly selected), yielding new group maps. The spatial correlation between each initial group map and new group map was calculated. The initial map with the highest correlation coefficient with its new bootstrap map was retained (except if the correlation value was below 0.30). As a result of this bootstrap procedure, which was repeated 100 times, a number of t-maps for the classes of interest were retained. The remaining classes represented functionally coherent brain networks across the entire sample.
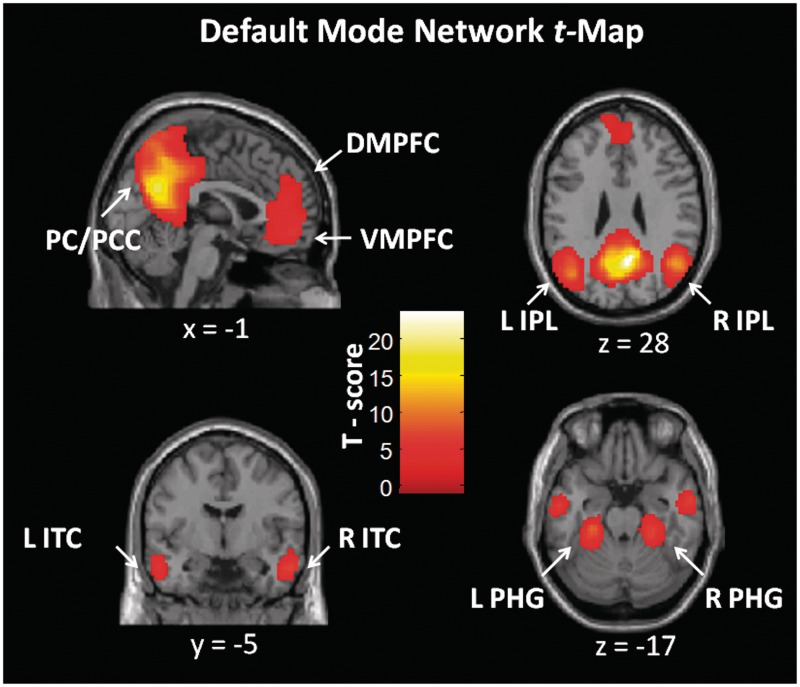
Next, each network map was manually inspected. Based on previous reports (Buckner et al., 2008; Perlbarg et al., 2008), the map which best corresponded to the DMN was selected for the functional connectivity analyses. Regions of interest (ROIs) were selected based on the peak voxels identified in the DMN t-map. Each region selected was composed of 10 voxels, delimited by a region-growing algorithm (Bellec et al., 2006) from the given peak and was located at least 30 mm apart from another ROI. Similarly to previous studies (Fransson and Marrelec, 2008; Perlbarg et al., 2008), the network comprised nine nodes: the PC / PCC, the VMPFC, the DMPFC, the left and right IPL, the left and right ITC and the left and right parahippocampal gyrus (PHG). Regions of the DMN selected for the analyses are shown in Figure 1 and the coordinates are shown in Table 1.
Fig. 1.
Default mode network t-map identified at the group level using NEDICA across all participants. Peaks revealed in the t-map were chosen as seed regions for the functional connectivity analyses, to compare connectivity between regions of the network for experienced relative to beginner meditators. PCC = posterior cingulate cortex; PC = precuneus; DMPFC = dorso-medial prefrontal cortex; VMPFC = ventro-medial prefrontal cortex; IPL = inferior parietal lobule; ITC = inferolateral temporal cortex; PHG = parahippocampal gyrus; R = right; L = left.
The procedure to identify group maps using NEDICA was performed within each group, and the peak locations of all seed regions were very similarly located. As participants were all healthy individuals and did not significantly differ with respect to age, it seemed more appropriate to identify DMN regions based on the group map computed across the entire sample (for both experienced and beginner meditators). This approach was also considered best suited for the present study given that our main interest was to examine functional connectivity between seed regions of the DMN.
After DMN seed regions were identified, CORrection of Structured noise using spatial Independent Component Analysis (CORSICA; Perlbarg et al., 2007) was applied to remove components related to physiological noise. Then, pairwise correlations between the time-course of each seed regions were calculated within the group of experienced meditators, and within the group of beginner meditators, using the correlation coefficient cc (Biswal et al., 1995). Significant correlations between groups were evaluated based on a Bayesian sampling scheme (Marrelec et al., 2006, 2008). The probability threshold was set at P = 0.95.
Finally, in the same manner, partial correlations were also computed, using the partial coefficient denoted by Π, as previously described by Marrelec et al. (2006). The partial correlation between two regions has the advantage of reflecting the covariation between the time series of these two regions after removal of the part of variance explained by any other seed region. In this sense, the relationship between two regions as measured by partial correlation cannot be explained by the contribution of a third seed region.
RESULTS
Group differences in correlations between DMN regions
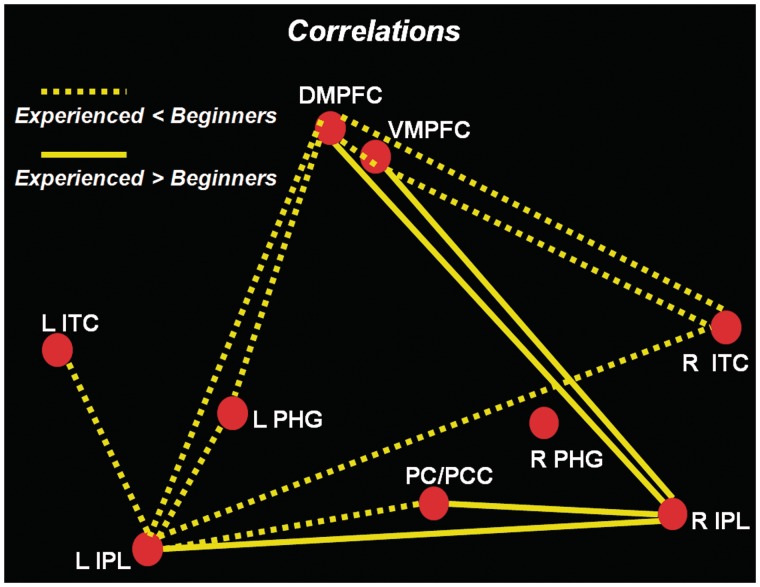
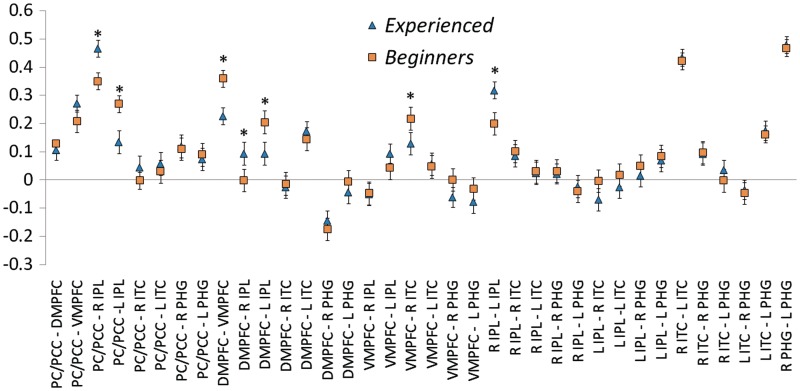
The results of the analyses revealed that correlations between nodes of the DMN were significantly increased for beginners, relative to experienced meditators (P > 0.95), between the DMPFC (BA 10) and the following regions: left IPL (BA 39), right ITC (BA 21) and left PHG. Weaker correlations were also found, for experienced compared to beginner meditators, between the VMPFC (BA 10) and the following regions: DMPFC (BA 10), right ITC (BA 21) and left PHG (BA 36). Finally, other stronger correlations for beginners vs. experienced meditators were measured between the left IPL and the following regions: PC/PCC (BA 39), right PHG (BA 36), left ITC (BA 21) and left PHG (BA 36).
The analyses also revealed stronger correlations for experienced meditators, relative to beginners (P > 0.95), between the right IPL and the following regions: PC/PCC (BA 31), DMPFC (BA 10) and left IPL (BA 39) (Tables 2 and 3, Figures 2 and 3).
Table 4.
Partial correlation matrix between nodes of the default mode network for experienced and beginner meditators
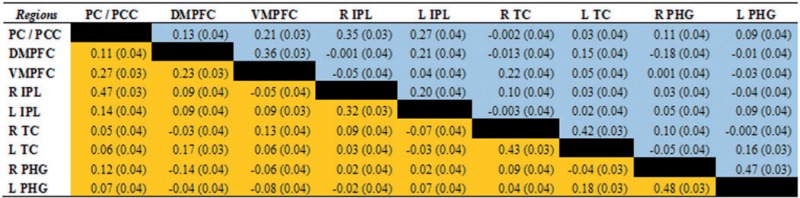 |
Table 2.
Coordinates for seed regions within the default mode network
| Region | BA | x | y | z |
|---|---|---|---|---|
| PC/PCC | 31 | 8 | −53 | 27 |
| DMPFC | 10 | −10 | 57 | 19 |
| VMPFC | 10 | −2 | 47 | −10 |
| R IPL | 39 | 48 | −56 | 27 |
| L IPL | 39 | −40 | −67 | 34 |
| R ITC | 21 | 56 | −4 | −22 |
| L ITC | 21 | −56 | −10 | −17 |
| R PHG | 36 | 26 | −32 | −15 |
| L PHG | 36 | −26 | −29 | −18 |
Notes: Stereotaxic coordinates are derived from the human atlas of Talairach and Tournoux (1988), referring to the medial–lateral position (x) relative to the midline (positive = right), and anterior–posterior position (z) relative to the commissural line (positive = superior). Designations of Brodmann position (y) relative to the anterior commissure (positive = anterior), and superior–inferior areas for cortical areas are also based on this atlas. BA = Brodmann area; PCC = posterior cingulate cortex; PC = precuneus; DMPFC = dorso-medial prefrontal cortex; VMPFC = ventro-medial prefrontal cortex; IPL = inferior parietal lobule; ITC = inferolateral temporal cortex; PHG = parahippocampal gyrus; R = right; L = left.
Table 3.
Correlation matrix between nodes of the default mode network for experienced and beginner meditators
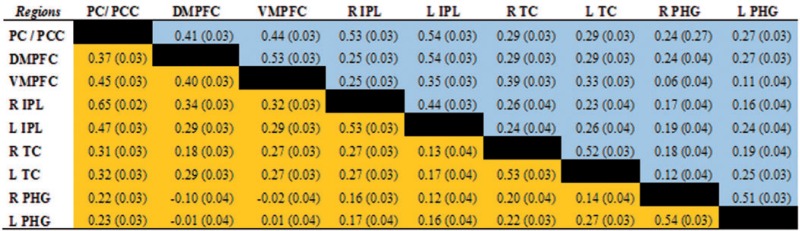 |
Fig. 2.
Diagram illustrating significant group differences (P > 0.95) in correlations (cc) between regions of the default mode network. Dotted lines represent significantly weaker correlations for experienced relative to beginner meditators, whereas full lines represent significantly stronger correlations for experienced meditators compared to beginners. PCC = posterior cingulate cortex; PC = precuneus; DMPFC = dorso-medial prefrontal cortex; VMPFC = ventro-medial prefrontal cortex; IPL = inferior parietal lobule; ITC = inferolateral temporal cortex; PHG = parahippocampal gyrus; R = right; L = left.
Fig. 3.
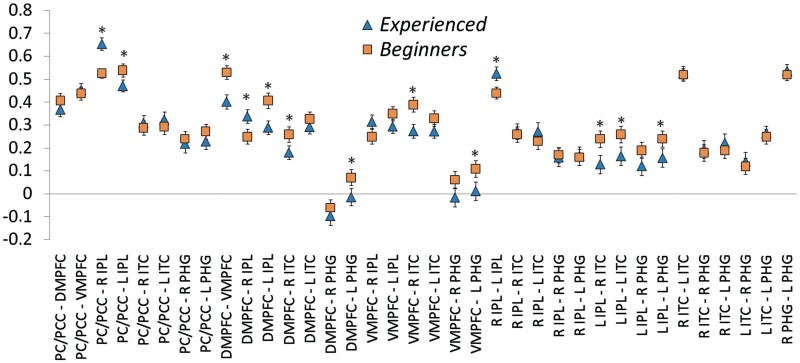
Correlation values (cc; y-axis) for all pairwise relationships between default network regions (x-axis). Blue triangles represent values for experienced meditators, and orange squares represent values for beginner meditators. PCC = posterior cingulate cortex; PC = precuneus; DMPFC = dorso-medial prefrontal cortex; VMPFC = ventro-medial prefrontal cortex; IPL = inferior parietal lobule; ITC = inferolateral temporal cortex; PHG = parahippocampal gyrus; R = right; L = left. *Significant group differences (P > 0.95).
Group differences in partial correlations between DMN regions
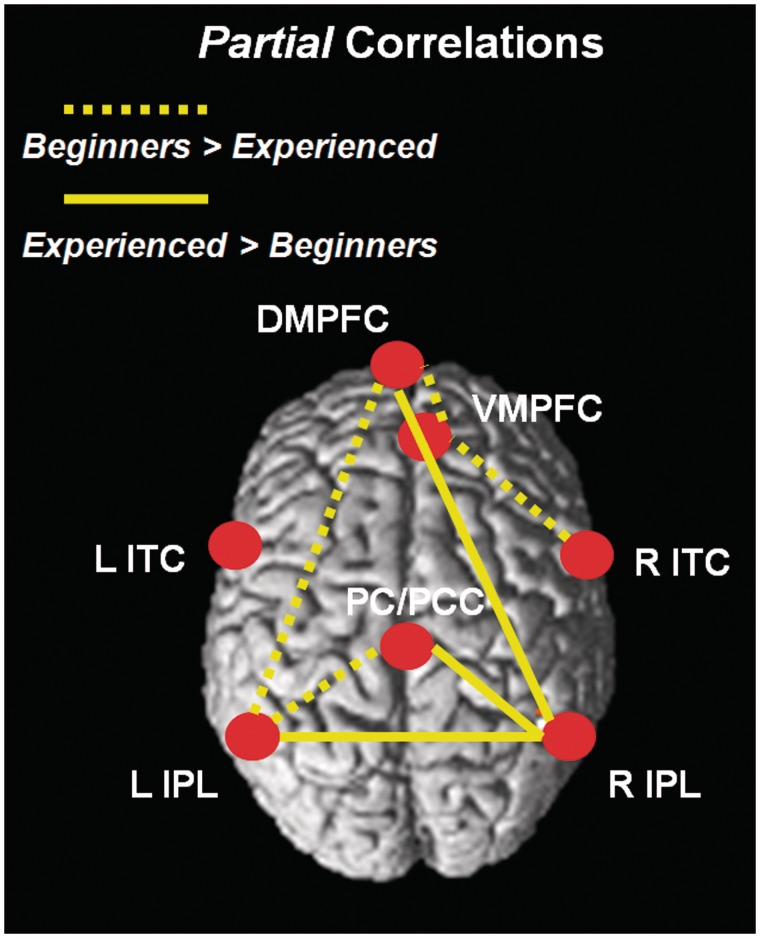
The stronger relationships between DMN regions observed in beginners vs. experienced meditators, which remained significant (P > 0.95) using the partial correlations measure, were found for the following pairs of regions: PC/PCC (BA 31) and left IPL (BA 39), DMPFC (BA 10) and left IPL (BA 39), DMPFC (BA 10) and VMPFC (BA 10), as well as VMPFC (BA 10) and right ITC (BA 21).
In addition, all of the stronger correlations for experienced meditators relative to beginners, which involved the right IPL (BA 39), remained significant using the partial correlation measure (P > 0.95), yielding the following coefficients with respect to these regions: PC/PCC (BA 31), DMPFC (BA 10) and PC / PCC (BA 31) (Figures 4 and 5).
Fig. 4.
Diagram illustrating significant group differences (P > 0.95) in partial correlations (Π) between default mode network regions. Dotted lines represent significantly weaker partial correlation coefficients for experienced compared to beginner meditators, and full lines represent significantly stronger partial correlation values for experienced meditators relative to beginners. PCC = posterior cingulate cortex; PC = precuneus; DMPFC = dorso-medial prefrontal cortex; VMPFC = ventro-medial prefrontal cortex; IPL = inferior parietal lobule; ITC = inferolateral temporal cortex; PHG = parahippocampal gyrus; R = right; L = left.
Fig. 5.
Partial correlation coefficients (Π; y-axis) for all pairwise relationships between default mode network regions (x-axis). Triangles represent the group of experienced meditators, and squares represent the group of beginner meditators. PCC = posterior cingulate cortex; PC = precuneus; DMPFC = dorso-medial prefrontal cortex; VMPFC = ventro-medial prefrontal cortex; IPL = inferior parietal lobule; ITC = inferolateral temporal cortex; PHG = parahippocampal gyrus; R = right; L = left. *Significant group differences (P > 0.95).
Correlations with hours of meditation practice in experienced meditators
Correlational analyses were conducted to examine whether the results obtained in the functional connectivity analyses were also related to the number of hours of meditation practice in the group of experienced meditators. Pearson r correlation coefficients were computed between the number of hours of meditation practice and the seven partial correlations which were significant in the between-group comparisons given the exploratory nature of these analyses, and therefore to avoid the occurrence of false positive results. The only significant correlation with the number of meditation hours was the DMPFC–VMPFC partial correlation, which was negatively related to the extent of meditation experience without the inclusion of the outlier with the most hours of meditation practice (r = −0.54, P = 0.008). Nonetheless, when including the outlier case and transforming the number of meditation practice hours into a rank ordered variable, the correlation remained significant (r = −0.50, P = 0.011). In sum, the partial correlation between the DMPFC and VMPFC was the only between-region connection to be significantly related to the number of hours of meditation practice.
DISCUSSION
The results of this study can be summarized as follows. First, the DMN was successfully identified using the NEDICA approach at the group level. Second, our hypotheses that decreased connectivity would be observed between regions of the MPFC and other DMN regions was only partially supported, as some connections were also found to be increased in experienced meditators relative to beginners. Thus, as hypothesized, functional connectivity between regions of the MPFC and other DMN nodes was weaker for the group of experienced meditators compared with beginners (such as the relationship between the DMPFC (BA 10) and three other DMN regions (left IPL [BA 39], VMPFC [BA 10]), as well as the relationship between the VMPFC (BA 10) and the right ITC [BA 21]). Weaker functional connectivity for experienced meditators was also found between the left IPL (BA 39) and PC/PCC (BA 31). However, contrary to our hypotheses, experienced meditators exhibited stronger functional connectivity between the right IPL (BA 39) and three other DMN regions (DMPFC [BA 10], left IPL [BA 39] and PC / PCC [BA 31), relative to beginners. These group differences in the connectivity between DMN regions for experienced meditators relative to beginners were significant when assessed using both correlation and partial correlation coefficients. This finding indicates that these functional coupling differences remained significant after controlling for the interaction with other DMN nodes.
Identification of the default mode network using NEDICA
The NEDICA approach successfully identified the DMN at the group level, across the entire sample. This is consistent with the extensive literature demonstrating that specific prefrontal, temporal, temporolimbic and parietal brain regions have correlated time-courses during a restful state (Greicius et al., 2003; Damoiseaux et al., 2006; De Luca et al., 2006; Buckner et al., 2008; Fransson and Marrelec, 2008; Perlbarg et al., 2008). However, our seed for the DMPFC (BA 10) was slightly lateralized to the left, and the seed for the right IPL (BA 39) was slightly inferior compared with those used in other studies (Fransson and Marrelec, 2008). Currently, it remains unclear as to the optimal method for identifying seed regions in functional connectivity analyses, which can be determined using foci obtained from univariate analyses of task-related paradigms, anatomical landmarks or seed regions previously reported in the literature. However, it has recently been shown that using a priori coordinates based on the literature or spatial ICA to identify DMN seed regions leads to essentially similar results with respect to differences in DMN connectivity between a continuous working memory task and a state of rest (Marrelec and Fransson, 2011). For the purpose of the present study, we selected seed regions based on the peaks revealed in the DMN map identified across groups, to reflect more ecological validity with respect to the particular DMN function of our sample. Finally, it is noteworthy that NEDICA is a tool designed to detect multiple networks across sessions and subjects, and the analyses conducted in the present study revealed the presence of several other networks across resting state runs (visual, motor and auditory, for example). We chose, however, to examine functional connectivity only within regions of the DMN for the sake of conciseness, in addition to our clear a priori hypotheses with respect to this network and its relationship with meditation training.
Increased connectivity for experienced meditators compared to beginners
First, our findings are in contrast with those from a recent study (Kilpatrick et al., 2011) in which differences between intrinsic connectivity resting state networks were examined between a group of participants having completed an 8-week MBSR training program and a wait-listed control group. Though Kilpatrick et al. (2011) did not observe any differences between the two groups in functional connectivity with respect to the DMN, the discrepancy with our findings may arise in part from the different statistical analytic procedures employed as well as from the extent of experience from the group of meditators. As such, it is possible that differences in DMN connectivity emerge after more than 8 weeks of training.
Nonetheless, the stronger functional connectivity observed in the present study between the right IPL (BA 39) and DMPFC (BA 10) in experienced meditators, relative to beginners, is consistent with previous studies (Lutz et al., 2004; Fell et al., 2010). For instance, it has been reported that, compared to control subjects with no meditative experience, Buddhist meditators (with 10 000–40 000 h of meditation experience) exhibit greater gamma synchrony between medial prefrontal and parietal areas during a resting state (Lutz et al., 2004). Interestingly, increased gamma wave synchrony between frontal and parietal lobes has been interpreted as reflecting enhanced conscious awareness of the present moment (Tononi and Edelman, 1998; Engel et al., 1999), a central characteristic of the mindful state.
The strengthened correlation between the right IPL (BA 39) and the DMPFC (BA 10) for experienced meditators (relative to beginner meditators) might reflect adaptive consequences of mindfulness training, as this connection has been shown to be hypofunctional in individuals with high basal cortisol levels (Schutter et al., 2002). Indeed, it has been found that elevated baseline levels of the steroid hormone cortisol, previously associated with depression (Holsboer et al., 2000), are correlated with reduced functional connectivity between the left PFC and right parietal cortex. These findings are consistent with evidence showing that transcranial magnetic stimulation (TMS) applied locally to the left prefrontal or to the right parietal cortex (to increase activity in these regions) reduces depressive symptoms (George et al., 1995; 1996; 2010; van Honk et al., 2003). Given this, the increased connectivity between the DMPFC and the right IPL found here in experienced meditators may reflect a beneficial impact of mindfulness training in terms of emotional resources and conscious awareness of the present moment. This hypothesis is consistent with evidence that mindfulness is accompanied by increased mood and well-being, enhanced attention and cognitive performance, as well as reduced stress, depressive symptoms, anger and cortisol levels (Baer, 2003; Tang et al., 2007; Jung et al., 2010).
Increased connectivity was also noted for experienced meditators, compared to beginners, between right IPL (BA 39) and two other DMN nodes (PC/PCC [BA 31] and left IPL [BA 39]). The heightened connectivity between the right IPL and the PC/PCC is in accordance with a recent study (van Buuren et al., 2010), which demonstrated that self-referential processing is associated with reduced coupling between the right parietal cortex and the precuneus. This finding supports the view that mindfulness training induces brain function changes that are accompanied by a reduction of self-referential thoughts during rest. Alternatively, as the parietal cortex is involved in working memory and visuo-spatial attention (Culham and Kanwisher, 2001), this finding may reflect greater global attention and moment-to-moment awareness in experienced meditators during a restful state. Nonetheless, these interpretations remain speculative, at best, until being tested using behavioural paradigms assessing these specific processes.
Individuals with MDD and a concomitant anxiety disorder display greater right vs. left parietal alpha activity (Culham and Kanwisher, 2001). In contrast, depressed individuals without a comorbid anxiety disorder exhibit greater left vs. right alpha activity over parietal areas. Consequently, the increased connectivity between the left and right parietal regions measured in experienced meditators, relative to beginners, may reflect the greater emotional stability that results from long-term practice of mindfulness (Bruder et al., 1997; Broderick, 2005; Sheline et al., 2009).
Decreased connectivity for experienced meditators relative to beginners
Greater coherence in the theta range between the PFC and the left parietal cortex has been measured during working memory tasks involving verbally related content, whereas theta coherence enhancement between the PFC and the right parietal cortex is seen in working memory tasks implicating spatial features (Sarnthein et al., 1998). Therefore, the decreased connectivity between the DMPFC (BA 10) and the left IPL (BA 39) in experienced meditators, compared to beginners, may be related to a diminution of analytic self-referent processes (Northoff et al., 2006).
For experienced meditators relative to beginners, reduced connectivity was also measured between the VMPFC (BA 10) and DMPFC (BA 10). Since these medial prefrontal areas are adjacent to each other, it is difficult to tease apart their distinct contributions to DMN functioning. Both areas play a role in self-relatedness (Schneider et al., 2008), self-referential processing (van Buuren et al., 2010), emotional judgements (Northoff et al., 2004) and in the appraisal of stimuli relative to the self (Ochsner et al., 2004; Ochsner and Gross, 2005). The VMPFC (BA 10), which has dense projections to the amygdala (Amaral et al., 1992), is also thought to be implicated in the extinction of conditioned fear, as well as the down-regulation of emotional responses (LaBar et al., 1998; Davidson, 2002; Phelps et al., 2004). It thus appears plausible that the decreased coupling between the DMPFC (BA 10) and the VMPFC (BA 10) noted in experienced meditators may reflect a reduction in emotional appraisal during self-referent processes, consistent with the view that mindfulness is intended to promote acceptance of thoughts, perceptions and feelings (Bishop, 2004). In addition, since the connectivity between these regions was also negatively correlated to the number meditation practice hours in the group of experienced meditators, the decreased DMPFC–VMPFC connectivity found for experienced meditators relative to beginners may specifically be related to the extent of meditation training.
Finally, experienced meditators had reduced connectivity between the VMPFC and the right TC, which may reflect reduced retrieval or encoding of self-referent memories. As such, the anterior temporal cortex has been associated with retrieval of autobiographical emotional memories (compared with neutral autobiographical memories) (Dolan et al., 2000). Thus, the reduced functional coupling between the right TC and the VMPFC in experienced vs. beginner meditators may reflect reduced emotional autobiographical retrieval during rest for the experienced meditators. Future studies are needed to investigate the extent and nature of autobiographical memory retrieval during rest as a result of meditation training.
Correlations and partial correlations between DMN regions
Distinct patterns of functional connectivity within the DMN regions were revealed, in experienced vs. beginner meditators, by both correlations and partial correlations. Several correlations, however, involving the VMPFC (BA 10), the right IPL (BA 39), the PHG (BA 36) and the left ITC (BA 21) were no longer different, between the two groups, when analyzed using partial correlations. This finding is consistent with the notion that the DMN is segregated into functional subsystems (Perlbarg et al., 2007; Buckner et al., 2008), and that the power of low-frequency BOLD fluctuations in different regions of the DMN is rank-ordered, with the ITC exhibiting the lowest power, and the PC/PCC the highest power, followed by the VMPFC and the DMPFC (Jiao et al., 2011).
LIMITATIONS AND FUTURE DIRECTIONS
This study is nonetheless limited in some respects. First, the two groups of participants may have differed in other aspects (personality traits, lifestyle, etc.); therefore, longitudinal studies examining the relationship between meditation training and functional connectivity are needed to rule out these potential confounds. Second, the partial correlation method used in this study does not allow for causal inferences to be made about the relationships between different regions. Thus, further studies using effective connectivity methods, such as dynamic causal modelling, are required to investigate causal relationship between DMN regions as a result of meditation training. Third, this study assessed differences regarding functional connectivity between DMN regions at rest, and did not examine any behavioural processes; hence, interpretations relating brain connectivity differences between the two groups remain speculative, at best, until being replicated in studies using paradigms specifically assessing the behavioural mechanisms involved. Moreover, though age differences between the two groups did not attain statistical significance, there was a slight difference between the mean ages of the two groups but it was not possible to include a covariate in the independent component analyses using the NEDICA software. In addition, given our relatively small sample size for an fMRI experiment, the results of the present study should be interpreted with caution until being replicated in larger samples precisely matched with respect to age. Finally, it is possible that the state of rest may have differed qualitatively between the two groups; therefore, future studies examining the relationship between meditation training and resting state connectivity should acquire qualitative measures of thought content and conscious processes occurring during the rest period to aid in interpretation of brain imaging functional connectivity data.
CONCLUSION
In conclusion, this study demonstrates that individuals with extensive mindfulness training exhibit significant differences in functional connectivity between regions of the DMN. Our findings suggest that mindfulness training leads to changes in the functional dynamics of the DMN that extend beyond a state of meditation per se.
Conflict of Interest
None declared.
Acknowledgments
The authors thank the staff of the Unité de Neuroimagerie Fonctionnelle (UNF), Institut universitaire de gériatrie de Montréal (IUGM), for their skilful technical assistance. This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) (M.B.).
REFERENCES
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Wiley-Liss. 1992. pp. 1–66. [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: A conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–43. [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23:137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7:1129–59. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bellec P, Perlbarg V, Jbabdi S, et al. Identification of large-scale networks in the brain using fMRI. Neuroimage. 2006;29:1231–43. doi: 10.1016/j.neuroimage.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Bishop RS. Mindfulness: a proposed operational definition. Clinical Psychology: Science and Practice. 2004;11:230–41. [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Broderick PC. Mindfulness and coping with dysphoric mood: Contrasts with rumination and distraction. Cognitive Therapy Research. 2005;29:501–10. [Google Scholar]
- Bruder GE, Fong R, Tenke CE, et al. Regional brain asymmetries in major depression with or without an anxiety disorder: a quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–48. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG. Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology. 2001;11:157–63. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–67. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Lane R, Chua P, Fletcher P. Dissociable temporal lobe activations during emotional episodic memory retrieval. Neuroimage. 2000;11:203–9. doi: 10.1006/nimg.2000.0538. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P, Konig P, Brecht M, Singer W. Temporal binding, binocular rivalry, and consciousness. Conscious Cognition. 1999;8:128–51. doi: 10.1006/ccog.1999.0389. [DOI] [PubMed] [Google Scholar]
- Esposito F, Scarabino T, Hyvarinen A, et al. Independent component analysis of fMRI group studies by self-organizing clustering. Neuroimage. 2005;25:193–205. doi: 10.1016/j.neuroimage.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J, Axmacher N, Haupt S. From alpha to gamma: electrophysiological correlates of meditation-related states of consciousness. Medical Hypotheses. 2010;75:218–24. doi: 10.1016/j.mehy.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Archives of General Psychiatry. 2010;67:507–16. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–6. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- George MS, Wassermann EM, Williams WA, et al. Changes in mood and hormone levels after rapid-rate transcranial magnetic stimulation (rTMS) of the prefrontal cortex. Journal of Neuropsychiatry and Clinical Neuroscience. 1996;8:172–80. doi: 10.1176/jnp.8.2.172. [DOI] [PubMed] [Google Scholar]
- Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152:150–6. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Kang DH, et al. Increased default mode network connectivity associated with meditation. Neuroscience Letters. 2011;487(3):358–62. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Jiao Q, Lu G, Zhang Z, et al. Granger causal influence predicts BOLD activity levels in the default mode network. Human Brain Mapping. 2011;32:154–61. doi: 10.1002/hbm.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YH, Kang DH, Jang JH, et al. The effects of mind-body training on stress reduction, positive affect, and plasma catecholamines. Neuroscience Letters. 2010;479:138–42. doi: 10.1016/j.neulet.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Wherever you go there you are. New York, USA: Hyperion Books; 1994. [Google Scholar]
- Kilpatrick LA, Suyenobu BY, Smith SR, et al. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. Neuroimage. 2011;56(1):290–8. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16369–73. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelec G, Bellec P, Krainik A, et al. Regions, systems, and the brain: hierarchical measures of functional integration in fMRI. Medical Image Analysis. 2008;12:484–96. doi: 10.1016/j.media.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Fransson P. Assessing the influence of different ROI selection strategies on functional connectivity analyses of fMRI data acquired during steady-state conditions. Plos One. 2011;6(4):1–14. doi: 10.1371/journal.pone.0014788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelec G, Krainik A, Duffau H, et al. Partial correlation for functional brain interactivity investigation in functional MRI. Neuroimage. 2006;32:228–37. doi: 10.1016/j.neuroimage.2005.12.057. [DOI] [PubMed] [Google Scholar]
- Meindl T, Teipel S, Elmouden R, et al. Test-retest reproducibility of the default-mode network in healthy individuals. Human Brain Mapping. 2010;31(2):237–46. doi: 10.1002/hbm.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, et al. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Human Brain Mapping. 2004;21:202–12. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Bellec P, Anton JL, Pelegrini-Issac M, Doyon J, Benali H. CORSICA: correction of structured noise in fMRI by automatic identification of ICA components. Magnetic Resonance Imaging. 2007;25:35–46. doi: 10.1016/j.mri.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Marrelec G, Doyon J, Pélégrini-Issac M, Lehéricy S, Benali H. NEDICA: detection of group functional networks in fMRI using spatial independent component analysis. 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 2008:1247–50. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M. L'art de la meditation. 2008. (Nil éditions): Paris, France. [Google Scholar]
- Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7092–6. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Bermpohl F, Heinzel A, et al. The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience. 2008;157:120–31. doi: 10.1016/j.neuroscience.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, Van Honk J, Koppeschaar H, Kahn R. Cortisol and reduced interhemispheric coupling between the left prefrontal and the right parietal cortex. Journal of Neuropsychiatry and Clinical Neuroscience. 2002;14:89–90. doi: 10.1176/jnp.14.1.89. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cerebral Cortex. 2009;19(10):2209–29. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1942–7. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17152–6. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thich Nhat Hanh. Le miracle de la pleine conscience. 1994. (L'espace bleu) [Google Scholar]
- Tononi G, Edelman GM. Consciousness and complexity. Science. 1998;282:1846–51. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- van Buuren M, Gladwin TE, Zandbelt BB, Kahn RS, Vink M. Reduced functional coupling in the default-mode network during self-referential processing. Human Brain Mapping. 2010;31:1117–27. doi: 10.1002/hbm.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Putman P, de Haan EH, d'Alfonso AA. Reductions in phenomenological, physiological and attentional indices of depressive mood after 2 Hz rTMS over the right parietal cortex in healthy human subjects. Psychiatry Research. 2003;120:95–101. doi: 10.1016/s0165-1781(03)00114-8. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–77. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]