Abstract
Background
Low serum magnesium has been linked to increased risk of atrial fibrillation (AF) following cardiac surgery. It is unknown whether hypomagnesemia predisposes to AF in the community.
Methods and Results
We studied 3,530 participants (mean age, 44 years; 52% women) from the Framingham Offspring Study who attended a routine examination, and were free of AF and cardiovascular disease. We used Cox proportional hazard regression analysis to examine the association between serum magnesium at baseline and risk of incident AF. Analyses were adjusted for conventional AF risk factors, use of antihypertensive medications, and serum potassium. During up to 20 years of follow-up, 228 participants developed AF. Mean serum magnesium was 1.88 mg/dl. The age- and sex-adjusted incidence rate of AF was 9.4 per 1,000 person-years (95% confidence interval, 6.7 to 11.9) in the lowest quartile of serum magnesium (≤1.77 mg/dl), compared with 6.3 per 1,000 person-years (95% confidence interval, 4.1 to 8.4) in the highest quartile (≥1.99 mg/dl). In multivariable-adjusted models, individuals in the lowest quartile of serum magnesium were approximately 50% more likely to develop AF (adjusted hazard ratio, 1.52, 1.00 to 2.31; P=0.05), compared with those in the upper quartiles. Results were similar after excluding individuals on diuretics.
Conclusion
Low serum magnesium is moderately associated with the development of AF in individuals without cardiovascular disease. Because hypomagnesemia is common in the general population, a link with AF may have potential clinical implications. Further studies are warranted to confirm our findings and elucidate the underlying mechanisms.
Keywords: arrhythmia, epidemiology, atrial fibrillation, magnesium
Hypomagnesemia has been linked to the pathogenesis of arrhythmias in experimental studies.1 For instance, in rodents, magnesium deficiency potentiates the proarrhythmic effect of hypokalemia,2 and high magnesium prevents the development of ventricular reperfusion arrhythmias.3 In humans, low serum magnesium is associated with the development of atrial fibrillation (AF) after coronary artery bypass surgery.4 Some, but not all, studies suggest that magnesium supplementation reduces the incidence of post-operative AF.5–11
Prior clinical studies examining the relation between serum magnesium and AF have focused on hospitalized, post-surgical patients. It is unknown whether low serum magnesium is associated with the development of AF in ambulatory individuals, particularly those without existing cardiovascular disease. Such a link could have public health implications, because low magnesium status is relatively common and potentially correctable. Thus, we examined the association between serum magnesium levels and future AF, using data from more than 2 decades of longitudinal follow-up in the Framingham Offspring Study.
METHODS
Study sample
The Framingham Heart Study was established in 1948. In 1971, 5,124 children (or their spouses) of the Original Framingham Heart Study participants were enrolled in the Framingham Offspring Study.12 Participants attending the second examination of the Offspring cohort (n=3,863), which took place between 1979 and 1983, were eligible for the current investigation. We excluded participants lacking a serum magnesium measurement (n=176) or with prevalent cardiovascular disease (n=156) or AF (n=2). A total of 3,526 participants were eligible for the analysis. The study protocol was approved by the Boston University Medical Center Institutional Review Board, and all participants provided written informed consent.
Clinical assessment
Participants underwent a complete medical history, anthropometry and laboratory assessment of cardiovascular disease risk factors at entrance into the study and at each examination. Prevalent cardiovascular disease (myocardial infarction, unstable angina, heart failure, or stroke) was determined based upon blinded review of the medical history, hospital records and outpatient visit notes by 3 investigators. Blood pressure was measured twice by a physician after the participant had been sitting for 5 minutes, and the mean of the two blood pressures was used for analysis. Body mass index was calculated as weight in kilograms divided by the square of height in meters. Current smoking was defined as regular cigarette smoking in the year prior to the examination. Alcohol consumption was assessed by self-report of ounces per week. Moderate-heavy alcohol consumption was defined as ≥ 14 drinks/week for men and ≥ 7 drinks/week for women (1 drink = 13 grams of alcohol). Caffeine consumption was measured in cups of caffeinated coffee or tea per day. We classified a heart murmur as significant if at least 3 out of six intensity systolic or any diastolic murmur was present.
Blood was obtained from participants in the fasting state. Measurements were made of total and HDL cholesterol, glucose, albumin, hemoglobin, high-sensitivity C-reactive protein, and electrolytes. Medication use was by self-report. Diabetes was defined as a fasting glucose level greater than or equal to 126 mg/dL, or the use of oral hypoglycemic agents or insulin. Glomerular filtration rate was estimated from serum creatinine using the simplified Modification of Diet in Renal Disease equation.13 All participants underwent a 12-lead electrocardiogram, and the electrocardiographic PR interval was measured manually by a Framingham clinic physician.
Measurement of serum magnesium
All participants had serum magnesium measured using a standard colorimetric assay (Roche Diagnostics, Alameda, California). Potassium was measured using flame-emission spectrophotometry.14
Measurement of AF outcome
All participants were under routine surveillance for the development of AF. AF was diagnosed if atrial fibrillation or atrial flutter was present on an electrocardiogram obtained from a Framingham clinic visit, outpatient physician visit, inpatient hospitalization, or Holter monitor. All potential cases of AF were adjudicated by a Framingham Heart Study cardiologist.
Statistical analysis
Descriptive statistics were performed for key clinical risk factors using means and standard deviations for continuous variables and percentages for categorical variables. Pearson correlations were used to summarize the associations between continuous risk factors and serum magnesium. Mean serum magnesium levels were assessed in participants with and without dichotomous risk factors. Age- and sex-adjusted incidence rates were calculated by quartiles of serum magnesium.
We assessed the relation of clinical factors to serum magnesium with linear regression. Cox proportional hazard regression analysis was used to examine the relation of baseline serum magnesium concentrations with incident AF. Follow-up was censored at 20 years. The proportionality of hazards assumption was confirmed. Magnesium concentration, which was approximately normally distributed, was analyzed in quartiles. Because there was no evidence of effect modification by sex, sex-pooled models were constructed. Covariates in the multivariable models included age, sex, body mass index, diabetes, systolic blood pressure, total/high density lipoprotein ratio, smoking status, and anti-hypertensive treatment. We additionally adjusted for measures potentially associated with magnesium status: hemoglobin, serum albumin, estimated glomerular filtration rate and alcohol consumption. Caffeine consumption was not included in multivariable models because it has previously been found not to be associated with incident AF in this cohort.15
Cox proportional hazards regression was also used to examine the relation of baseline serum potassium and calcium and AF. In secondary analyses, models were estimated with and without adjustment for serum potassium, C-reactive protein, heart failure, significant heart murmur, and electrocardiographic PR interval. We also conducted Cox proportional hazard models excluding individuals who developed AF after a diagnosis of heart failure, cardiac surgery, or myocardial infarction, and those on diuretic therapy at baseline. In further analyses we tested for effect modification between moderate to heavy alcohol use and magnesium concentration and risk of AF. We conducted a series of sensitivity analyses examining various approaches to categorizing magnesium concentrations, including examining the lowest quartile vs. the upper 3 quartiles and the lowest decile vs. the upper 9 deciles. We also performed a spline analysis to assess the linearity of the association between serum magnesium and AF risk.
All analyses were performed using SAS 9.1.3 (SAS Institute, Cary, N.C.). Two-tailed P <0.05 were considered statistically significant. The authors had full access to the data and take responsibility for its integrity. All of the authors have read the article as written and agreed with submission in its current form.
RESULTS
Characteristics of the study sample are shown in Table 1. The baseline mean age of the sample was 44 years and the sample consisted of 52% women. The mean serum magnesium level was 1.88 mg/dl with a range of 1.41 to 2.40 mg/dl in men, and 1.86 mg/dl with a range of 1.15 to 2.46 mg/dl in women. The association of baseline factors with serum magnesium is shown in Table 2.
Table 1.
Characteristics of study participants.
| Quartile of Serum Magnesium | ||||
|---|---|---|---|---|
| 1 (N=936) | 2 (N=906) | 3 (N=839) | 4 (N=849) | |
| Age, years | 45.0±10.2 | 44.0±10.0 | 44.0±9.9 | 44.4±9.9 |
| Body mass index, kg/m2 | 26.0±4.8 | 25.7±4.3 | 25.7±4.3 | 25.9±4.1 |
| Systolic blood pressure, mm Hg | 123±17 | 121±16 | 122±17 | 121±16 |
| Diastolic blood pressure, mm Hg | 78±10 | 78±10 | 78±10 | 78±10 |
| Antihypertensive medications, % | 12 | 7 | 8 | 8 |
| Total/HDL cholesterol ratio | 4.3±1.5 | 4.4±1.6 | 4.6±1.6 | 4.7±1.6 |
| Creatinine, mg/dL | 1.0±0.2 | 1.1±0.2 | 1.2±0.2 | 1.2±0.3 |
| Albumin, g/dL | 4.4±0.3 | 4.4±0.3 | 4.5±0.3 | 4.5±0.3 |
| Hemoglobin, g/dL | 14.4±1.4 | 14.5±1.3 | 14.7±1.4 | 14.8±1.3 |
| Phosphorus, mg/dL | 3.2±0.5 | 3.2±0.4 | 3.1±0.4 | 3.2±0.4 |
| Calcium, mg/dL | 9.5±0.4 | 9.6±0.4 | 9.6±0.4 | 9.7±0.4 |
| Magnesium, mg/dL | 1.7±0.1 | 1.8±0.03 | 1.9±0.03 | 2.1±0.1 |
| Diabetes, % | 8 | 4 | 2 | 3 |
| Smoking, % | 35 | 35 | 36 | 39 |
| Potassium, mEq/L | 4.6±0.5 | 4.7±0.5 | 4.7±0.5 | 4.7±0.5 |
| C-reactive protein, mg/L | 2.6±5.1 | 2.4±5.0 | 2.3±4.3 | 2.3±4.3 |
| Alcohol consumption, drinks/week | 7.6±11.4 | 8.6±12.5 | 7.9±10.0 | 8.8±12.1 |
| Caffeine consumption, cups/day | 3.6±3.0 | 3.8±2.7 | 4.0±2.8 | 3.8±2.7 |
| Significant murmur, N(%) | 1 | 0.4 | 0.2 | 0.4 |
| Interim myocardial infarction, N(%) | 2 | 2 | 1 | 1 |
| Interim heart failure, N(%) | 2 | 2 | 2 | 2 |
| Interim cardiac surgery, N(%) | 1 | 1 | 1 | 1 |
Clinical characteristics are expressed as mean±SD, or %
Table 2.
Associations between baseline risk factors and serum magnesium concentration
| Correlation of serum magnesium with baseline characteristics in the entire sample (N=3,530). | ||
|---|---|---|
| r | P-value | |
| Age, years | −0.027 | 0.11 |
| Body mass index, kg/m2 | −0.019 | 0.27 |
| Systolic blood pressure, mm Hg | −0.053 | 0.002 |
| Diastolic blood pressure, mm Hg | 0.005 | 0.77 |
| Total/HDL cholesterol ratio | 0.093 | 0.0001 |
| Creatinine, mg/dL | 0.310 | 0.0001 |
| Albumin, g/dL | 0.167 | 0.0001 |
| Hemoglobin, g/dL | 0.097 | 0.0001 |
| Phosphorus, mg/dL | 0.011 | 0.51 |
| Calcium, mg/dL | 0.154 | 0.0001 |
| Potassium, mEq/L | 0.098 | 0.0001 |
| Alcohol consumption, oz/week | 0.022 | 0.19 |
| Mean magnesium concentration in mg/dl according to the presence of selected risk factors.
| |||
|---|---|---|---|
| Risk Factor | P-value | ||
| Present Mean (SD) | Absent Mean (SD) | ||
| Male Sex | 1.89 (0.15) | 1.86 (0.16) | 0.0001 |
| Antihypertensive medications, % | 1.83 (0.18) | 1.88 (0.15) | 0.0001 |
| Diabetes, % | 1.80 (0.19) | 1.88 (0.16) | 0.0001 |
| Smoking, % | 1.88 (0.16) | 1.87 (0.16) | 0.009 |
| Chronic kidney disease* | 1.93 (0.21) | 1.87 (0.16) | 0.005 |
| Diuretic use | 1.84 (0.19) | 1.88 (0.15) | 0.0001 |
Chronic kidney disease defined as estimated glomerular filtration rate below the 5th percentile by sex.
Over a mean follow-up time of 18.6 ± 3.7 years, 228 participants (5%) developed new-onset AF. The cumulative incidence of AF by quartile of serum magnesium is shown in Table 3. The age-and sex-adjusted incidence of AF was 9.4 per 1,000 person-years (95% confidence interval [CI], 6.7 to 11.9) in the lowest quartile of serum magnesium, compared with 6.3 per 1,000 person-years in the highest quartile (95% CI, 4.1 to 8.4).
Table 3.
Age- and sex-adjusted incidence of AF, according to magnesium quartile
| Quartile | Serum magnesium range, mg/dl | No. of Events | Person-years | Incidence rate (95% CI), per 1,000 person-years |
|---|---|---|---|---|
| Lowest | ≤1.77 | 80 | 17,209 | 9.4 (6.7–11.9) |
| Second | 1.78–1.88 | 53 | 16,933 | 6.9 (4.6–9.0) |
| Third | 1.89–1.98 | 50 | 15,619 | 7.1 (4.8–9.3) |
| Highest | ≥1.99 | 45 | 15,908 | 6.3 (4.1–8.4) |
Results of multivariable Cox proportional hazard regression analyses are shown in Table 4. In age- and sex-adjusted analyses, the risk of incident AF was highest in the lowest quartile of serum magnesium (hazard ratio compared with the highest quartile, 1.54, 95% CI, 1.06 to 2.22; P=0.02). No gradient in risk of AF was observed across the upper three quartiles of serum magnesium. A similar pattern was observed in the multivariable-adjusted analyses (Table 4).
Table 4.
Multivariable regression models
| Age-and Sex-Adjusted | Multivariable-Adjusted* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| In entire sample (n=3530) | ||||||
| Quartile 1 | 1.54 | (1.06–2.22) | 0.02 | 1.45 | (0.99–2.12) | 0.06 |
| Quartile 2 | 1.11 | (0.75–1.66) | 0.60 | 1.11 | (0.74–1.67) | 0.61 |
| Quartile 3 | 1.15 | (0.77–1.72) | 0.49 | 1.14 | (0.76–1.71) | 0.53 |
| Quartile 4 | 1.00 | Referent | - | 1.00 | Referent | - |
| In individuals not on diuretics (n=3239) | ||||||
| Quartile 1 | 1.53 | (1.02–2.27) | 0.04 | 1.52 | (1.00–2.31) | 0.05 |
| Quartile 2 | 1.02 | (0.66–1.58) | 0.92 | 1.09 | (0.69–1.69) | 0.72 |
| Quartile 3 | 1.07 | (0.69–1.66) | 0.76 | 1.08 | (0.69–1.69) | 0.73 |
| Quartile 4 | 1.00 | Referent | - | 1.00 | Referent | - |
| With magnesium as a dichotomous variable (n=3530) | ||||||
| Quartile 1 | 1.41 | (1.07–1.86) | 0.01 | 1.34 | (1.01–1.77) | 0.05 |
| Quartiles 2–4 | 1.00 | Referent | - | 1.00 | Referent | - |
| By decile (n=3530) | ||||||
| Decile 1 | 1.69 | (1.18–2.41) | 0.004 | 1.48 | (1.02–2.14) | 0.04 |
| Deciles 2–10 | 1.00 | Referent | 1.00 | Referent | ||
Multivariable models are adjusted for the following covariates: age, sex, body mass index, systolic blood pressure, diabetes, total/high density lipoprotein ratio, smoking status, anti-hypertensive treatment, hemoglobin, albumin, glomerular filtration rate, and alcohol consumption.
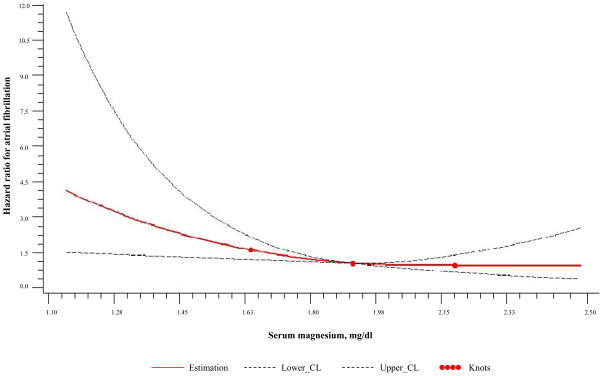
Because the excess risk of AF appeared confined to individuals in the lowest magnesium quartile, we performed additional analyses with magnesium as a dichotomous variable. An increased risk of AF was noted in individuals in the lowest quartile (adjusted hazard ratio, 1.34, 95% CI, 1.01 to 1.77; P=0.05) or lowest decile (adjusted hazard ratio, 1.48, 95% CI, 1.02 to 2.14; P=0.04) of serum magnesium, compared with the remaining participants (Table 4). We also performed a spline analysis to further assess the shape of the relation between baseline magnesium and AF risk (Figure 1).
Figure 1.
Spline analysis.
There was no association between serum potassium and AF in age- and sex-adjusted (HR per SD of serum potassium, 0.92, 95% CI, 0.81–1.04; p=0.18) or multivariable-adjusted (HR, 0.97; 95% CI, 0.85–1.10; p=0.62) models. Similarly, there was no association between serum calcium and AF in either age- and sex-adjusted (HR per SD of serum calcium, 0.96, 95% CI, 0.84–1.09; p=0.54) or multivariable-adjusted (HR, 0.93, 95% CI, 0.81–1.06; P=0.26) models.
The association of serum magnesium with AF persisted in models further adjusting for serum potassium concentration (multivariable-adjusted HR for the lowest quartile, 1.33, 95% CI, 1.00–1.77; P=0.05), and C-reactive protein (HR, 1.34, 95% CI, 1.00–1.80; P=0.05). In models adjusted for PR interval or heart murmur, the hazard ratios were minimally attenuated (HR after adjustment for PR interval, 1.32, 95% CI, 0.99–1.75; P=0.06; HR after adjustment for heart murmur, 1.27, 95% CI, 0.95–1.69; P=0.11). The hazard ratio was also similar in analyses excluding any case of AF that developed after the diagnosis of heart failure (N= 29), myocardial infarction (N=5), or cardiac surgery (N=29) (HR, 1.33, 95% CI, 0.95–1.85; P=0.09).
There was no association between moderate-to-heavy alcohol consumption and magnesium concentration (P=0.99). Furthermore, there was no interaction between moderate-to-heavy alcohol use and magnesium concentration on the risk of AF (interaction P=0.72).
DISCUSSION
In summary, low serum magnesium was associated with the development of AF in a longitudinal, community-based cohort. Although previous studies have reported an association between low serum magnesium and AF risk in the context of cardiac surgery,4 the present study is the first, to our knowledge, to demonstrate this association in the broader community.
Magnesium has several effects on the cardiac conduction system. It is an essential cofactor for the Na-K ATP pump, which controls the movement of sodium and potassium across the cell membrane.16 Disruption or alteration in the function of this pump in the setting of hypomagnesemia may impact myocardial excitability. Magnesium also may prolong the effective refractory period and alter the function of the inward rectifying potassium channel, although this has not been shown in all studies.17–22 Magnesium infusion prolongs atrioventricular node conduction time,23 whereas low serum magnesium concentration increases sinus node automaticity.24 Clinical studies have shown that intravenous magnesium can augment rate control in AF, and facilitate maintenance of sinus rhythm.25 In contrast, hypomagnesemia increases the dose of digoxin required for rate control,26 and lowers the threshold for digoxin-related arrhythmias.27 In small metabolic unit studies, restriction of dietary magnesium to less than one-half of the recommended daily allowance increased supraventricular ectopy28 and risk of AF.29 Another recent study reported an inverse relation between magnesium status and sudden cardiac death, potentially lending further support to the link between hypomagnesemia and cardiac arrhythmias.30
Magnesium and potassium deficiency frequently coexist,31 and in experimental models, hypomagnesemia has been shown to potentiate the electrophysiologic effects of hypokalemia.2 Because of the association between magnesium and potassium, we performed secondary analyses adjusting for serum potassium, and the magnesium-AF relation remained unchanged. Furthermore, baseline serum potassium was unrelated to incident AF in our data. Hence, it seems unlikely that potassium concentrations explain the serum magnesium - AF association.
Our analyses suggest that the association with serum magnesium and AF is not linear, but observes a threshold. The excess risk of AF appears primarily in those in the lowest quartile of serum magnesium.4 Although residual confounding from chronic illnesses or malnutrition that predispose to both hypomagnesemia and AF cannot be excluded, several features of the present study attenuate this concern. First, our cohort consisted primarily of ambulatory, young to middle-aged adults with little comorbidity. Second, even the lower magnesium values observed in our study were not in the range found with malnutrition or severe deficiency. Third, if low serum magnesium was simply serving as a marker of chronic illness, then we would expect it to be associated with other adverse outcomes. However, we previously found that magnesium status was not associated with all-cause mortality or vascular risk in the Framingham Offspring cohort.32 Thus, our findings with regard to the risk associated with low magnesium status appear specific for AF, a biologically-plausible endpoint in view of the experimental data and studies in the acute hospital setting.
Causality is difficult to infer from the data. Nonetheless, we believe our study has significant strengths, including the use of a large, well-characterized cohort, rigorous adjudication of AF and other cardiovascular outcomes, the long period of follow-up and standardized ascertainment of baseline magnesium concentrations in a single laboratory.
Several limitations deserve mention. Serum magnesium concentration may not fully reflect total body magnesium stores, although serum magnesium correlates well with intracellular magnesium levels.33 Serum magnesium was only measured at one time point, and magnesium levels fluctuate over time. Nonetheless, random misclassification of magnesium status would be expected to bias the results toward the null. Since AF can be paroxysmal and asymptomatic, it is possible that not all cases of AF were detected. We did not differentiate between types of AF (paroxysmal, persistent or permanent, AF or atrial flutter); hence, we cannot comment as to whether the relation between magnesium and AF varies by subtype.
Also, we were unable to examine the relation between dietary magnesium intake and AF, because dietary magnesium records were not available at the index examination. However, direct measurements of serum magnesium levels avoid reporting bias, which is a limitation of dietary recall studies.34 Alcohol consumption may have been underestimated because it was obtained by self-report. In addition, we did not specifically ask about binge drinking, which could also influence serum magnesium concentrations. We did not assess sleep apnea at the index examination. Although sleep apnea is associated with incident AF,35 its relation with magnesium status is unknown. Family history of AF was not available on a significant proportion of the sample. Thus, we did not include this variable as a covariate. We submit that it is unlikely that parental AF is a strong correlate of offspring magnesium status. Our study sample was largely of European ancestry, so the generalizability of our results to other races/ethnicities is uncertain.
We observed that low serum magnesium was associated with the development of AF. If confirmed, our observations may have important public health implications, because the prevalence of AF is increasing, and magnesium deficiency is common and potentially modifiable.36, 37 Further studies are warranted to determine whether the association is present in other populations, and whether magnesium supplementation lowers AF risk.
Clinical Perspective.
Data from both experimental and human studies suggest that low magnesium may be linked to the development of arrhythmias. While hypomagnesemia has been associated with the development of atrial fibrillation (AF) after cardiac surgery, it is unknown whether serum magnesium is associated with the development of AF in healthy, ambulatory individuals. In a longitudinal, community-based study of 3,530 individuals without known cardiac disease, we identified an association between low serum magnesium and the development of AF over 20 years of follow-up. We found that those in the lowest quartile of serum magnesium were approximately 30% more likely to develop AF than those in the upper three quartiles. This association persisted despite adjustment for known AF risk factors and the interim development of heart failure, myocardial infarction, or cardiac surgery. Because magnesium deficiency is relatively common and easily treatable with dietary supplementation, a link with AF in the general population has potential clinical implications.
Acknowledgments
Funding Sources: This work was supported in part by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195). NIH grants and contracts N01-HC-25195, 6R01-NS17950, 1R01HL092577, 1RC1HL101056, and 1R01HL102214, the Evans Center for Interdisciplinary Biomedical Research ARC on Atrial Fibrillation at Boston University (http://www.bumc.bu.edu/evanscenteribr/).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Iseri LT, Allen BJ, Ginkel ML, Brodsky MA. Ionic biology and ionic medicine in cardiac arrhythmias with particular reference to magnesium. Am Heart J. 1992;123:1404–1409. doi: 10.1016/0002-8703(92)91059-a. [DOI] [PubMed] [Google Scholar]
- 2.Evans SJ, Levi AJ, Jones JV. Low magnesium enhances the pro-arrhythmic effect of low potassium in the hypertrophied rat heart but not in the normal rat heart. J Hypertens. 1996;14:635–644. doi: 10.1097/00004872-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Ponce Zumino A, Risler NR, Schanne OF, Ruiz Petrich E, Carrion A. Magnesium: effects on reperfusion arrhythmias and membrane potential in isolated rat hearts. Mol Cell Biochem. 1997;171:85–93. doi: 10.1023/a:1006863311967. [DOI] [PubMed] [Google Scholar]
- 4.Zaman AG, Alamgir F, Richens T, Williams R, Rothman MT, Mills PG. The role of signal averaged P wave duration and serum magnesium as a combined predictor of atrial fibrillation after elective coronary artery bypass surgery. Heart. 1997;77:527–531. doi: 10.1136/hrt.77.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alghamdi AA, Al-Radi OO, Latter DA. Intravenous magnesium for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and meta-analysis. J Card Surg. 2005;20:293–299. doi: 10.1111/j.1540-8191.2005.200447.x. [DOI] [PubMed] [Google Scholar]
- 6.Hazelrigg SR, Boley TM, Cetindag IB, Moulton KP, Trammell GL, Polancic JE, Shawgo TS, Quin JA, Verhulst S. The efficacy of supplemental magnesium in reducing atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2004;77:824–830. doi: 10.1016/j.athoracsur.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Cook RC, Humphries KH, Gin K, Janusz MT, Slavik RS, Bernstein V, Tholin M, Lee MK. Prophylactic intravenous magnesium sulphate in addition to oral {beta}-blockade does not prevent atrial arrhythmias after coronary artery or valvular heart surgery: a randomized, controlled trial. Circulation. 2009;120:S163–169. doi: 10.1161/CIRCULATIONAHA.108.841221. [DOI] [PubMed] [Google Scholar]
- 8.Burgess DC, Kilborn MJ, Keech AC. Interventions for prevention of post-operative atrial fibrillation and its complications after cardiac surgery: a meta-analysis. Eur Heart J. 2006;27:2846–2857. doi: 10.1093/eurheartj/ehl272. [DOI] [PubMed] [Google Scholar]
- 9.Miller S, Crystal E, Garfinkle M, Lau C, Lashevsky I, Connolly SJ. Effects of magnesium on atrial fibrillation after cardiac surgery: a meta-analysis. Heart. 2005;91:618–623. doi: 10.1136/hrt.2004.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiga T, Wajima Z, Inoue T, Ogawa R. Magnesium prophylaxis for arrhythmias after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Med. 2004;117:325–333. doi: 10.1016/j.amjmed.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Toraman F, Karabulut EH, Alhan HC, Dagdelen S, Tarcan S. Magnesium infusion dramatically decreases the incidence of atrial fibrillation after coronary artery bypass grafting. Ann Thorac Surg. 2001;72:1256–1261. doi: 10.1016/s0003-4975(01)02898-3. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 14.Worth HG. A comparison of the measurement of sodium and potassium by flame photometry and ion-selective electrode. Ann Clin Biochem. 1985;22:343–350. doi: 10.1177/000456328502200402. [DOI] [PubMed] [Google Scholar]
- 15.Shen J, Johnson VM, Sullivan LM, Jacques PF, Magnani JW, Lubitz SA, Pandey S, Levy D, Vasan RS, Quatromoni PA, Junyent M, Ordovas JM, Benjamin EJ. Dietary factors and incident atrial fibrillation: the Framingham Heart Study. Am J Clin Nutr. 2011;93:261–266. doi: 10.3945/ajcn.110.001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skou JC, Butler KW, Hansen O. The effect of magnesium, ATP, Pi, and sodium on the inhibition of the (Na + + K + )-activated enzyme system by g-strophanthin. Biochim Biophys Acta. 1971;241:443–461. doi: 10.1016/0005-2736(71)90044-7. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Dreifus LS. Electrophysiological effects of magnesium and its interactions with potassium. Cardiovasc Res. 1972;6:79–88. doi: 10.1093/cvr/6.1.79. [DOI] [PubMed] [Google Scholar]
- 18.Vandenberg CA. Inward rectification of a potassium channel in cardiac ventricular cells depends on internal magnesium ions. Proc Natl Acad Sci U S A. 1987;84:2560–2564. doi: 10.1073/pnas.84.8.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christiansen EH, Frost L, Andreasen F, Mortensen P, Thomsen PE, Pedersen AK. Dose-related cardiac electrophysiological effects of intravenous magnesium. A double-blind placebo-controlled dose-response study in patients with paroxysmal supraventricular tachycardia. Europace. 2000;2:320–326. doi: 10.1053/eupc.2000.0123. [DOI] [PubMed] [Google Scholar]
- 20.Stark G, Stark U, Pilger E, Honigl K, Bertuch H, Tritthart HA. The influence of elevated Mg2+ concentrations on cardiac electrophysiologic parameters. Cardiovasc Drugs Ther. 1989;3:183–189. doi: 10.1007/BF01883863. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen HS, Thomsen PE. The electrophysiological effects of intravenous magnesium on human sinus node, atrioventricular node, atrium, and ventricle. Clin Cardiol. 1989;12:85–90. doi: 10.1002/clc.4960120204. [DOI] [PubMed] [Google Scholar]
- 22.Kulick DL, Hong R, Ryzen E, Rude RK, Rubin JN, Elkayam U, Rahimtoola SH, Bhandari AK. Electrophysiologic effects of intravenous magnesium in patients with normal conduction systems and no clinical evidence of significant cardiac disease. Am Heart J. 1988;115:367–373. doi: 10.1016/0002-8703(88)90483-8. [DOI] [PubMed] [Google Scholar]
- 23.DiCarlo LA, Jr, Morady F, de Buitleir M, Krol RB, Schurig L, Annesley TM. Effects of magnesium sulfate on cardiac conduction and refractoriness in humans. J Am Coll Cardiol. 1986;7:1356–1362. doi: 10.1016/s0735-1097(86)80157-7. [DOI] [PubMed] [Google Scholar]
- 24.Op’t Hof T, Mackaay AJ, Bleeker WK, Jongsma HJ, Bouman LN. Differences between rabbit sinoatrial pacemakers in their response to Mg, Ca and temperature. Cardiovasc Res. 1983;17:526–532. doi: 10.1093/cvr/17.9.526. [DOI] [PubMed] [Google Scholar]
- 25.Onalan O, Crystal E, Daoulah A, Lau C, Crystal A, Lashevsky I. Meta-analysis of magnesium therapy for the acute management of rapid atrial fibrillation. Am J Cardiol. 2007;99:1726–1732. doi: 10.1016/j.amjcard.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 26.DeCarli C, Sprouse G, LaRosa JC. Serum magnesium levels in symptomatic atrial fibrillation and their relation to rhythm control by intravenous digoxin. Am J Cardiol. 1986;57:956–959. doi: 10.1016/0002-9149(86)90738-1. [DOI] [PubMed] [Google Scholar]
- 27.Seller RH, Cangiano J, Kim KE, Mendelssohn S, Brest AN, Swartz C. Digitalis toxicity and hypomagnesemia. Am Heart J. 1970;79:57–68. doi: 10.1016/0002-8703(70)90394-7. [DOI] [PubMed] [Google Scholar]
- 28.Klevay LM, Milne DB. Low dietary magnesium increases supraventricular ectopy. Am J Clin Nutr. 2002;75:550–554. doi: 10.1093/ajcn/75.3.550. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen FH, Milne DB, Klevay LM, Gallagher S, Johnson L. Dietary magnesium deficiency induces heart rhythm changes, impairs glucose tolerance, and decreases serum cholesterol in post menopausal women. J Am Coll Nutr. 2007;26:121–132. doi: 10.1080/07315724.2007.10719593. [DOI] [PubMed] [Google Scholar]
- 30.Chiuve SE, Korngold EC, Januzzi JL, Jr, Gantzer ML, Albert CM. Plasma and dietary magnesium and risk of sudden cardiac death in women. Am J Clin Nutr. 2011;93:253–260. doi: 10.3945/ajcn.110.002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whang R, Oei TO, Aikawa JK, Ryan MP, Watanabe A, Chrysant SG, Fryer A. Magnesium and potassium interrelationships, experimental and clinical. Acta Med Scand Suppl. 1981;647:139–144. doi: 10.1111/j.0954-6820.1981.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 32.Khan AM, Sullivan L, McCabe E, Levy D, Vasan RS, Wang TJ. Lack of association between serum magnesium and the risks of hypertension and cardiovascular disease. Am Heart J. 2010;160:715–720. doi: 10.1016/j.ahj.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huijgen HJ, Sanders R, van Olden RW, Klous MG, Gaffar FR, Sanders GT. Intracellular and extracellular blood magnesium fractions in hemodialysis patients; is the ionized fraction a measure of magnesium excess? Clin Chem. 1998;44:639–648. [PubMed] [Google Scholar]
- 34.Westerterp KR, Goris AH. Validity of the assessment of dietary intake: problems of misreporting. Curr Opin Clin Nutr Metab Care. 2002;5:489–493. doi: 10.1097/00075197-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Mehra R, Benjamin EJ, Shahar E, Gottlieb DJ, Nawabit R, Kirchner HL, Sahadevan J, Redline S. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whang R, Ryder KW. Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. JAMA. 1990;263:3063–3064. [PubMed] [Google Scholar]
- 37.Whang R, Chrysant S, Dillard B, Smith W, Fryer A. Hypomagnesemia and hypokalemia in 1,000 treated ambulatory hypertensive patients. J Am Coll Nutr. 1982;1:317–322. doi: 10.1080/07315724.1982.10719001. [DOI] [PubMed] [Google Scholar]