Abstract
Long-term Vipassana meditators sat in meditation vs. a control (instructed mind wandering) states for 25 min, electroencephalography (EEG) was recorded and condition order counterbalanced. For the last 4 min, a three-stimulus auditory oddball series was presented during both meditation and control periods through headphones and no task imposed. Time-frequency analysis demonstrated that meditation relative to the control condition evinced decreased evoked delta (2–4 Hz) power to distracter stimuli concomitantly with a greater event-related reduction of late (500–900 ms) alpha-1 (8–10 Hz) activity, which indexed altered dynamics of attentional engagement to distracters. Additionally, standard stimuli were associated with increased early event-related alpha phase synchrony (inter-trial coherence) and evoked theta (4–8 Hz) phase synchrony, suggesting enhanced processing of the habituated standard background stimuli. Finally, during meditation, there was a greater differential early-evoked gamma power to the different stimulus classes. Correlation analysis indicated that this effect stemmed from a meditation state-related increase in early distracter-evoked gamma power and phase synchrony specific to longer-term expert practitioners. The findings suggest that Vipassana meditation evokes a brain state of enhanced perceptual clarity and decreased automated reactivity.
Keywords: meditation, Vipassana, EEG, wavelet, theta, alpha, gamma, spectral power, phase synchrony
MEDITATION AND ELECTROENCEPHALOGRAPHIC MEASURES
Meditation brain effects are as yet not well characterized, although this topic has received considerable attention, is growing at an accelerating rate over the past 10 years, and some areas of growing consensus are emerging (Andresen, 2000; Lazar et al., 2003; Cahn and Polich, 2006; Lutz et al., 2007, 2008; Pagnoni et al., 2008; Slagter et al., 2011; Farb et al., 2012). From a neuroscience perspective, meditation can be conceived as the interaction of diverse and distinct attentional mechanisms. Recent reports have begun to focus on well-characterized neural measures of attentional engagement during (state) and from (trait) meditation, which have begun to delineate specific effects of these ancient practices on brain activity and its subsequent influence on cognitive and emotional processing.
A major finding using electroencephalographic (EEG) methods indicates that theta and alpha power are related to proficiency of practice. However, the alteration in the dynamics of these rhythms with extended meditative practice is non-linear and topographically specific. These observations imply that increases in alpha power as a state effect of meditation may be related to learning meditation in the early stages for some subjects, but long-term practitioners demonstrate little enhancement of alpha state effects (Cahn et al., 2010). Theta band activity increase seems to be a marker of meditation across a number of different practice types, although it appears more specifically related to the focused attention meditative forms (Hebert and Lehmann, 1977; Aftanas and Golocheikine, 2001; Cahn and Polich, 2006; Lutz et al., 2009; Baijal and Srinivasan, 2010; Cahn et al., 2010). In addition, growing evidence indicates that increased gamma band fast amplitude activity can be observed in advanced practitioners, which supports the interpretation that many meditative practices involve active up-regulation of attentional capacities (Lutz et al., 2004; Cahn et al., 2010; Slagter et al., 2011).
Early EEG studies of meditation and stimulus processing focused on assessing alpha blocking, which refers to the characteristic decrease in alpha activity after stimulus presentation that is related to active stimulus processing (Niedermeyer, 1997). During concentrative Yogic practices, some highly experienced meditators failed to demonstrate alpha blocking to auditory clicks or aversive stimuli such as placing the hands in cold water (Anand et al., 1961; Wenger and Bagchi, 1961). Later studies assaying a mantra meditation and Zen practice indicated that alpha power was less disrupted in meditation than control states in response to loud aversive stimuli (Lehrer et al., 1980) and ‘name calling’ (Kinoshita, 1975). It was hypothesized that practitioners during meditative states could adjust the relevant attentional networks thereby controlling neural activation. This theoretical view is consistent with observations of late alpha desynchronization as reflecting attentional engagement (Klimesch, 1996; Schurmann and Basar, 2001).
In contrast, studies of Japanese Zen monks, schooled in a practice tradition similar to the mindfulness-focus of Vipassana demonstrated distinctly different outcomes. With repetitive but relatively infrequent (>10 s) auditory stimulation, long-term Zen practitioners demonstrated a decrease in the normal alpha blocking habituation compared with novices (Kasamatsu and Hirai, 1966; Hirai, 1974). This result implied that long-term open-awareness practice in the Zen tradition was associated with a ‘de-automization’ of sensory and cognitive processing, such that successive auditory stimuli were perceived as relatively novel even with repeated presentation. These early event-related alpha dynamics studies alternately showed a decrease vs. increase in alpha desynchronization, and in both cases they were taken to indicate that meditation may lead to neurophysiologic states marked by decreased stimulus-driven automated processing, either by blocking the access to such evaluative processing or interfering with normal habituation of such processing. It is important to note, however, that the manual alpha power assessments employed were not fully reliable. A later technically more comprehensive attempt to replicate these findings in practitioners of Transcendental Meditation, Zen and another Yogic tradition incorporating mantra practice found no such replication of the alpha suppression or habituation findings across these different groups (Becker and Shapiro, 1981).
Contemporary quantitative analyses of neuroelectric responsiveness as modulated by meditation are beginning. Wavelet-based time frequency decomposition analyses have been applied to data from one cohort of advanced meditator subjects relative to novices. The meditator cohort was assessed before and after a 3-month full-time Vipassana meditation retreat whereas a control group of beginners did not participate in any intensive training in between assessments (Lutz et al., 2009; Slagter et al., 2009). Participants in the retreat engaged in both periods of concentrative (‘focused attention’) meditation on breath sensations as well as periods of open-awareness (‘open monitoring’) meditation on body sensations and the contents of awareness generally. Behavioral and brain activity from a dichotic auditory oddball and a visual attentional blink task were obtained. The dichotic auditory oddball paradigm involved the presentation of frequent standard tones to both ears with instruction to detect intermittent rare deviant tones, which were presented to either the attended or the unattended ear. Participants were instructed to press a button when such deviants were detected in the attended ear only. The visual attentional blink paradigm presented visual stimulus sets in quick succession, with target stimulus pairs embedded in the stimulus train and instruction for participants to respond by identifying both embedded targets (‘T1’ and ‘T2’). Previous research has consistently verified that when the second target (T2) stimulus falls in a critical time window after first target (T1), the attentional systems are less sensitive to the incoming T2 stimulus resulting in lower detection performance.
The meditation retreat resulted in the practitioners but not the control group showing increased theta phase synchrony in the first ∼500 ms post-stimulus presentation to both attended tones in the dichotic listening task and to the perceived second targets (‘T2’ stimuli) embedded in the attentional blink task stimulus trains. After the intensive meditation retreat, in the dichotic auditory oddball task beta (15–30 Hz) activity was also found to show less event-related desynchronization to attended tones, and broad band (1–30 Hz) activity was found to show enhanced inter-trial coherence (phase synchronization) to both attended and non-attended tones (Lutz et al., 2009). In the attentional blink paradigm, a meditation-related enhancement was also obtained for event-related alpha power in the first ∼200 ms evoked by the first stimulus of the target stimulus pairs in no blink compared with blink trials (Slagter et al., 2009). Taken together, the findings suggested that meditative training enhances attentional capacities and that one common neural signature of the enhanced processing evoked through intensive meditation training is indexed by event-related enhancements in theta phase consistency across trials to attended and perceived stimuli.
PRESENT STUDY
This study assessed meditation state effects using a within-subject design on a single day of testing. Meditation state dynamics were quantified with spectral decomposition of the EEG recorded during a three-stimulus auditory oddball stimulus train while subjects sat in meditation vs. in the control instructed ‘mind-wandering’ state with no task imposed. This no-task design has the advantage of allowing practitioners to engage in their meditative practice while recording brain activity during such practice. The control task instructions were to let the mind wander freely through non-emotional memories and thoughts. This procedure was devised to mimic a mind-wandering ‘default mode’ with the ecological validity of cognitive engagement in normal everyday life (Smallwood and Schooler, 2006; Christoff et al., 2009).
All participants engaged in Vipassana meditation, a traditional Buddhist practice that involves focusing on present-moment sensory awareness with an equanimous and non-reactive mental set (Hart, 1987; Gunaratana, 2002). This tradition has served as the foundation for contemporary ‘mindfulness’ meditation techniques underlying adaptations of meditative practices such as Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy, which are used as the basis for clinical interventions (Kabat-Zinn, 1982; Teasdale et al., 1995; Teasdale et al., 2000; Davidson, 2003; Kabat-Zinn, 2003; Grossman et al., 2004). The development of greater awareness of (and concomitant non-reactivity to) interoceptive and exteroceptive sensory stimuli during formal Vipassana/mindfulness meditation is hypothesized to enhance self-awareness, such that adaptive responding is facilitated at the expense of automated non-adaptive reactions thereby promoting more successful management of stressful life situations (Hart, 1987; Segal et al., 2002; Kabat-Zinn, 2003; Lutz et al., 2007). State effect assessments of Vipassana meditation have provided EEG and event-related potential (ERP) evidence that enhancements of awareness and concomitant decreases in automated reactivity are measurable brain effects of such practice (Cahn and Polich, 2009; Cahn et al., 2010).
To elucidate further the distinct effects, similarities and differences amongst the wide variety of meditative practices, it is important to distinguish the specific characteristics of any given meditation technique (Cahn and Polich, 2006; Lutz et al., 2008; Slagter et al., 2011). The majority of the Vipassana practitioners in the present study had been taught in the tradition of S.N. Goenka (Hart, 1987). This school of practice involves attentional absorption in the sensations throughout the body in an iterative and cyclic fashion, mentally scanning body sensations from the top of the head to the toes and back again repeatedly. The technique includes the concomitant adoption detached attitude with attentive observation and non-reactivity to any elaborative sensations/thoughts that may arise.
Event-related spectral dynamics have been found to accompany processing of the auditory oddball paradigm with a variety of time/frequency effects observed for each stimulus condition (Klimesch, 1999; Basar et al., 2001; Neuper and Pfurtscheller, 2001; Mazaheri and Picton, 2005; Ishii et al., 2009). Moreover, the three-stimulus oddball task in which a target is detected among non-changing standard and distractor stimuli provides insight into the attention/memory brain circuit (Katayama and Polich, 1998; Comerchero and Polich, 1999; Demiralp et al., 2001; Polich and Criado, 2006), which time-frequency analyses are beginning to characterize in normal cognition (Klimesch et al., 2007; Polich, 2007).
Given the above neuroelectric meditation findings, several outcomes for this study were predicted: (1) As a reflection of decreased automated attentional engagement, the increased frontal theta/delta power and/or phase consistency to distracting stimuli should be reduced in meditation relative to control conditions. (2) As a reflection of enhanced sensory representation and decreased habituation to environmental stimuli, some measures of enhanced processing of the standard stimuli and/or enhanced stimulus-representation neural activity should be obtained. (3) Previous finding of tonically enhanced gamma activity during Vipassana practitioners with the most years of daily practice implies that this subgroup might also show enhanced stimulus-related gamma phase consistency and/or power measures in response to one or more of the stimulus classes. (4) Theta inter-trial phase synchrony should also provide a reliable measure of meditation practice.
METHODS
Participants
A total of N = 16 Vipassana (F = 5, M = 11) meditators were assessed (M = 45.5, s.d. = 9.8, 24–56 years). These individuals had been meditating for a considerable period (M = 20.0, s.d. = 12.1, 2.5–40 years): All had been meditating daily (7 days/week) for at least 1 year (M = 13.0, s.d. = 10.7, 1–30 years), and at least 0.5+ h/day (M = 1.3, s.d. = 0.7, 0.5–3 h). Participants were recruited from a local Vipassana meditation community and compensated $40 for the 3-h study.
Recording conditions
EEG data were collected using tin sensors embedded in a 19-channel Electro-Cap International (ECI) electrode cap from the following electrode locations: Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, T7, T8, Cz, P3, P4, P7, P8, Pz, O1 and O2. The electrodes were referenced to linked earlobes, using a forehead ground. Eye movement (EOG) activity was assessed with electrodes placed at the outer canthi and above/below the left eye in line with the pupil for horizontal and vertical EOG monitoring using bipolar reference. Impedances were kept below 10 kΩ. The signals were recorded with a band pass of 0.01–70 Hz (6 dB octave/slope) and digitization rate of 256 Hz.
Procedure
Participants were instructed to sit on cushions and meditate within the Vipassana meditation tradition or engage in the control neutral thinking state, with the order of the tasks counterbalanced across individuals. Participants were instructed to sit in the same posture for both the meditation and control task recordings. Headphones were placed over the cap the outset of the recording session and worn throughout the recording. Pilot testing indicated that some participants found it difficult to refrain from engaging in their meditative practice when sitting in the meditative posture. Instructions therefore specified to think about emotionally neutral past events if they began slipping into meditative practice. The participants were otherwise to let their mind wander freely through non-emotional neutral thoughts.
This cognitive engagement control was chosen to emulate a ‘mind-wandering’ state with high ecological validity can be contrasted with the purposeful and highly practiced attentional engagement on present-moment sensations of the body, and reflects the meta-cognitive monitoring characteristic the meditation state (Hart, 1987; Smallwood and Schooler, 2006; Christoff et al., 2009). Participants were informed that after 21 min of the eyes-closed control thinking or meditation practice, they would hear the tone series from the auditory three-stimulus paradigm and were instructed to continue the control thinking/meditation practice and ignore the auditory stimuli: ‘At the completion of the silent period, you will hear a series of tones. When these tones start, please ignore them to the best of your ability and instead continue to engage with the Vipassana meditation practice vs. engagement with thoughts about neutral past memories’. It is important to note that Vipassana practice encourages an open state of awareness wherein internal and environmental stimuli are not purposefully blocked from attentional processing. Thus, the instructions did not emphasize trying to exclude the auditory stimuli but rather just to refrain from paying explicit attention to them.
The auditory paradigm consisted of pseudorandom presentation of 250 stimuli. Standard tones were 500 Hz and occurred with a probability of 0.80, oddballs were 1000 Hz tones presented with a probability of 0.10, and distracters were white noise bursts that occurred with a probability of 0.10 (cf. Combs and Polich, 2006). All stimuli were presented over headphones at 80 dB sound pressure level (SPL), a duration of 60 ms (5 ms r/f) and an inter-stimulus interval of 1 s. At the conclusion of the first recording period, participants were given the opportunity to stand up and stretch before taking the same seating position for the second recording of equal length.
Immediately after each condition, participants completed a short rating form (1–10) indicating whether they had experienced drowsiness or sleep onset during the session. The depth of meditative experience at ‘1’ indicating the normal waking state, and ‘10’ indicating the deepest level of meditative absorption ever experienced. One-half the participants meditated first, and one-half the participants performed the control task first. After the two experimental periods with passive presentation of the auditory stimuli were finished, the auditory stimuli were presented for a third time as an active task condition. Participants were instructed to press a mouse-button with the right hand index finger when oddball stimuli (1000 Hz tone) were detected and to refrain from responding to the standard (500 Hz) and distracter (white noise) sounds.
Data analysis
The data were filtered at 0.5 Hz (Rabiner and Gold, 1975). Each epoch was inspected visually for muscle activity or other phasic noise contamination, with few epochs removed. Of a total of 20 standards and 25 each for the oddball and distracter stimuli, the number of trials (mean ± s.d.) in the time frequency analysis for each stimulus and condition type was: standard control = 185.5 ± 18.7, standard meditation = 189.7 ± 9.4, standard active task = 188.8 ± 14.9, oddball control = 23.7 ± 1.9, oddball meditation = 24.1 ± 1.3, oddball active task = 24.0 ± 2.4, distracter control = 23.0 ± 2.5, distracter meditation = 23.6 ± 1.2, distracter active task = 23.2 ± 2.5. A 3 stimulus × 2 condition analysis of variance (ANOVA) found no statistically significant difference between states for the number of included trials were found (P > 0.4 in all cases).
The extended ICA algorithm was then run on the data using the runica algorithm implemented within EEGLAB in Matlab (Delorme and Makeig, 2004; Delorme et al., 2007). The resultant independent components accounting for horizontal and vertical eye movements were then marked and removed epochs (Jung et al., 2000a,b). The data were then organized according to stimulus class (standards, oddballs, distracters) from −1000 ms prior to stimulus to +2000 ms following each stimulus, with the Morlet wavelet decomposition applied to the 3-s epochs time-locked to auditory stimuli (Goupillaud et al., 1984). The 3-s epochs were employed to facilitate calculation of low-frequency power and phase synchrony dynamics, as longer epochs increase resolution of these measures. Baseline power and phase synchrony measures were taken over the −300 to −50 ms time range relative to stimulus presentation. A total of 150 linearly spaced time points and a series of 65 log-spaced frequencies ranging from 1.5 to 65 Hz were employed, with 1 cycle at the lowest frequency increasing linearly and capping at 8 cycles at 30 Hz and higher.
Spectral power was assessed in decibel (dB) units obtained with the log power transformation, −10 × log10(X), such that X is absolute power at a given time-frequency point after subtracting the pre-stimulus (−300 to −50 ms) average baseline log-power at each frequency (Delorme and Makeig, 2004). Data reflecting event-related spectral perturbation and inter-trial coherence (phase locking) were derived from delta (2–4 Hz), theta (4–8 Hz), alpha-1 (8–10 Hz) and alpha-2 (10–12 Hz) frequency bands the time windows of interest were defined based on established auditory stimulus event-related spectral dynamics in combination with observed time-frequency effects from the Fz and Cz electrodes, where such effects were observed to be strongest (Figures 1A–C). The inter-trial coherence and spectral power calculations were obtained using the complex Gaussian wavelet as (Tallon-Baudry and Bertrand, 1999). The wavelet transformation was applied on each single trial and time frequency powers were then averaged across all trials and inter-trial coherence values calculated based on relative phase consistency across all trials.
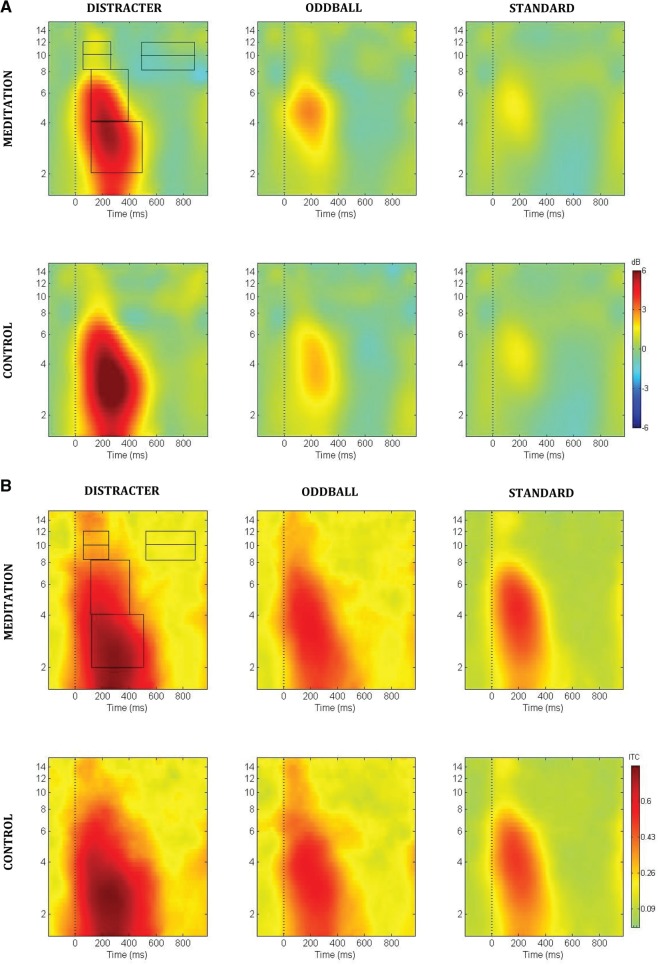
Fig. 1.
(A) Event-related spectral perturbation power (dB) at electrode Cz. Overlaid boxes are of time-frequency decomposition analyzed. (B) Event-related inter-trial coherence (0–1 phase synchrony value) at electrode Cz. Boxes are of time-frequency decomposition analyzed. (C) Gamma frequency event-related inter-trial coherence (0–1 phase synchrony value) at electrode Fz.
The data were then exported to STATISTICA, and repeated-measures ANOVA analyses were performed on the amplitude and inter-trial coherence values for channels F3, Fz, F4, C3, Cz, C4, P3, Pz and P4 at each frequency band and time window of interest. The ANOVA was structured as 3-stimulus types (standard, oddball and distracter) × 2 condition states (control and meditation) × 3 frontal/parietal electrode positions (frontal, central and parietal) × 3 coronal locations (left, central and right). Subsequent ANOVA sub-analyses were conducted for each stimulus class independently, using a 2 state (meditation and control) × 3 frontal/parietal electrode positions (frontal, central and parietal) × 3 coronal locations (left, central and right).
A second set of analyses designed to characterize significant meditation state-related findings for standard and distracter stimuli were performed. These purposefully evaluated the data from the active task condition in comparison with the control and meditation event-related spectral dynamics. A repeated-measures ANOVA was structured as 3 (passive control, passive meditation and active task) × 3 frontal/parietal electrode positions (frontal, central and parietal) × 3 coronal locations (left, central and right).
Greenhouse–Geisser df corrections were employed to correct for violations of the sphericity assumptions. The Tukey post-hoc means comparisons procedure was used to assess specific main effects and interaction outcomes of interest. Covariate analysis using experimental session order (control vs. meditation first), years of daily meditation practice and self-reported meditative depth also were conducted for each finding and are reported where significant.
RESULTS
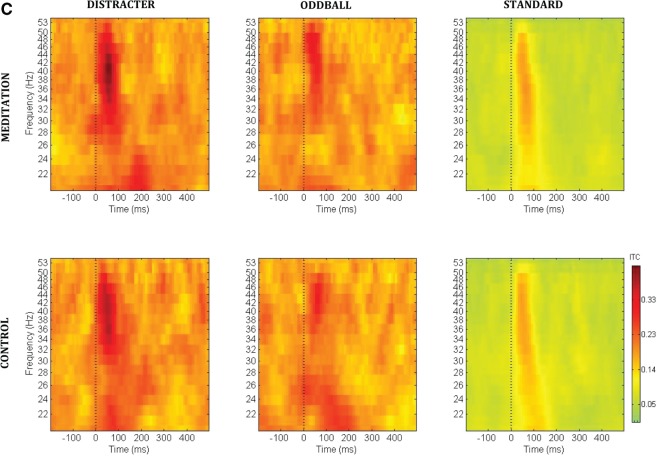
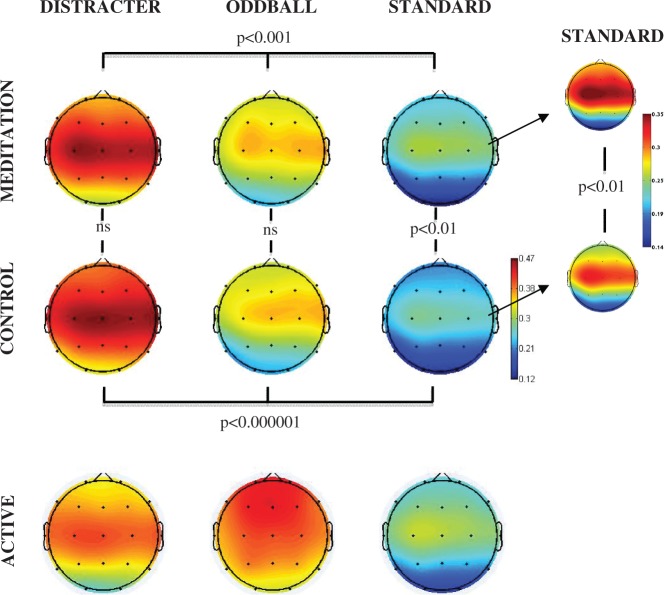
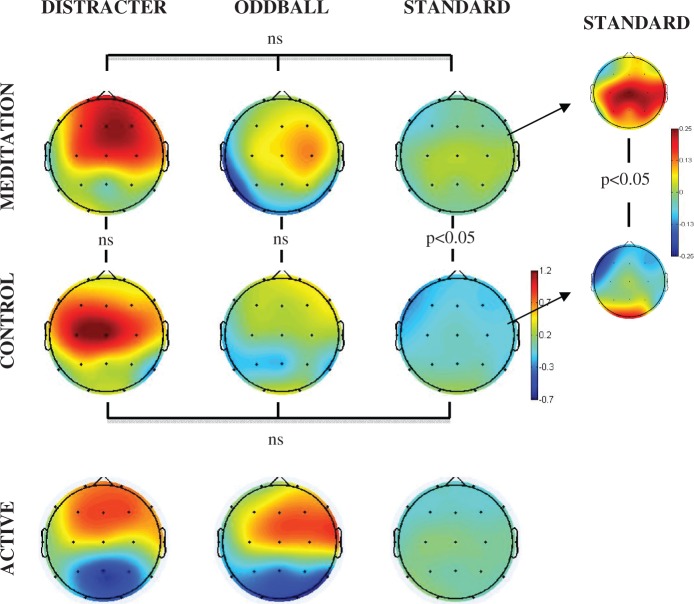
Figures 1A illustrates the event-related spectral perturbation power for each stimulus condition at the Cz electrode. Figure 1B illustrates the inter-trial coherence across the control and meditation conditions for each stimulus condition at the Cz electrode. The box outlines indicate the analyzed areas of the time frequency decomposition from the distracter stimulus during meditation in the upper left of each figure. Figure 1C illustrates the event-related inter-trial coherence at electrode Fz for the gamma (35–45 Hz) frequency range. Note that the corresponding inter-trial coherence color scale is reduced in range relative to the scale at lower frequencies to promote comparison among conditions. Figures 2–6 illustrate the scalp topography distributions for the time frequency decomposition at the maximally responsive latencies for the frequency bands of interest. Figure 2 shows the event-related spectral power in the 2–4 Hz delta band (100–500 ms). Figure 3 shows the inter-trial coherence in the 4–8 Hz theta band (100–400 ms). Figure 4 shows the early-alpha event-related spectral power in the 8–10 Hz band (50–250 ms). Figure 5 shows the late-alpha event-related spectral power in the 8–10 Hz band (500–900 ms). Figure 6 shows the inter-trial coherence (6A) and event-related spectral power (6B) in the 35–45 Hz gamma band.
Fig. 2.
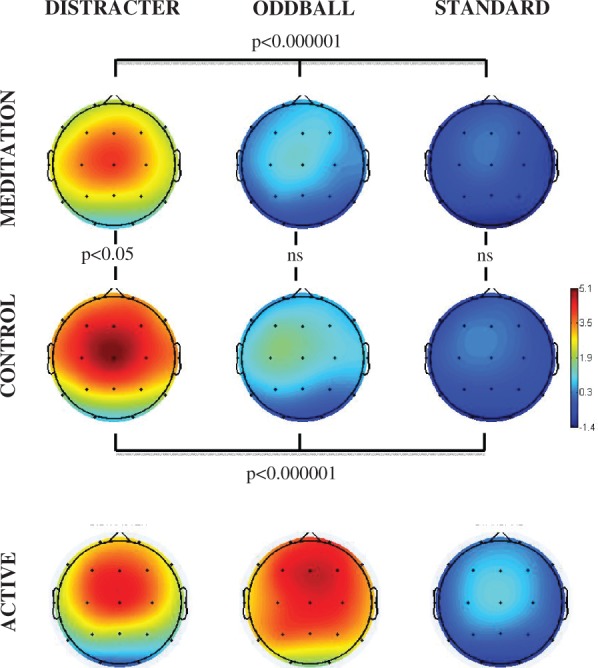
Delta (2–4 Hz, 100–500 ms post-stimulus) event-related spectral perturbation power (dB) scalp topography for each stimulus type (distracter, oddball and standard) and task (meditation, control and active) condition. The head plots indicate the post stimulus event-related spectral power change relative to baseline or the post stimulus inter-trial coherence (0–1 phase synchrony value). Omnibus ANOVA and post-hoc statistical outcomes (ns = not significant).
Fig. 6.
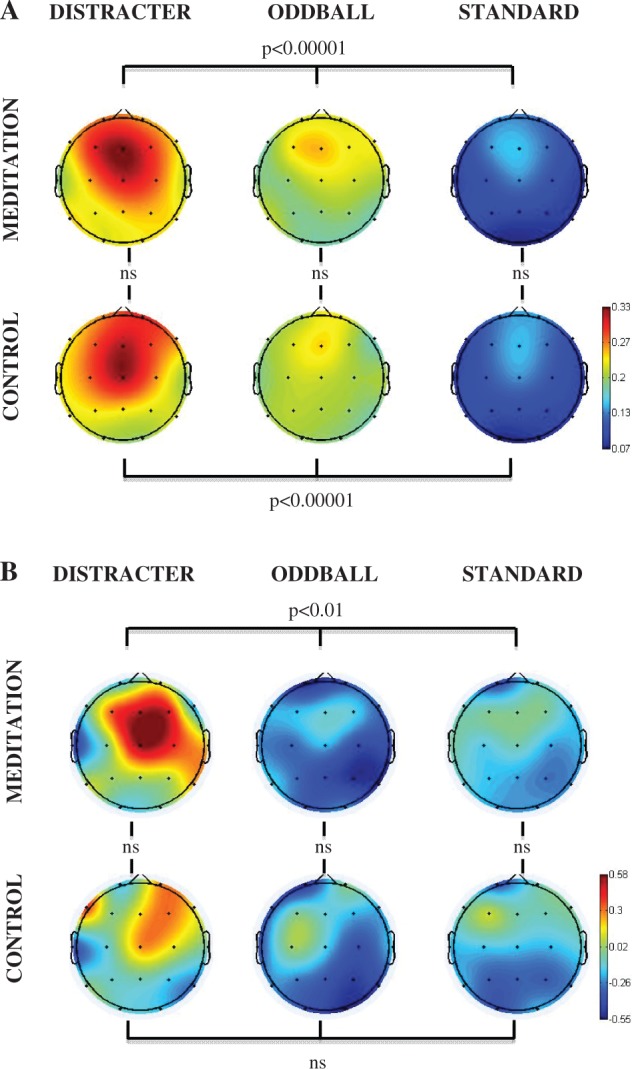
(A) Gamma (35–45 Hz, 20–100 ms post-stimulus) event-related inter-trial coherence (0–1 phase synchrony value) scalp topography for each stimulus type (distracter, oddball and standard) and task (meditation, control and active) condition. (B) Gamma (35–45 Hz, 20–100 ms post-stimulus) event-related spectral perturbation power (dB) scalp topography for each stimulus type (distracter, oddball and standard) and task (meditation, control and active) condition. The head plots indicate the post stimulus event-related spectral power (dB) change relative to baseline. Omnibus ANOVA and post-hoc statistical outcomes (ns = not significant). Please note that as detailed in the text, while there was no overall state effect of meditation on these gamma band measures, there were significant covariant interactions between meditation state and meditation expertise.
Fig. 3.
Theta inter-trial coherence (4–8 Hz, 100–400 ms post stimulus) scalp topography for each stimulus type (distracter, oddball and standard) and task (meditation, control and active) condition. The head plots indicate the post stimulus inter-trial coherence (0–1 phase synchrony value). Omnibus ANOVA and post-hoc statistical outcomes (ns = not significant). The smaller plots to the right of the main figure indicate the coherence strength for the standard stimuli, which is replotted with reduced scale to illustrate the difference between control and meditation state.
Fig. 4.
Early alpha-1 (8–10 Hz, 50–250 ms post stimulus) event-related spectral perturbation (dB) scalp topography for each stimulus type (distracter, oddball and standard) and task (meditation, control and active) condition. The head plots indicate the post stimulus event-related spectral power change relative to baseline. Omnibus ANOVA and post-hoc statistical outcomes (ns = not significant). The smaller plots to the right of the main figure indicate the event-related alpha power for the standard stimuli, which is replotted with reduced scale to illustrate the difference between control and meditation state.
Fig. 5.
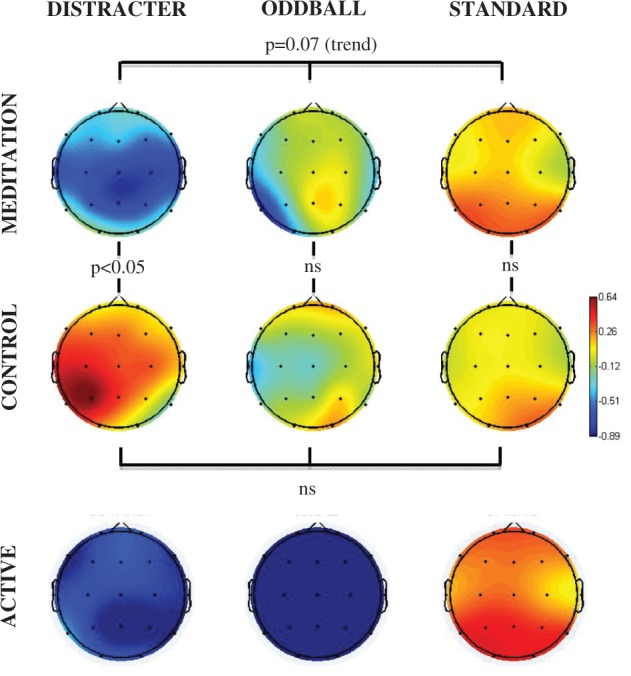
Late alpha-1 (8–10 Hz, 500–900 ms post stimulus) event-related spectral perturbation (dB) scalp topography for each stimulus type (distracter, oddball and standard) and task (meditation, control and active) condition. The head plots indicate the post stimulus event-related spectral power change relative to baseline. Omnibus ANOVA and post-hoc statistical outcomes (ns = not significant).
Standard stimulus-related processing
Theta (4–8 Hz) band
Figure 3 indicates that the inter-trial coherence in the theta frequency range from 100 to 400 ms post-stimulus was greatest following distracters, followed by oddballs and then standards. Standard stimuli also evinced significantly greater theta inter-trial coherence during meditation compared with control state. Statistical analysis of the theta inter-trial coherence across stimulus types was significant for both control [F(2,30) = 18.5, P < 0.00001] and meditation [F(2,30) = 9.23, P < 0.001] states. Comparing theta phase synchrony response with standards across control vs. meditation state yielded significant effect of state [F(1,15) = 9.01, P < 0.01], with no reliable outcomes found for the oddballs or distracters across state. A state × location interaction was obtained for the theta synchrony response to standards as well [F(2,30) = 4.4, P < 0.05]. Post-hoc analyses indicated that this was produced by significant meditation-state-related enhancement of theta phase locking to standards at frontal (P < 0.001) and central (P < 0.001) but not parietal (P = 0.27) locations. Although activity in the 4–8 Hz theta band did reflect enhanced power in the initial 400 ms post-stimulus and enhanced phase locking, no difference was found between states for event-related spectral power changes in this frequency band.
Alpha-1 (8–10 Hz)
Meditation effects were found for stimulus-related processing in the slow alpha 8–10 Hz (alpha-1) frequency range, one in response to standard stimuli and the second in response to distracter stimuli. No reliable meditation effects were obtained for the fast alpha frequency 10–12 Hz (alpha-2) range.
Early (50–250 ms) alpha-1 (8–10 Hz) band synchronization
Figure 4 indicates that the stimulus-related power in the early (50–250 ms) alpha band was greatest after the distracter stimuli followed by oddballs and then standards. The inlaid topography plots for the standard stimuli indicate an alpha synchronization response that was bilateral and central in topography and enhanced for the meditation relative to control state. Statistical analysis of the early alpha stimulus-related power across stimulus types was not significant for either the control (P = 0.17) or meditation (P = 0.64) state. However, comparing standards across control vs. meditation state did yield a significant effect of state [F(1,15) = 6.64, P < 0.05], whereas no reliable outcome was found comparing oddballs or distracters across state. Although activity in the 8–10 Hz alpha-1 band also showed enhanced inter-trial coherence (phase locking) during this time period, no differences were found comparing the event-related phase-locking values across states.
Distracter-related processing
Delta (2–4 Hz) band
Figure 2 illustrates that distracter stimuli were associated with significantly greater 2–4 Hz evoked power than oddball stimuli and oddball stimuli led to greater 2–4 Hz evoked power than standard stimuli in the 100–500 ms post-stimulus time range. This activity was enhanced for the oddball stimuli in the active task condition, wherein a response to the oddball stimulus was required of participants. Of note, during meditation compared with control state the relative increase in evoked 2–4 Hz power was not as pronounced. The 2–4 Hz spectral power across stimulus types was highly significant for both control [F(2,30) = 56.0, P < 0.000001] and meditation [F(2,30) = 33.3, P < 0.000001] states. Comparing distracters across control vs. meditation states yielded a significant effect of state [F(1,15) = 4.90, P < 0.05], but no effect was found comparing standards or oddballs between states. Although the activity in the 2–4 Hz delta band did show enhanced phase locking for distracter stimuli as well, no difference was seen comparing the phase locking in this frequency band across states.
Late alpha-1 desynchronization (500–900 ms)
Figure 5 illustrates the stimulus-related power in the late (500–900 ms) alpha-1 (8–10 Hz) band, which decreased after the distracter stimuli significantly more in the meditation compared with control state. The late alpha-1 stimulus-related spectral perturbation across stimulus types was not significant for control (P = 0.54) but did approach significance for meditation the state (P = 0.07). Moreover, comparing distracter outcomes across control vs. meditation state did yield a significant effect of state [F(1,15) = 5.57, P < 0.05], with a significantly less desynchronization found in alpha-1 power (event-related desynchronization) for the control relative to the meditation state. No difference was found comparing the late alpha response to standards or oddballs across state.
Early gamma power and phase synchrony (20–100 ms)
Figures 6A and B indicate that distracter stimuli evoked greater 35–45 Hz gamma synchrony (Figure 6A) and power (Figure 6B) compared to oddball and standard stimuli in the 20–100 ms post-stimulus time range. The statistical analysis of the 35–45 Hz phase synchrony data across stimulus types yielded significant outcomes for both control [F(2,30) = 24.0, P < 0.00001] and meditation [F(2,30) = 25.5, P < 0.00001] states. Gamma phase synchrony values did not produce any main effect of meditation state. During control compared with meditation state, the relative increase in stimulus-related gamma power was less pronounced across the three classes of auditory stimuli. The 35–45 Hz post-stimulus power across stimulus types was not significant for control [F(2,30) = 0.65, P > 0.5) state, but it was significant for meditation state [F(2,30) = 5.3, P < 0.01]. No state effects were obtained in assessing the stimulus-evoked gamma powers between states for each of the three stimulus types.
Individual differences
Categorization of participants into those with a history of >10 years (N = 10, M = 19.3, s.d. = 8.6, range 10–20 years) and those with <5 years (N = 6, M = 2.5, s.d. = 1.4, range 1–5 years) of daily meditation practice yielded significant interactions for event-related gamma phase synchrony changes to the distracter stimuli. This approach was applied instead of a median split to accommodate the appreciable difference of daily practice years between the six relative beginners and the more experienced practitioners. The interaction between meditation expertise status × state × frontality was significant for gamma phase synchrony [F(2,2,28) = 5.83, P < 0.01]. Post-hoc analysis indicated that for the shorter term meditator cohort yielded no significant differences comparing control and meditation states for gamma phase synchrony (P > 0.99, all comparisons). In contrast, for the longer term practitioners, meditation state yielded higher gamma phase synchrony values during meditation at frontal (P < 0.05), but not central or parietal sites (P > 0.97, all comparisons). Further, a marginal effect was obtained for the interaction among meditation expertise status × stimulus type × state [F(2,28) = 3.01, P = 0.075]. The shorter term meditator cohort tended towards greater distracter-related gamma phase synchrony in control state whereas the longer term meditator cohort tended toward greater distracter-related gamma phase synchrony in meditation state.
Years of daily meditation practice was also significantly related to the gamma power effects. When including number of practice years as a covariate in assessing the stimulus-evoked gamma power across the three stimulus types, a significant meditation expertise status × stimulus type × state × frontality interaction was obtained [F(4,56) = 3.61, P < 0.05]. Post-hoc assessment indicated that although no significant differences occurred between stimulus-related gamma power for standards or oddballs across groups, the longer-term meditator subjects showed increased distracter-evoked gamma power at frontal (P < 0.01), central (P < 0.001) and parietal (P < 0.001) sites during meditation. In contrast, the shorter term daily meditators showed no difference across states in distracter-evoked gamma power at frontal (P = 1.0) or central (P = 0.51) sites, and decreased distracter-related gamma power at parietal sites (P < 0.05) during meditation.
Meditation state and active task activity
Subsequent ANOVAs were performed with the inclusion of the active task measures at the frequency/latency combinations for each obtained meditation state effect. These included for standards—enhanced early alpha-1 power and theta synchrony, for distracters—decreased stimulus-related delta power and greater late alpha power reductions.
For the theta phase synchrony (100–400 ms) measure to standards, a significant state effect was obtained [F(2,30) = 6.36, P < 0.01]. Post-hoc analysis revealed that the passive control state was associated with significantly less theta synchrony to standards compared with active task activity (P < 0.005), but the passive meditation state was not significantly different from active task theta synchrony (P = 0.42). For the early (0–250 ms) alpha-1 power to standards, no significant state effect was found when including the active task comparator [F(2,30) = 1.44, P = 0.25].
For the delta power (100–500 ms) to distracters, a trend state effect was found when including the active task comparator [F(2,30) = 2.93, P = 0.075] with the control activity tending to be less than both the meditation (P = 0.1) and active task (P = 0.12) activity for this parameter, but missing statistical significance. For the late 500–900 ms alpha-1 (100–500 ms) power from distracter processing, a state effect was obtained when including the active task comparator [F(2,30) = 3.98, P < 0.05]. Post-hoc analysis indicated that active task late alpha-1 power decreases to distracter stimuli were significantly greater than the control state (P < 0.05) but indistinguishable from the meditation state (P = 0.97).
For the oddball stimuli, no significant state effect in the delta range was revealed comparing control and meditation states. Nonetheless, given the prominent oddball/target-evoked delta power in the active task, an ANOVA across all three states for the oddball stimulus was conducted revealing that for the delta power (100–500 ms) to oddballs a highly significant state effect was obtained [F(2,30) = 24.9, P < 0.00001]. Post-hoc analyses demonstrated that the active task condition was greater than both control (P < 0.0001) and meditation (P < 0.0001) conditions.
DISCUSSION
Vipassana meditative practice involves the adoption of a mindful and receptive mental awareness, with attentional absorption on present-moment body sensations and meta-cognitive reframing of ongoing experience as impersonal phenomena to be observed without reacting (Hart, 1987; Gunaratana, 2002; Lutz et al., 2007). The present findings describe further the neuroelectric brain state produced by this meditation practice (Cahn and Polich, 2006), and the results imply that such practice involves enhancement in early stimulus-representation of the habituated standard stimuli (Cahn et al., 2010). Also found was decreased frontal responsivity to distracting stimuli accompanied by a dissociation between the normal positive relationship between early enhanced P3/evoked delta and later event-related desynchronization of alpha power (Bernat et al., 2007; Cahn and Polich, 2009).
Meditation-related effects on processing of the habituated standard stimuli were demonstrated by increased early alpha power and increased theta phase synchrony. Early alpha-evoked power has been shown to be related to N1 amplitude and attentional engagement (Klimesch et al., 2004; Hanslmayr et al., 2007). Here, we show that during meditation early-evoked alpha power increased in amplitude specifically to the standard stimuli but not the oddball or distracter stimuli. This outcome is consistent with the notion of enhanced stimulus representation of the habituated standard stimuli and a previously reported trend towards enhanced frontal N1 amplitude to standards in this same dataset (Cahn and Polich, 2009). It is also of note that in a previous study assessing the effects of intensive meditation retreat on time-frequency EEG effects in the attentional blink paradigm an increase in early alpha power to the first stimulus of the two-stimulus sets was observed (Slagter et al., 2009).
Enhanced inter-trial coherence (phase-locking) in the theta frequency range (4–8 Hz) was found to accompany deviant stimuli thereby indexing attentional engagement and incorporation of sensory stimuli into conscious awareness (Kahana et al., 2001). This measure has been previously shown to be enhanced to target stimuli after a three-month period of intensive meditation in a cohort of long-term meditators (Lutz et al., 2009; Slagter et al., 2009). For the passive auditory oddball stimulus presentation in the present study, theta phase locking was specifically greater in meditation than control state for the habituated standard stimuli with no difference between states observed for the oddball or distracter stimuli. These alpha power and theta phase synchrony findings imply that in the meditation state the standard stimuli are processed to a greater degree than in the control state, thereby providing evidence for a de-automatization of habituation processes and indicative of an enhanced ‘present-minded’ brain state. In line with this interpretation, theta phase synchrony to standard stimuli during meditation was equivalent to that observed when the participants were more fully engaged with the auditory stimulus train during the active task condition, whereas in the control passive state there was a significant reduction in theta phase synchronization relative to both meditative and active states.
Specific findings for distracter-related processing were evinced in the delta, alpha and gamma frequency ranges. Evoked early gamma power was found to be differentially sensitive to the three stimulus types in meditation state but not the control state. This effect was driven by enhanced gamma processing to distracters in longer term meditators, with the shorter term meditator cohort showing no such tendency. Similar early gamma responses have been found to correlate with enhanced stimulus representation (Varela, 1995; Basar-Eroglu et al., 1996; Engel and Singer, 2001; Kang et al., 2005). Despite this early marker of enhanced stimulus representation of distracters, subsequent evoked power in the delta (2–4 Hz) range accompanied distracter stimulus processing but was decreased in amplitude during meditation relative to the control state. Evoked delta activity has been linked to the P3 event-related potential, attentional engagement and cognitive elaboration (Basar et al., 2001; Ishii et al., 2009).
Finally, the late alpha event-related desynchronization to distracters was of greater magnitude in meditation compared with control state. This alpha frequency appears related to fundamental cognitive processing such that the degree of earlier low frequency/P3 activity reflects attentional engagement and tends to predict greater late alpha power reductions (Intriligator and Polich, 1994, 1995; Krause et al., 1994; Yordanova et al., 2001; Krause, 2006; Bernat et al., 2007; Digiacomo et al., 2007). Comparing these distracter-related effects with the activity during active task processing indicated that the evoked delta power is a reliable measure of cognitive engagement, as it was highly enhanced in response to oddball stimuli when participants performed the detection task and responded to oddballs as targets. Delta power effects also suggest that the late alpha desynchronization is related to more active processing of the stimuli as this spectral power decrease was of greatest magnitude during active task processing of oddballs. Of note, the degree of late alpha desynchronization to distracters in the active task was shown to be indistinguishable from the meditation state and in both cases greater than the control state.
This latter result seems to imply that while there was a decrease in the mechanism of frontal attentional engagement to the distracting stimuli in meditation, a simultaneous and somewhat paradoxically increased widespread active processing occurred for these stimuli as indexed by the alpha desynchronization effect. The greater alpha power reductions obtained for the distracters in the meditation state also support the hypothesis that there may be a relationship between this measure and the early findings of decreased habituation to infrequent stimuli reported with Zen meditators, although not replicated by all groups (Kasamatsu and Hirai, 1966; Becker and Shapiro, 1981). In those early studies, auditory stimuli were presented approximately once every 10 seconds, with alpha desynchronization measured in the seconds after stimulus presentation. In contrast, this study used a once per second stimulus inter-trial interval, but the overall time between distracter stimuli was still on the order of 10 s. A decrease in alpha power was discernible in the 500–900 ms post-stimulus time frame to those stimuli engaging attention more strongly, evidenced most clearly by observing the very significant alpha power reduction to the targets in the active task condition. The greater decrease in alpha power in the meditation state compared with the control state may reflect a lack of habituation of the alpha desynchronizing effects induced by the distracters. Thus, it may be the case that in both passive states the early stimulus train distracters evoked some alpha desynchronization, but this effect decreased with time in control but not meditative state. It is also possible that a differential degree of evoked delta power habituation and/or gamma phase synchrony and power habituation was partly responsible for the meditation state (and expertise related) effects on these neural measures. Future studies that compare successive stimuli across different paradigms in meditation vs. control tasks would help identify whether differential habituation across stimuli is meaningfully related to these neural signatures of altered processing in the meditative state.
In sum, these distracter-processing effects indicate a tendency towards greater stimulus representation that is indexed by enhanced gamma phase synchrony and power in the first 100 ms post-stimulus specific to longer-term meditators. In addition, the subsequent decrease in distracter-related frontal processing is associated with reduced evoked delta power and a simultaneous increase in late alpha desynchronization. Taken together with the effects on enhanced measures of processing the habituated standard stimuli, this study supports the notion that Vipassana meditation leads to enhanced clarity of awareness across background stimuli, with simultaneously reduced frontal mechanisms of reactivity to distracting and/or aversive stimuli.
Significance of active task comparison
This study involved the presentation of the auditory oddball paradigm three separate times to each participant, the first two times with instructions to the participants to ignore the stimuli while meditating or engaging in the control cognitive task, and the final time with instruction to actively engage in the task and respond to the oddball stimuli. A detailed comparison between active and passive task processing was not pursued, given that these comparisons have been made previously (Polich and McIsaac, 1994; Mertens and Polich, 1997; Jeon and Polich, 2001) and are not particularly informative regarding the specific effects of meditation. Rather the active task condition is included to highlight the functional role played by the various stimulus-induced effects on spectral power and phase synchrony.
The distracter-related increase in evoked delta power that decreased in the meditation state can be interpreted as index of attentional engagement, and this functional perspective is supported by the large delta power increase to the oddball targets in the active task. More important are the meditation-related effects showing a greater similarity between the meditation state and active task processing. In response to the standard stimuli, theta phase synchrony was increased in meditation and can be interpreted as a measure of enhanced processing engaged by these frequent and repeated stimuli during the meditation state. This outcome is similar to the theta phase synchrony to the standards during the active task, which was also found to be increased and indistinguishable from the meditation state but of greater amplitude in the active and control tasks. Similarly, meditation compared with control task enhanced the distracter-related late alpha event-related desynchronization. The active task demonstrated a similar distracter-related late alpha decrease in power that was statistically equivalent to the meditation state. This finding is consistent with the view that the late alpha desynchronization that was enhanced in the meditative state reflects active distracter processing.
Theoretical perspectives
EEG activity during auditory oddball stimuli processing demonstrates decreased engagement of the frontal attentional systems to distracters during meditation relative to the control period. This result is consistent with the present decrease in evoked 2–4 Hz activity and previously reported decrease in P3a distracter amplitude and P2 oddball amplitude. These were obtained concomitantly with an increase in theta phase consistency and early alpha event-related synchronization (power) to the standards. Hence, the Vipassana meditation state is associated with enhanced early sensory processing/representation and simultaneous top-down control of the elaborative attentional engagement with the contents of awareness. These finding are consistent with enhancement of neural signatures of attentional stability and efficiency due to meditative interventions (Jha et al., 2007; Slagter et al., 2007; Lutz et al., 2009; Slagter et al., 2009; van Vugt and Jha, 2011).
Previous findings on spontaneous EEG in these same practitioners revealed the primary effects of the Vipassana meditation state as producing decreased frontal delta power likely originating from frontal activation and increased in parietooccipital gamma power originating from parietooccipital activation, and moderate relative increase in frontal theta power. Given the association of increased slow delta activity during deep sleep and the decrease in gamma power during sleep, these overall state effects may likely reflect enhanced ‘awakeness’ (Maloney et al., 1997; Cantero et al., 2004; Cantero and Atienza, 2005). It is therefore reasonable to suppose that Vipassana meditative practice promotes a more active brain state, which is implied by the finding that both the standard and distracter processing were more similar during active task conditions to the passive meditation state than to the passive control cognitive state.
The present time-frequency findings also appear to indicate that mindfulness/open-monitoring practices such as Vipassana widen the ‘attentional spotlight’ on present-moment sensory input. This phenomenon may be characterized by cortical engagement that mediates enhanced stimulus representation and subsequent clarity of awareness. This may account for the present findings of enhanced spontaneous and evoked gamma to distracters in longer-term practitioners, as well as the early alpha synchronization and theta phase coherence to standards across all participants. These empirical effects occur with concomitant decreases in cognitive elaboration on the stimulus environment to affect distracter-related evoked 2–4 Hz power and P3a amplitude. Practices involving the narrowing of the attentional spotlight, such as mantra and breath-focused attention, may be associated with greater baseline frontal midline theta engagement and less enhanced measures of external stimulus representation such as theta inter-trial coherence to standard stimuli. Such concentrative meditative practices may not be accompanied by the unique dissociation between early-evoked delta power and late alpha desynchronization to distracters found here, as processing of such distracter stimuli might be even further inhibited during such practice possibly leading to both decreased evoked delta power and late alpha desynchronization.
The present findings in relation to the processing of habituated standard and distracting deviant stimuli over the course of the 1 second following stimulus presentation are consonant with a rich and growing field of research describing the effects of mindfulness training. Findings indicating decreased default mode network activation indicative of state and trait decreases in the tendency toward both stimulus-induced and baseline self-narrative-generating cognitive processes are growing at an accelerating pace (Pagnoni et al., 2008; Brewer et al., 2011; Taylor et al., 2011; Hasenkamp and Barsalou, 2012; Hasenkamp et al., 2012; Taylor et al., 2012). In addition, in relation with affective processes, it has been demonstrated that meditative training (Farb et al., 2007, 2010) as well as trait mindfulness characteristics (Creswell et al., 2007) demonstrate parallel findings to those presented here on the processing of affect and automated self-related cognitive reactivity. Specifically, it has been shown repeatedly that mindfulness/meditative training correlates with enhanced ‘early’ affect representation at the level of insular and somatosensory activation while simultaneously resulting in a decrease in elaborative self-related cognitive processing and both amygdala and medial prefrontal reactivity to cognitive and affective content (Brefczynski-Lewis et al., 2007; Creswell et al., 2007; Farb et al., 2007, 2010, 2012; Way et al., 2010). Similarly, studies of the effects of pain processing from long term (Grant and Rainville, 2009; Grant et al., 2011) as well as short term (Zeidan et al., 2011) mindfulness-type meditation point toward the combination of enhanced stimulus representation at the level somatosensory/insular cortex activation and decreased evaluative attentional circuitry in combination with increased pain tolerance. Similar to our interpretation of the time-frequency findings presented here, these meditation-related findings on affect and pain have been taken to be indicative of greater present-moment awareness and body state representation occurring simultaneously with a mode of neurophysiologic processing with decreased cognitive and emotional automated reactivity. It is of some theoretical interest to verify whether meditative training does indeed engage brain changes related to the altered fast (sub-second) processing of basic auditory stimuli as detailed here, and the temporal and/or causative relationship between such changes and the changes in functional activation measurable on the scale of seconds to minutes in the default mode network activation, affective and pain-related processing resulting from such training.
CONCLUSIONS
The present time frequency analysis of the three-stimulus auditory paradigm indicates that sensory encoding-related theta phase consistency and early event-related alpha synchronization are increased to standard stimuli in meditation relative to control state. In addition, the attention-related recruitment of 2–4 Hz power to distracters was decreased in meditation, which indicates decreased distracter-related frontal delta power. This was complemented by the late event-related alpha desynchronization to the distracters that was actually increased in meditation. Thus, Vipassana practice may index a meditation-related transition in the cognitive processing of distraction from an early frontally focused activity to a later mechanism involving widespread centroparietal areas.
Further studies contrasting Vipassana with focused attention practices such as those involving purely breath awareness, mantra and/or visualization would help define specificity of the delta, theta and alpha time-frequency effects in relation to auditory stimuli seen in these other modes of meditative engagement. Indeed, the particular Vipassana meditation teaching tradition assayed in this study may involve a more concentrative focus than some other forms of Vipassana practice, as the latter incorporate more specific injunctions to welcome other aspects of ongoing internal/external experience outside of somatic sensations alone into the attentional spotlight. Given that such meditative practices are clinically efficacious, how Vipassana and other meditation practices modulate the spectral dynamics associated with perception in patients with psychiatric symptoms and whether meditation state-related changes in processing may correlate with improvements in clinical status is of medical and theoretical import (Cahn and Polich, 2006; Rubia, 2009; Farb et al., 2012).
In sum, Vipassana meditation leads to a state of altered attentional engagement with the sensory surround marked by enhanced stimulus encoding to both standard and distracter stimuli. An altered form of attentional engagement to distracting stimuli yields a unique dissociation between early-evoked delta/P3 activity, which is decreased, and later event-related alpha desynchronization, which is actually increased. These data support the conclusion that this meditative practice may lead to enhanced sensitivity and awareness with decreased automated stimulus reactivity. If so, the decreased automated brain reactivity to distraction may reflect decreased obligatory recruitment of frontal circuitry and the processing of stimuli to be mediated through a more widespread cortical network.
ACKNOWLEDGEMENTS
B.R.C. was supported by a grant by the Fetzer Institute to himself and Franz X. Vollenweider. B.R.C. was also supported by the NIH Medical Scientist training grant T32 GM07198 and the NIH Post-doctoral training grant T32 MH019934. J.P. was supported by the NIH grants DA018262 and P50 AA06420.
REFERENCES
- Aftanas LI, Golocheikine SA. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neuroscience Letters. 2001;310(1):57–60. doi: 10.1016/s0304-3940(01)02094-8. [DOI] [PubMed] [Google Scholar]
- Anand BK, Chhina GS, Singh B. Some aspects of electroencephalographic studies in yogis. Electroencephalography and Clinical Neurophysiology. 1961;13:452–6. [Google Scholar]
- Andresen J. Meditation meets behavioural medicine: the story of experimental research on meditation. Journal of Consciousness Studies. 2000;7(11–12):17–73. [Google Scholar]
- Baijal S, Srinivasan N. Theta activity and meditative states: spectral changes during concentrative meditation. Cognitive Processing. 2010;11(1):31–8. doi: 10.1007/s10339-009-0272-0. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. International Journal of Psychophysiology. 1996;24(1–2):101–12. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology. 2001;39(2–3):241–8. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Becker DE, Shapiro D. Physiological responses to clicks during Zen, Yoga, and TM meditation. Psychophysiology. 1981;18(6):694–9. doi: 10.1111/j.1469-8986.1981.tb01846.x. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Malone SM, Williams WJ, Patrick CJ, Iacono WG. Decomposing delta, theta, and alpha time-frequency ERP activity from a visual oddball task using PCA. International Journal of Psychophysiology. 2007;64(1):62–74. doi: 10.1016/j.ijpsycho.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski-Lewis JA, Lutz A, Schaefer HS, Levinson DB, Davidson RJ. Neural correlates of attentional expertise in long-term meditation practitioners. Proceedings of National Academy of Science of United States of America. 2007;104(27):11483–8. doi: 10.1073/pnas.0606552104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of National Academy of Science of United States of America. 2011;108(50):20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Delorme A, Polich J. Occipital gamma activation during Vipassana meditation. Cognitive Processing. 2010;11(1):39–56. doi: 10.1007/s10339-009-0352-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychological Bulletin. 2006;132(2):180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation (Vipassana) and the P3a event-related brain potential. International Journal Psychophysiology. 2009;72(1):51–60. doi: 10.1016/j.ijpsycho.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Atienza M. The role of neural synchronization in the emergence of cognition across the wake-sleep cycle. Reviews in the Neurosciences. 2005;16(1):69–83. doi: 10.1515/revneuro.2005.16.1.69. [DOI] [PubMed] [Google Scholar]
- Cantero JL, Atienza M, Madsen JR, Stickgold R. Gamma EEG dynamics in neocortex and hippocampus during human wakefulness and sleep. Neuroimage. 2004;22(3):1271–80. doi: 10.1016/j.neuroimage.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of National Academy of Science of United States of America. 2009;106(21):8719–24. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs LA, Polich J. P3a from auditory white noise stimuli. Clinical Neurophysiology. 2006;117(5):1106–12. doi: 10.1016/j.clinph.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a and P3b from typical auditory and visual stimuli. Clinical Neurophysiology. 1999;110(1):24–30. doi: 10.1016/s0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69(6):560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective neuroscience and psychophysiology: toward a synthesis. Psychophysiology. 2003;40(5):655–65. doi: 10.1111/1469-8986.00067. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34(4):1443–9. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A, Comerchero M, Polich J. Wavelet analysis of P3a and P3b. Brain Topography. 2001;13(4):251–67. doi: 10.1023/a:1011102628306. [DOI] [PubMed] [Google Scholar]
- Digiacomo MR, Gomez CM, Flores AB. Alpha reduction and event-related potentials, theta and gamma increase linked to letter selection. Neuroreport. 2007;18(8):729–33. doi: 10.1097/WNR.0b013e3280c1e370. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cognitive Science. 2001;5(1):16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Mayberg H, Bean J, McKeon D, Segal ZV. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Segal ZV. The mindful brain and emotion regulation in mood disorders. Canadian Journal of Psychiatry. 2012;57(2):70–7. doi: 10.1177/070674371205700203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Mayberg H, Bean J, McKeon D, Fatima Z, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2(4):313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goupillaud P, Grossman A, Morlet J. Cycle-octave and related transforms in seismic signal analysis. Geoexploration. 1984;23:85–102. [Google Scholar]
- Grant JA, Courtemanche J, Rainville P. A non-elaborative mental stance and decoupling of executive and pain-related cortices predicts low pain sensitivity in Zen meditators. Pain. 2011;152(1):150–6. doi: 10.1016/j.pain.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Grant JA, Rainville P. Pain sensitivity and analgesic effects of mindful states in Zen meditators: a cross-sectional study. Psychosomatic Medicine. 2009;71(1):106–14. doi: 10.1097/PSY.0b013e31818f52ee. [DOI] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. Journal of Psychosomatic Research. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Gunaratana H. Mindfulness in Plain English. Boston, MA: Wisdom Publications; 2002. [Google Scholar]
- Hanslmayr S, Klimesch W, Sauseng P, et al. Alpha phase reset contributes to the generation of ERPs. Cerebral Cortex. 2007;17(1):1–8. doi: 10.1093/cercor/bhj129. [DOI] [PubMed] [Google Scholar]
- Hart W. The Art of Living: Vipassana Meditation as Taught by S. N. Goenka. New York, NY: HarperOne; 1987. [Google Scholar]
- Hasenkamp W, Barsalou LW. Effects of meditation experience on functional connectivity of distributed brain networks. Frontiers Human Neuroscience. 2012;6:38. doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. Neuroimage. 2012;59(1):750–60. doi: 10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hebert R, Lehmann D. Theta bursts: An EEG pattern in normal subjects practising the transcendental meditation technique. Electroencephalography and Clinical Neurophysiology. 1977;42(3):397–405. doi: 10.1016/0013-4694(77)90176-6. [DOI] [PubMed] [Google Scholar]
- Hirai T. Psychophysiology of Zen. Tokyo: Igaku Shoin; 1974. [Google Scholar]
- Intriligator J, Polich J. On the relationship between background EEG and the P300 event-related potential. Biological Psychology. 1994;37(3):207–18. doi: 10.1016/0301-0511(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Intriligator J, Polich J. On the relationship between EEG and ERP variability. International Journal Psychophysiology. 1995;20(1):59–74. doi: 10.1016/0167-8760(95)00028-q. [DOI] [PubMed] [Google Scholar]
- Ishii R, Canuet L, Herdman A, et al. Cortical oscillatory power changes during auditory oddball task revealed by spatially filtered magnetoencephalography. Clinical Neurophysiology. 2009;120(3):497–504. doi: 10.1016/j.clinph.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. P3a from a passive visual stimulus task. Clinical Neurophysiology. 2001;112(12):2202–8. doi: 10.1016/s1388-2457(01)00663-0. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive Affective & Behavavioral Neuroscience. 2007;7(2):109–9. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000a;37(2):163–78. [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000b;111(10):1745–58. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. General Hospital Psychiatry. 1982;4(1):33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Mindfulness-based interventions in context: Past, present, and future. Clinical Psychology: Science and Practice. 2003;10(2):144–58. [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Current Opinions in Neurobiology. 2001;11(6):739–44. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kang K, Williams LM, Hermens D, Gordon E. Neurophysiological markers of contextual processing: the relationship between P3b and Gamma synchrony and their modulation by arousal, performance and individual differences. Brain Research, Cognitive Brain Research. 2005;25(2):472–83. doi: 10.1016/j.cogbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Kasamatsu A, Hirai T. An electroencephalographic study on the zen meditation (Zazen) Folia Psychiatrica et Neurologica Japonica. 1966;20(4):315–36. doi: 10.1111/j.1440-1819.1966.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Katayama J, Polich J. Stimulus context determines P3a and P3b. Psychophysiology. 1998;35(1):23–33. [PubMed] [Google Scholar]
- Kinoshita K. A study on response of EEG during Zen meditation—alpha-blocking to name calling (in Japanese) Seishin Shinkeigaku Zasshi. 1975;77(9):623–58. [PubMed] [Google Scholar]
- Klimesch W. Memory processes, brain oscillations and EEG synchronization. International Journal of Psychophysiology. 1996;24(1–2):61–100. doi: 10.1016/s0167-8760(96)00057-8. [DOI] [PubMed] [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research. Brain Research Reviews. 1999;29(2–3):169–95. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neuroscience and Biobehavioral Reviews. 2007;31(7):1003–16. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1-N1 complex and are related to memory performance. Brain Research Cognitive Brain Research. 2004;19(3):302–16. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Krause CM. Cognition- and memory-related ERD/ERS responses in the auditory stimulus modality. Progress in Brain Research. 2006;159:197–207. doi: 10.1016/S0079-6123(06)59013-2. [DOI] [PubMed] [Google Scholar]
- Krause CM, Lang HA, Laine M, Helle SI, Kuusisto MJ, Porn B. Event-related desynchronization evoked by auditory stimuli. Brain Topography. 1994;7(2):107–12. doi: 10.1007/BF01186768. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Rosman IS, Vangel M, et al. Functional brain imaging of mindfulness and mantra-based meditation. Paper presented at the Society for Neuroscience, New Orleans, LA. 2003 [Google Scholar]
- Lehrer PM, Schoicket S, Carrington P, Woolfolk RL. Psychophysiological and cognitive responses to stressful stimuli in subjects practicing progressive relaxation and clinically standardized meditation. Behaviour Research & Therapy. 1980;18(4):293–303. doi: 10.1016/0005-7967(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Lutz A, Dunne JD, Davidson RJ. Meditation and the neuroscience of consciousness. In: Zelazo PD, Moscovitch M, Thompson E, editors. The Cambridge Handbook of Consciousness. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Long-term meditators self-induce high-amplitude gamma synchrony during mental practice. Proceedings of National Academy of Science United States Academy. 2004;101(46):16369–73. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Science. 2008;12:163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, Davidson RJ. Mental training enhances attentional stability: neural and behavioral evidence. Journal of Neuroscience. 2009;29(42):13418–27. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76(2):541–55. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Picton TW. EEG spectral dynamics during discrimination of auditory and visual targets. Brain Research and Cognitive Brain Research. 2005;24(1):81–96. doi: 10.1016/j.cogbrainres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Mertens R, Polich J. P300 from a single-stimulus paradigm: passive versus active tasks and stimulus modality. Electroencephalography and Clinical Neurophysiology. 1997;104(6):488–97. doi: 10.1016/s0168-5597(97)00041-5. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. International Journal of Psychophysiology. 2001;43(1):41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. Alpha rhythms as physiological and abnormal phenomena. International Journal of Psychophysiology. 1997;26(1–3):31–49. doi: 10.1016/s0167-8760(97)00754-x. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M, Guo Y. “Thinking about not-thinking”: neural correlates of conceptual processing during Zen meditation. PLoS One. 2008;3(9):e3083. doi: 10.1371/journal.pone.0003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60(2):172–85. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Polich J, McIsaac HK. Comparison of auditory P300 habituation from active and passive conditions. International Journal of Psychophysiology. 1994;17(1):25–34. doi: 10.1016/0167-8760(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Rabiner LR, Gold B. Theory and Application of Digital Signal Processing. Englewood Cliffs, NJ: Prentice-Hall; 1975. [Google Scholar]
- Rubia K. The neurobiology of meditation and its clinical effectiveness in psychiatric disorders. Biological Psychology. 2009;82(1):1–11. doi: 10.1016/j.biopsycho.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar E. Functional aspects of alpha oscillations in the EEG. International Journal of Psychophysiology. 2001;39(2–3):151–8. doi: 10.1016/s0167-8760(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press; 2002. [Google Scholar]
- Slagter HA, Davidson RJ, Lutz A. Mental training as a tool in the neuroscientific study of brain and cognitive plasticity. Frontiers in Human Neuroscience. 2011;5:17. doi: 10.3389/fnhum.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, et al. Mental training affects distribution of limited brain resources. PLoS Biology. 2007;5(6):1228–35. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Nieuwenhuis S, Davidson RJ. Theta phase synchrony and conscious target perception: impact of intensive mental training. Journal of Cognitive Neuroscience. 2009;21(8):1536–49. doi: 10.1162/jocn.2009.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychological Bulletin. 2006;132(6):946–58. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Science. 1999;3(4):151–62. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Taylor VA, Daneault V, Grant J, et al. Impact of meditation training on the default mode network during a restful state. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nsr087. http://www.ncbi.nlm.nih.gov/pubmed/22446298 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor VA, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. Neuroimage. 2011;57(4):1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal Z, Williams JMG. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behaviour Research & Therapy. 1995;33(1):25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting & Clinical Psychology. 2000;68(4):615–23. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Jha AP. Investigating the impact of mindfulness meditation training on working memory: A mathematical modeling approach. Cognitive Affective & Behavioral Neuroscience. 2011;11(3):344–53. doi: 10.3758/s13415-011-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ. Resonant cell assemblies: a new approach to cognitive functions and neuronal synchrony. Biological Research. 1995;28(1):81–95. [PubMed] [Google Scholar]
- Way BM, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10(1):12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger MA, Bagchi BK. Studies of autonomic functions in practitioners of Yoga in India. Behavioral Science. 1961;6:312–23. doi: 10.1002/bs.3830060407. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Polich J. P300 and alpha event-related desynchronization (ERD) Psychophysiology. 2001;38(1):143–52. [PubMed] [Google Scholar]
- Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience. 2011;31(14):5540–8. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]