Abstract
An extensive body of research defines the default-mode network (DMN) to be one of the critical networks of the human brain, playing a pivotal functional role in processes of internal mentation. Alterations in the connectivity of this network as a function of aging have been found, with reductions associated with functional ramifications for the elderly population. This study examined associations between integrity of the DMN and trait levels of mindfulness disposition, defined by our ability to exert attentional and emotional control in the present moment, and, thereby, bring awareness to immediate experiences. Twenty-five older adults participated in the study and underwent a brief functional magnetic resonance imaging session and filled out questionnaires related to their overall health and mindfulness disposition. Mindfulness disposition was associated with greater connectivity of the DMN, specifically, in the dorsal posterior cingulate cortex and the precuneus. Mindfulness disposition, thus, explains variance in the connectivity of one of the more intrinsic networks of the human brain, known to be critical for promoting self-relevant mental explorations and building cognitive and affective control.
Keywords: mindfulness, default network connectivity, individual differences, aging
INTRODUCTION
The supersonic rise of technology has fostered a modern day lifestyle characterized by digital connectivity, fast-paced intercommunication and constant hustle and bustle, both in the workplace and home life. The constant ‘doing’ and ‘multi-tasking’ that typifies day-to-day life in the modern age has the potential to not only diminish the richness of our experiences but also reduce focused attention to the present, waking moments of our lives. Mindfulness is becoming increasingly popular both in the scientific world and the general public for its potential to provide an alternate mode of ‘being’ (Williams, 2010). Defined as the ability to experience the present moment in its entirety, mindfulness involves paying attention to internal and external phenomena as they unfold, moment by moment (Kabat-Zinn, 1994), and is, thus, distinguished by its direct diametric association with tangential, mind-wandering behavior (Mrazek et al., 2012). Although the study of mindfulness is still in its infancy, both cross-sectional studies, treating mindfulness disposition as an individual difference variable (Way et al., 2010), and randomized controlled trials, designed to teach the principles and practice of mindfulness, have been conducted (Jha et al., 2007; Goldin and Gross, 2010) to examine the impact of mindfulness on affective and cognitive functioning, along with general well-being.
Expert meditators have been found to perform significantly better than novices on tasks of selective and sustained attention (Moore and Malinowski, 2009; van den Hurk et al., 2010), show greater gray matter volumes in the prefrontal and temporal cortices (Holzel et al., 2008; Luders et al., 2009), and greater cortical thickness in the frontal cortices and the anterior insula (Lazar et al., 2005). Furthermore, practitioners of Zen meditation have also been shown to counter normal age-related decline in sub-cortical gray matter volume, thus providing preliminary support for the role of meditation and mindfulness practices as a putative buffer against the neurobiological cascades of aging (Pagnoni and Cekic, 2007). Cross-sectional examinations of mindfulness disposition in healthy young adults also provide evidence for a positive association between trait levels of mindfulness disposition and an efficient neural recruitment of the cortico-subcortico circuitry engaged in emotional regulation (Creswell et al., 2007; Way et al., 2010). Extending these findings, randomized controlled studies have found such training programs to be prophylactic for enhancing positive mood (Ortner et al., 2007) and emotional regulation (Goldin and Gross, 2010), decreasing perseverative cognition and overall negative affect (Kuyken et al., 2008; Craigie and Nathan, 2009) and increasing working memory and attentional capacities (Jha et al., 2007, 2010). Taken together, these results lend preliminary support for the prophylaxis afforded by mindfulness interventions for both cognitive and emotional control, influencing behavior and neural functioning.
In this study, our goal was to examine the association between mindfulness disposition, as assessed by the Mindfulness Attention and Awareness Scale (MAAS; Brown and Ryan, 2003), and connectivity of one of the more critical networks of the human brain, the default-mode network (DMN), in community-dwelling older adults. The DMN peaked the curiosity of the scientific world after a seminal meta-analysis published by Shulman et al. (1997), where they noted a set of midline cortical regions to show consistent deactivation across tasks and populations. A decade of scientific exploration on the functional significance of this network provides evidence for this system being one of the core networks defining the functional architecture of the brain, with some of the regions augmenting integrative processing (Buckner et al., 2009). The activation of this network is reliably observed when attention is oriented away from external stimuli to internally directed thoughts (Greicius et al., 2003; Buckner et al., 2008), thus suggesting the DMN’s potential role in mental self-explorations and consolidation of activity during exogenous processing (Raichle, 2010). Thus, given the engagement of this network in internal channels of thoughts, and that of mindfulness on awareness of the present thoughts through attentional regulation and interoceptive awareness, we reasoned that individuals with higher levels of mindfulness disposition would demonstrate greater integrity in areas of the DMN that show an age-related decline in connectivity, relative to individuals lying on the lower end of the mindfulness continuum. Here, we note the multi-faceted nature of mindfulness as a theoretical construct and the high degree of divergence associated with the operationalization of this term (Grossman, 2011). The complexities of mindfulness have spurred the development of a multitude of measures aimed at quantifying different aspects of this construct, from mindful awareness to openness to experience. To maintain a consistent line of research, this study specifically chose to employ the MAAS, a standard questionnaire targeting attention to day-to-day experiences and one of the most extensively used measures in the mindfulness literature. This measure has been associated with sound psychometric properties and has also shown a strong association with cortical areas involved in affective and self-referential processing (Way et al., 2010).
METHODS AND MATERIALS
Participant characteristics
Twenty-five older participants (mean age = 65.88 years, 76% female, mean education = 14. 8 years) were recruited for this study. The age range of our participants was 60–75 years with an s.d. of 3.78 years. Inclusionary criteria included a score >23 on the Mini-Mental Status Examination (maximum score = 30; Folstein et al., 1975), corrected (near and far) acuity 20/40 or better, right-handedness as assessed by the Edinburgh Handedness Inventory, between the ages of 60–75 years, no history of psychiatric or neurological disorders, and suitability for participation in a magnetic resonance imaging (MRI) environment. The Ohio State Institutional Review Board approved this study and all participants provided written informed consent.
Measures
Mindfulness disposition—All participants were administered the MAAS (Brown and Ryan, 2003) to assess trait levels of mindfulness disposition. MAAS is a 15-item, single-factor questionnaire designed to assess the ability to focus on present moment experiences and disengage from a mechanical, ‘auto-pilot’ mode of functioning. All items are rated on a six-point likert scale (almost always—almost never) and the questionnaire has been shown to have good internal consistency and test–retest reliability (Brown and Ryan, 2003). Cronbach’s alpha for our study for this measure was 0.90.
Please note that this study was embedded in the context of a larger database of neuroimaging, neuropsychological and questionnaire data that was collected to examine the association between mindfulness disposition and cognitive and emotional health.
Functional and structural MRI parameters
Participants were scanned at the Wright Center of Innovation on Ohio State campus using a 3 T Philips full body scanner. High-resolution structural images were collected for each participant using a three-dimensional magnetization-prepared rapid gradient-echo imaging protocol with 160 contiguous sagittal slices [time to echo (TE)/time to repetition (TR)/TI 3.7/8.2/1017 ms], collected in an ascending fashion, parallel to the anterior and posterior commissures using a spoiled gradient sequence (240 × 240 mm field of view; 1 mm thick slices, with a 1 × 1 × 1 mm in-plane resolution) with a flip angle of 8°. Resting-state dataset was acquired following a fairly standard protocol in which participants were asked to lie down still on the scanner bed. Ambient light was minimized, and participants were asked to lie with their eyes open viewing a fixation cross, think of nothing in particular and not fall asleep. These are standard resting state instructions that have been employed throughout the literature (Damoiseaux et al., 2008; Prakash et al., 2011), resulting in robust characterization of the functional networks. For the functional resting-state scan, T2*-weighted echo planar images were acquired with the following sequence parameters: TR = 2000 ms, TE = 24 ms, flip angle = 80°, number of slices = 34 and voxel size = 3 mm isotropic. We acquired one 6 min functional scan with 180 volumes.
Image analyses
All functional MRI data were analyzed using the fMRIB software library (FSL version 4.1.4, 33) and preprocessed using statistics described earlier (Smith et al., 2004).
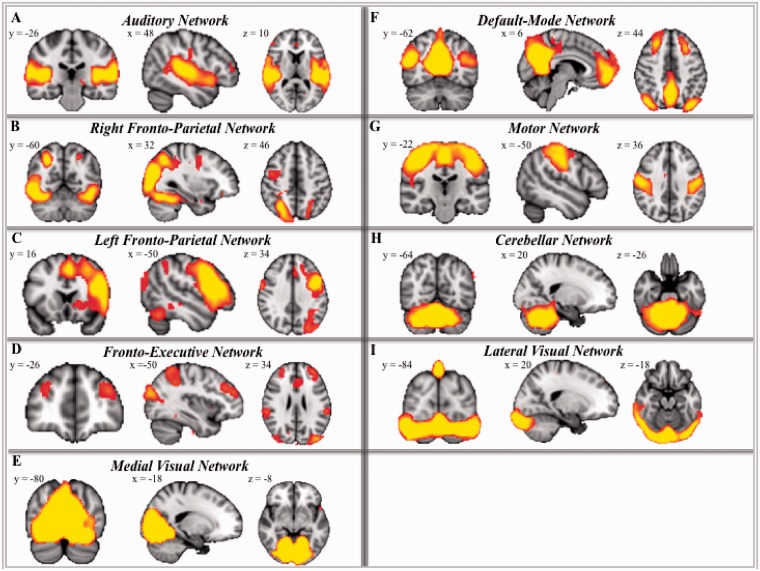
Given that the primary focus of this study was the examination of individual differences in connectivity of the DMN, we first generated a group-level DMN map by conducting a group temporal concatenation independent components analysis (ICA) using fMRIB’s MELODIC toolbox (Beckmann et al., 2005). The preprocessed data from all 25 participants were temporally concatenated to yield group-level independent component networks, which represented common spatial patterns across this group of participants without assumptions of common temporal responses across participants for the independent components. This analysis resulted in 26 independent components, which were all thresholded at P = 0.5 probability of activation to include voxels above that threshold. The analysis resulted in nine meaningful networks, known through previous research to represent functionally segregated systems showing co-activation (Beckmann et al., 2005; Damoiseaux et al., 2008). These networks presented in Figure 1 included the following: auditory network, the right fronto-parietal network, the left fronto-parietal network, the fronto-executive network, the medial visual network, the DMN, the motor network, the cerebellar network and the lateral visual network.
Fig. 1.
Nine network components extracted from ICA. Components were thresholded at P = 0.5 probability of activation to include voxels above that threshold. All subsequent analyses were conducted with the DMN mask generated through this ICA analysis. For all components, axial, coronal and sagittal views are presented in radiological orientation. (A) auditory network, (B) the right fronto-parietal network, (C) the left fronto-parietal network, (D) the fronto-executive network, (E) the medial visual network, (F) the DMN, (G) the motor network, (H) the cerebellar network and (I) the lateral visual network.
All subsequent functional connectivity analyses were performed with this DMN map, identified through the ICA. The DMN was identified first by visual inspection of the 26 independent components, and then by performing a spatial cross-correlation between the generated ICA maps and DMN template provided by the FSL group (Smith et al., 2009) and included the medial prefrontal cortex, the dorsal and ventral portions of the posterior cingulate cortex extending into the anterior and medial portions of the precuneus, the bilateral parietal cortices, the middle temporal gyri and the bilateral parahippocampal gyri. This group-level DMN reference map was then spatially regressed against each participant’s resting-state data to derive individual-level time course for the DMN for each subject. The time series of the DMN component was then variance normalized and entered as a temporal regressor in a general linear model, along with variables of no interest, to identify subject-specific spatial maps of DMN connectivity. To remove the potential influence of physiological noise and participant movement on low-frequency spontaneous oscillations, we entered the time series of head motion, global signal and signal from the white matter and cerebrospinal fluid (CSF) in our subject-level GLM analysis. The six motion parameters were computed by rigid body translation and rotation in preprocessing (Jenkinson et al., 2002). Employing FMRIB’s Automatic Segmentation Tool, we segmented the T1 image into partial volume estimates of gray matter, white matter and CSF probability maps. The global mask, white matter and CSF maps were then used as masks to extract mean timeseries data, which were also entered into the GLM to identify maps of DMN connectivity, independent of the confound of these variables.
The individual-level maps for the DMN were forwarded separately to a higher-level analysis, whereby inter-subject variability was treated as a random variable. The mixed effects analysis was performed using FMRIB’s Local Analysis of Mixed Effects (Beckmann et al., 2003). In this analysis, we were interested in examining if trait levels of mindfulness disposition were associated with greater connectivity of the DMN, independent of the variance associated with age, gender and education. For this, we entered mindfulness disposition as our independent variable of interest, while co-varying out the three demographic variables. This analysis yielded a contrast map that indicated areas of the DMN that demonstrated a positive association with mindfulness disposition. All contrast maps were thresholded at a voxel-wise threshold of z = 2.33 (P < 0.01) and for correction for multiple comparison, cluster thresholding at P < 0.05 using Gaussian Random Field Theory was employed (Worsley et al., 1992).
RESULTS
Mindfulness disposition and DMN connectivity
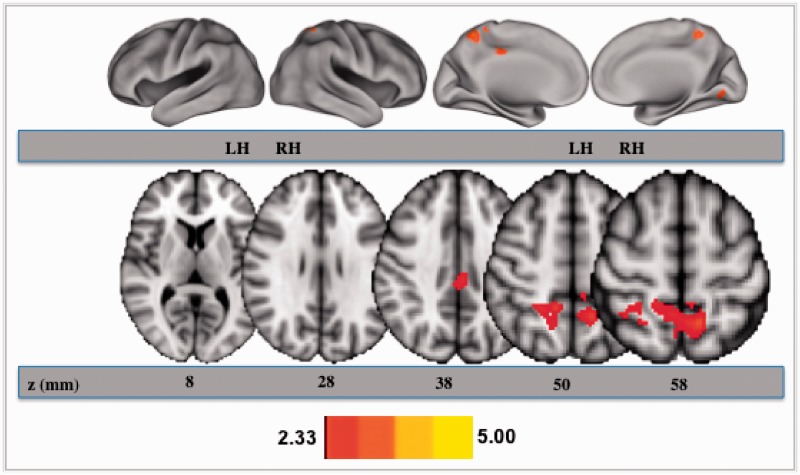
To examine associations between mindfulness disposition and DMN connectivity, we entered trait levels of mindfulness disposition in our higher-level model, after co-varying out age, education and gender. The results of this higher-order analysis are presented in Figure 2. Mindfulness disposition in older adults was associated with increased DMN connectivity in the dorsal posterior cingulate cortex, and the anterior and medial precuneus cortex. Table 1 presents the statistical peaks in Montreal Neurological Institute (MNI) space for both of these regions, along with the max z-stat values.
Fig. 2.
Cortical areas that showed a positive association with mindfulness disposition, after removing variance associated with age, education and gender. All axial slices are presented in radiological orientation.
Table 1.
Statistical peaks in MNI space from the positive association of MAAS scores with DMN connectivity
| Cortical area | Label | Max z-stat | MNI coordinates (mm) |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Posterior cingulate cortex | pCC | 3.09 | −2 | −24 | 38 |
| Precuneus | Precuneus | 2.69 | 8 | −56 | 36 |
DISCUSSION
The study of the human functional connectome has been gaining increasing prominence in the neuroimaging community (Biswal et al., 2010), with accumulating evidence suggesting a key role for these functionally segregated systems in explaining inter-individual differences in behavior (Kelly et al., 2008; Prakash et al., 2011). Aging research provides evidence for the disruption in the coordinated activity of such systems, such that older adults, compared with young adults, often show reduced correlations between the interacting regions of these functionally segregated networks (Damoiseaux et al., 2008; Koch et al., 2010; Voss et al., 2010).
The primary goal of this study was to examine the association between mindfulness disposition, a trait characteristic with critical ramifications for cognitive and affective control and integrity of the DMN. Elicited during undirected mental states, this network is now thought to play a part in self-referential processing, consolidating past events and anticipating future plans, and taking alternative perspectives (Gusnard and Raichle, 2001; Raichle, 2010). Though the network is active during passive states, such internal mentation and spontaneous cognition form the crux of human experience, and possibly explains individual differences in goal-directed behavior (Kelly et al., 2008; Prakash et al., in press). Furthermore, the posterior cingulate cortex and the medial prefrontal cortices, core components of the DMN, have been shown to be critical hubs or ‘way stations’ of the human brain (Buckner et al., 2009), integrating and augmenting information processing, thus resulting in high metabolic activity. Interestingly, these areas are also the known pathophysiological sites of Alzheimer’s disease, showing Pittsburgh Compound B deposition early in the disease course (Buckner et al., 2005; Mosconi et al., 2005), thus suggesting a critical role for the functioning of these nexuses in maintaining the functional connectome of the human brain. Mindfulness disposition, in our study, was found to explain variance in the coordinated activity of this essential network of the human brain in elderly participants, such that individuals with higher levels of this trait demonstrated enhanced connectivity in key nodes of this network, specifically in the posterior cingulate cortex, and the precuneus. These findings, therefore, provide preliminary support for the prophylactic influence of such present-focused attention orientation on strengthening connections between cortical areas.
A resurgence of interest in meditation and mindfulness-based practices has led to an escalation of research studies examining the putative impact of these Buddhist philosophy driven approaches in regulating emotional and cognitive control (Davidson, 2010; Williams, 2010). Employing neuroimaging methodology, recent research studies in the field of mindfulness training have examined the associations between such practices and functioning of the DMN. While still in its infancy, and rather varied in methodology employed, there is support for increased functional connectivity in meditators compared with novices in the medial prefrontal cortices (Jhang et al., 2011), suppression of these default regions in experienced meditators who habitually exercise disengagement from mind-wandering and other forms of self-referential processing (Brewer et al., 2011; Taylor et al., 2011), reduced post-stimulus tail in regions of the DMN in meditators during a semantic processing task (Pagnoni et al., 2008) and increased gray matter in key regions of this network following a mindfulness training intervention (Holzel et al., 2011). In this study, extending the previous literature, we report a positive association in older adults between mindfulness abilities and functional integration of the cortical areas of the default-network, specifically, the precuneus and the posterior cingulate cortex, thus, providing further support for the involvement of this network in mindfulness abilities.
An important point to note about the default network is the presence of functionally specialized, segregated sub-systems within this network, which tend to converge on three anatomical hubs: the posterior cingulate cortex, ventral medial prefrontal cortex and the lateral parietal cortices (Buckner et al., 2008). Of these three central nexuses, the posterior cingulate cortex demonstrates the highest levels of degree and betweenness centrality in both studies of functional connectivity (Achard et al., 2006; Fransson and Marrelec, 2008) and structural connectivity (Hagmann et al., 2008), suggesting a pivotal role for this region in modulating interactions between the different nodes of the DMN and between the DMN and other functional systems. Recent evidence also provides data on the posterior cingulate cortex being an interface between the different brain systems, enabling efficient modulation of both the DMN and the cognitive control network in response to increasing task demands (Leech et al., 2011). In fact, Leech et al. (2011), corroborating previous histological studies separating the posterior cingulate cortex into dorsal and ventral portions (Vogt et al., 2006), demonstrated separable functional roles of the dorsal and ventral portions in externally and internally directed cognition, respectively. In our study, mindfulness was found to correlate strongly with the integrity of the dorsal posterior cingulate cortex, thus providing a differential association of mindfulness with the sub-regions of the posterior cingulate cortex. While the ventral posterior cingulate cortex is involved in a more traditional role ascribed to the DMN of self-referential processing, the dorsal posterior cingulate cortex is considered as an interface between the task-negative or the DMN and the task-positive or the cognitive control network (Leech et al., 2011), thus affording a greater flexibility to switch between networks engaged in external and internal aspects of cognition. Given that a core facet of mindfulness is the ability to direct sustained attentional resources to immediate moment experiences, through attentional regulation and interoceptive awareness, the greater integrity of the dorsal posterior cingulate cortex, we suspect would facilitate a greater context-appropriate modulation of default-mode network and cognitive control network activity. It is also important to note, however, that while only the association with the dorsal posterior cingulate cortex survived cluster thresholding, lowering the threshold also provided evidence for an association between mindfulness disposition and greater functional integrity of the ventral posterior cingulate cortex. For future studies, it will be important to parse out the effect of mindfulness training on the functionally separable regions of the posterior cingulate cortex, providing empirical support for the role of mindfulness in internally directed aspects of cognition, subserved by the ventral posterior cingulate cortex or in sustained present-moment attention, via the efficient modulation enabled by the dorsal posterior cingulate cortex.
Another cortical area that we found to be associated with higher levels of mindfulness disposition in older adults was the precuneus. Forming the medial wall of the parietal lobe, the precuneus is often treated together with the posterior cingulate cortex in resting-state connectivity studies, thus showing connectivity with the other traditional regions within the DMN (Fransson and Marrelec, 2008; Margulies et al., 2009). This region has been purported to be involved in self-referential mental imagery and retrieval success during episodic memory tasks (Cavanna and Trimble, 2006). However, given that this region is traditionally subsumed within the posterior cingulate cortex, for future research it would be essential to parse out the two areas to better understand the functional purpose of this multi-modal region. This is especially critical given recent preliminary evidence suggesting a functional sub-division within the precuneus in humans and macaque monkeys, with separable functional connectivity across the different sub-divisions (Cavanna and Trimble, 2006).
As noted in the Introduction section, a potential limitation of this study may be its particular operationalization of mindfulness. The concept of mindfulness disposition currently lacks a solid technical definition in mainstream scientific discourse, and the proper characterization and quantification of this construct has involved much debate in the mindfulness literature, with some measures emphasizing certain qualities or experiential features more than others (Grossman, 2011). Falling under this grouping, the MAAS has been critiqued for its potentially narrow conceptualization of mindfulness which principally focuses on instances of everyday, moment-to-moment awareness, rather than tapping into other additional facets of mindfulness, such as non-judgementalness and/or emotional clarity. Although the MAAS has been a widely used index of mindfulness disposition in various research studies of mindfulness, we acknowledge the relative specificity of this measure in the context of this study and believe that it would be important for the field to continue to explore other paradigms of mindfulness, to more accurately characterize and fully represent this multi-faceted construct.
Conflict of Interest
None declared.
Acknowledgments
R.S.P. designed the study, analyzed the data and wrote the manuscript. A.A.D. and B.P. collected the data and edited the manuscript. M.K. and W.M. edited the manuscript.
This project was supported by Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The authors would like to thank the undergraduate research assistants Jamie Lukac, Daniel Snider, Danielle Rickert, Lyla Mournay, Trey Dossman and Luke McDonald for their help in data collection. We would also like to thank Amir Abduljalil, physicist at the Wright Center for Innovation in Imaging at Ohio State University for his help in designing the fMRI sequences and data collection.
REFERENCES
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. Journal of Neuroscience. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fMRI. NeuroImage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y, Weber J, Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20254–9. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. The Journal of Neuroscience. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Craigie MA, Nathan P. A nonrandomized effectiveness comparison of broad-spectrum group CBT to individual CBT for depressed outpatients in a community mental health setting. Behavior Therapy. 2009;40:302–14. doi: 10.1016/j.beth.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BA, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69:560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Sanz Arigita EJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. 2008;18:1856–64. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Empirical explorations of mindfulness: conceptual and methodological conundrums. Emotion. 2010;10:8–11. doi: 10.1037/a0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default network: evidence from a partial correlation network analysis. NeuroImage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Gross JJ. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10:83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re)invention of mindfulness: comment on Brown et al. (2011) Psychological Assessment. 2011;23:1034–40. doi: 10.1037/a0022713. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, et al. Mapping the structural core of the human cerebral cortex. PLoS Biology. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, et al. Mindfulness practice leads to increase in regional brain gray matter density. Psychiatry Research: Neuroimaging. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, et al. Investigation of mindfulness meditation practitioners with voxel-based morphometry. Social Cognitive and Affective Neuroscience. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective, and Behavioral Neuroscience. 2007;7:109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010;10:54–64. doi: 10.1037/a0018438. [DOI] [PubMed] [Google Scholar]
- Jhang JH, Jung WH, Kang DH, et al. Increased default mode network connectivity associated with meditation. Neuroscience Letters. 2011;487:358–62. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Wherever You Go, There You Are: Mindfulness Meditation in Everyday Life. New York: Hyperion; 1994. [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–37. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, et al. Effects of aging on default-mode network activity in resting state fMRI: does the method of analysis matter? NeuroImage. 2010;51:280–7. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, et al. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76:966–78. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893–7. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. Journal of Neuroscience. 2011;31:3217–24. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: larger hippocampal and frontal volumes of gray matter. NeuroImage. 2009;45:672–8. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Vincent JL, Kelly C, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20069–74. doi: 10.1073/pnas.0905314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A, Malinowski P. Meditation, mindfulness, and cognitive flexibility. Consciousness and Cognition. 2009;18:176–86. doi: 10.1016/j.concog.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Tsui W, De Santi S, et al. Reduced hippocampal metabolism in MCI and AD: automated FDG-PET image analysis. Neurology. 2005;64:1860–7. doi: 10.1212/01.WNL.0000163856.13524.08. [DOI] [PubMed] [Google Scholar]
- Mrazek MD, Smallwood J, Schooler JW. Mindfulness and mind-wandering: finding convergence through opposing constructs. Emotion. 2012;12:442–8. doi: 10.1037/a0026678. [DOI] [PubMed] [Google Scholar]
- Ortner CNM, Kilner SJ, Zelazo PD. Mindfulness meditation and reduced emotional interference on a cognitive task. Motivation and Emotion. 2007;31:271–83. [Google Scholar]
- Pagnoni G, Cekic M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiology of Aging. 2007;28:1623–7. doi: 10.1016/j.neurobiolaging.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Pagnoni G, Cekic M, Guo Y. “Thinking about not-thinking”: neural correlates of conceptual processing during Zen meditation. Plos One. 2008;3:e3083. doi: 10.1371/journal.pone.0003083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons JB, Mennin DS, Tucker DE. Common misconceptions about the nature and treatment of generalized anxiety disorder. Psychiatric Annals. 2001;31:501–7. [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF. Age-related differences in cortical recruitment and suppression: Implications for cognitive performance. Behavioural Brain Research. 2012;230:192–200. doi: 10.1016/j.bbr.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Prakash RS, Patterson B, Jannssen A, Abduljalil A, Boster A. Physical activity associated with increased resting-state functional connectivity in multiple sclerosis. Journal of the International Neuropsychological Society. 2011;17:986–97. doi: 10.1017/S1355617711001093. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends in Cognitive Sciences. 2010;14:180–90. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Fiez JA, et al. Searching for activations that generalize over tasks. Human Brain Mapping. 1997;5:317–22. doi: 10.1002/(SICI)1097-0193(1997)5:4<317::AID-HBM19>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementations as FSL. NeuroImage. 2004;23:S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Taylor AV, Grant J, Daneault V, et al. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage. 2011;57:1524–33. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- van den Hurk PAM, Giommi F, Gielen SC, Speckens AEM, Barendregt HP. Greater efficiency in attentional processing related to mindfulness meditation. The Quarterly Journal of Experimental Psychology. 2010;63:1168–80. doi: 10.1080/17470210903249365. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 2006;29:452–66. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Erickson KI, Prakash R, et al. Functional connectivity: a source of variance in the association between cardiorespiratory fitness and cognition? Neuropsychologia. 2010;48:1394–1406. doi: 10.1016/j.neuropsychologia.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BA, Cresswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10:12–24. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG. Mindfulness and psychological process. Emotion. 2010;10:1–7. doi: 10.1037/a0018360. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Evans AC, Marrett S, Neelin P. A three dimensional statistical analysis for CBF activation studies in human brain. Journal of Cerebral Blood Flow and Metabolism. 1992;12:900–18. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]