Abstract
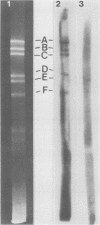
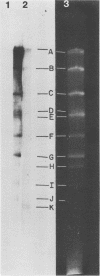
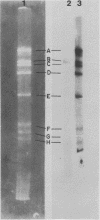
Previous kinetic and absorption hybridization experiments had demonstrated that the DNA of the B95-8 strain of Epstein-Barr virus was missing approximately 10% of the DNA sequences present in the DNA of the HR-1 strain (R.F. Pritchett, S.D. Hayward, and E. Kieff, J. Virol. 15:556-569, 1975; B. Sugder, W.C. Summers, and G. Klein, J. Virol. 18:765-775, 1976). The HR-1 strain differs from other laboratory strains, including the B95-8 and W91 strains, and from virus present in throat washings from patients with infectious mononucleosis in its inability to transform lymphocytes into lymphoblasts capable of long-term growth in culture (P. Gerber, Lancet i:1001, 1973; J. Menezes, W. Leibold, and G. Klein, Exp. Cell. Res. 92:478-484, 1975; G. Miller, D. Coope, J. Niederman, and J. Pagano, J. Virol. 18:1071-1080, 1976; G. Miller, J. Robinson, L. Heston, and M. Lipman, Proc. Natl. Acad. Sci. U.S.A. 71:4006-4010, 1974). In the experiments reported here, the restriction enzyme fragments of Epstein-Barr virus DNA which contain sequences which differ among the HR-1, B95-8, and W91 strains have been identified. The DNA of the HR-1, B95-8, and W91 strains each differed in complexity. The sequences previously shown to be missing in the B95-8 strain were contained in the EcoRI-C and -D and Hsu I-E and -N fragments of the HR-1 strain and in the EcoRI-C and Hsu I-D and -E fragments of the W91 strain. The HR-1 strain was missing DNA contained in EcoRI fragments A and J through K and Hsu I fragment B of the B95-8 strain and in the EcoRI-A and Hsu I-B fragments of the W91 strain. The relationship of these data to the linkage map of restriction enzyme fragments of the DNA of the B95-8 and W91 strains (E. Kieff, N. Raab-Traub, D. Given, W. King, A.T. Powell, R. Pritchett, and T. Dambaugh, In F. Rapp and G. de-The, ed., Oncogenesis and Herpesviruses III, in press; D. Given and E. Kieff, submitted for publication) and the possible significance of the data are discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber P., Nkrumah F. K., Pritchett R., Kieff E. Comparative studies of Epstein-Barr virus strains from Ghana and the United States. Int J Cancer. 1976 Jan 15;17(1):71–81. doi: 10.1002/ijc.2910170111. [DOI] [PubMed] [Google Scholar]
- Gerber P. Oral excretion of E.B. virus. Lancet. 1973 May 5;1(7810):1001–1001. doi: 10.1016/s0140-6736(73)91643-7. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. D. Epstein-Barr virus-specific RNA. I. Analysis of viral RNA in cellular extracts and in the polyribosomal fraction of permissive and nonpermissive lymphoblastoid cell lines. J Virol. 1976 May;18(2):518–525. doi: 10.1128/jvi.18.2.518-525.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. DNA of Epstein-Barr virus. II. Comparison of the molecular weights of restriction endonuclease fragments of the DNA of Epstein-Barr virus strains and identification of end fragments of the B95-8 strain. J Virol. 1977 Aug;23(2):421–429. doi: 10.1128/jvi.23.2.421-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Heston L., Grogan E., Miller G. Radiobiological inactivation of Epstein-Barr virus. J Virol. 1978 Jan;25(1):51–59. doi: 10.1128/jvi.25.1.51-59.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G., Zajac B. A., Pearson G., Waubke R., Scriba M. Differential reactivity of human serums with early antigens induced by Epstein-Barr virus. Science. 1970 Jul 10;169(3941):188–190. doi: 10.1126/science.169.3941.188. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Kieff E., Levine J. Homology between Burkitt herpes viral DNA and DNA in continuous lymphoblastoid cells from patients with infectious mononucleosis. Proc Natl Acad Sci U S A. 1974 Feb;71(2):355–358. doi: 10.1073/pnas.71.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G. Immunological aspects of Burkitt's lymphoma. Adv Immunol. 1971;14:187–250. doi: 10.1016/s0065-2776(08)60285-0. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Miller G., Coope D., Niederman J., Pagano J. Biological properties and viral surface antigens of Burkitt lymphoma- and mononucleosis- derived strains of Epstein-Barr virus released from transformed marmoset cells. J Virol. 1976 Jun;18(3):1071–1080. doi: 10.1128/jvi.18.3.1071-1080.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Comparison of the yield of infectious virus from clones of human and simian lymphoblastoid lines transformed by Epstein-Barr virus. J Exp Med. 1973 Dec 1;138(6):1398–1412. doi: 10.1084/jem.138.6.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K. High-frequency establishment of human immunoglobulin-producing lymphoblastoid lines from normal and malignant lymphoid tissue and peripheral blood. Int J Cancer. 1971 Nov 15;8(3):432–442. doi: 10.1002/ijc.2910080311. [DOI] [PubMed] [Google Scholar]
- Orellana T., Kieff E. Epstein-barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and prooductive infections. J Virol. 1977 May;22(2):321–330. doi: 10.1128/jvi.22.2.321-330.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett R. F., Hayward S. D., Kieff E. D. DNA of Epstein-Barr virus. I. Comparative studies of the DNA of Epstein-Barr virus from HR-1 and B95-8 cells: size, structure, and relatedness. J Virol. 1975 Mar;15(3):556–559. doi: 10.1128/jvi.15.3.556-559.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]