Abstract
Objective
To examine the relationship between large tumor size and breast cancer–specific mortality (BCSM), especially in a subset of patients with negative lymph nodes (LNs).
Patients and Methods
We used the Surveillance, Epidemiology and End Results registry to identify 107,705 female patients diagnosed from January 1, 1990, through December 31, 2003, as having invasive breast cancer and treated with surgery and LN dissection. Relevant issues unclear in the database were studied in an additional 335 patients with locally advanced disease treated with neoadjuvant chemotherapy.
Results
In the multivariable analysis, a significant interaction was found between tumor size and LN involvement (P<.001). In LN-negative diseases, the relationship between tumor size and BCSM was piecewise. Using 21- to 30-mm tumors as the reference, the hazard ratio (HR) of BCSM increased with increasing tumor size until a peak at 41 to 50 mm (HR, 1.49; P<.001), after which increasing tumor size was unexpectedly related to decreasing hazard, with a nadir at 61 to 80 mm (HR, 1.06; P=.70). The 61- to 80-mm tumors exhibited a significantly lower BCSM compared with the 41- to 50-mm (P=.02) and greater than 80-mm (P=.03) subgroups. This pattern remained after stratification by estrogen receptor status but was not observed in patients with LN-positive disease. Further analysis indicated that the survival advantage of 61- to 80-mm tumors in LN-negative disease might result from its low risk of distant metastasis.
Conclusion
A relatively larger tumor size without LN involvement may be a surrogate for biologically indolent disease of distant metastasis. Our findings, if validated in other large databases, may provide better understanding of breast cancer biology.
Abbreviations and Acronyms: AJCC, American Joint Committee on Cancer; BCSM, breast cancer–specific mortality; DRFS, distant relapse-free survival; ER, estrogen receptor; FDSCC, Fudan University Shanghai Cancer Center; HR, hazard ratio; LN, lymph node; NCT, neoadjuvant chemotherapy; pCR, pathological complete remission; PR, progesterone receptor; RFS, relapse-free survival; SEER, Surveillance, Epidemiology and End Results
Breast cancer is unfortunately a common and heterogeneous disease. Metastasis and breast cancer outcomes are still difficult to predict. Thus far, a number of prognostic factors have been identified, including local tumor size, regional lymph node (LN) involvement, the presence of distant metastasis, age, histologic grade, hormone receptor and HER2 status, and the presence of lymphovascular invasion.1 Local tumor size, regional LN status, and distant metastasis are the top 3 prognostic determinants and constitute the American Joint Committee on Cancer (AJCC) staging system.2 Increasing tumor size has been well established to be associated with a higher risk of axillary LN involvement and increased breast cancer–specific mortality (BCSM).3 However, these findings are from studies in which the investigators treated all the stage T3 tumors (>5 cm) as one category.3,4 Whether the linear relationship between tumor diameter and BCSM could be extrapolated to a specific subset of patients with a large tumor and negative LNs is unclear.
Recently, both preclinical and clinical evidence consistently revealed that the acquisition of metastatic potential and distant spread may occur during the early stage of cancer development.5,6 A piece of impressive evidence6 indicated that a very small tumor size is a surrogate for a biologically aggressive disease in cases with extensive LN involvement. These findings challenge the accepted notions of malignant progression that identify metastasis as a sequential process of malignant cellular expansion. We challenge the universal notion in another way, that is, a distant metastasis might not be a destined consequence of the local expansion of a primary tumor. We hypothesized that tumors that fail to metastasize to regional LNs even at a late stage (eg, a T3N0 tumor) may reflect a more biologically indolent phenotype and may thus paradoxically be associated with a relatively lower risk of distant spread and BCSM.
In the present study, we comprehensively examined the association between large tumor size and BCSM, especially in the subset of patients with negative LNs. To collect sufficient cases with large tumors, we used the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) cancer database.7 Moreover, because the SEER data lack information on the neoadjuvant chemotherapy (NCT) response, adjuvant therapies, and recurrence pattern, we further clarified these relevant issues in another set of patients with locally advanced breast cancer who uniformly received NCT from the Fudan University Shanghai Cancer Center (FDSCC).8
Patients and Methods
Patient Selection and Outcome Measures of the SEER Set
The current SEER database consists of 17 population-based cancer registries. We selected female patients diagnosed as having invasive breast cancer from January 1, 1990, through December 31, 2003. Patients diagnosed as having breast cancer before 1990 were excluded because of unavailable hormone receptor data; patients diagnosed as having breast cancer after 2003 were excluded to ensure an adequate follow-up time. SEER's coding instruction of tumor size and estrogen receptor (ER) and progesterone receptor (PR) status is given in the Supplemental Methods (available online at http://www.mayoclinicproceedings.org).
We identified 107,705 patients in the SEER database according to the following inclusion criteria: female, age of diagnosis between 18 and 74 years, surgical treatment with either mastectomy or breast-conserving surgery, AJCC stages I to III, pathologically confirmed invasive ductal carcinoma, at least 4 axillary LNs dissected, unilateral breast cancer, known ER and PR status, known time of diagnosis, breast cancer as the first and only cancer diagnosis, and known tumor size. Because SEER did not provide information on chemotherapy and endocrine therapy, we excluded the therapeutic factors in this analysis for consistency. The research on the SEER data was submitted and determined to be qualified for institutional review board exemption in FDSCC.
The primary study outcome of the SEER data was BCSM. Vital status (alive or dead) was obtained. The cause of death was categorized as breast cancer specific or non–breast cancer related. The BCSM was calculated from the date of diagnosis to the date of breast cancer death. Patients who died of other causes were censored at the date of death.
Patient Selection and Outcome Measures of the FDSCC Set
To validate the findings from the SEER set and to clarify relevant issues, we used data from the FDSCC, which included 395 consecutive patients diagnosed as having AJCC stages IIA to IIIC unilateral breast cancer and treated with anthracycline-based NCT between January 2000 and December 2007 at the FDSCC. We selected 335 patients fulfilling the inclusion criteria, which are given in the Supplemental Methods. Clinical response and pathologic complete remission (pCR) were evaluated (see criteria in Supplemental Methods).
The outcomes of interest were relapse-free survival (RFS) and distant relapse-free survival (DRFS), which were calculated from the date of diagnosis to the date of first event of local, regional, or distant metastasis of breast cancer and to the date of first distant metastasis, respectively. To determine distant relapse events, isolated local recurrence was further followed up until a metastatic event. The research protocol of this part was reviewed and approved by the Ethical Committee and Institutional Review Board of the FDSCC. All patients provided written informed consent.
Statistical Analyses
For the SEER data, study variables are given in Table 1. Tumor size was treated as a categorical variable. Tumors larger than 80 mm were combined because of limited case numbers. The basic analysis procedure has been described previously.6 To determine whether there was a significant interaction between the degree of tumor size and LN involvement in predicting BCSM, we defined an interaction term (ie, size × nodes).6 With a median follow-up of 99 months, we reported 8-year rates for BCSM. The nonlinear effect of continuous tumor size on BCSM was assessed using a B-spline transformation with evenly spaced knots.6 The overall presence of interaction between tumor size and node involvement was evaluated by the Wald test. Pairwise comparisons were performed between different combinations of LN involvement and tumor size to determine the presence of significant BCSM differences.
TABLE 1.
Characteristics of Patients from the SEER Database by LN Involvementa
| Characteristic | No. (%) of patientsb |
P value | ||
|---|---|---|---|---|
| Total (N=107,705) | LN negative (n=63,944) | LN positive (n=43,761) | ||
| Median follow-up (mo) (IQR) | 99 (72-140) | 106 (80-149) | 87 (63-122) | |
| Year of diagnosis | <.001 | |||
| 1990-1994 | 24,695 (22.9) | 15,415 (24.1) | 9280 (21.2) | |
| 1995-1999 | 34,497 (32.0) | 21,867 (34.2) | 12,630 (28.9) | |
| 2000-2003 | 48,513 (45.0) | 26,662 (41.7) | 21,851 (49.9) | |
| Patient age (y) | <.001 | |||
| ≤50 | 40,808 (37.9) | 21,516 (33.6) | 19,292 (44.1) | |
| >50 | 66,897 (62.1) | 42,428 (66.4) | 24,469 (55.9) | |
| Race | <.001 | |||
| White | 88,390 (82.1) | 53,253 (83.3) | 35,137 (80.3) | |
| Black | 9294 (8.6) | 4739 (7.4) | 4555 (10.4) | |
| Othersc | 9676 (9.0) | 5746 (9.0) | 3930 (9.0) | |
| Unknown | 345 (0.3) | 206 (0.3) | 139 (0.3) | |
| Tumor size (mm) | <.001 | |||
| 0-20 | 67,723 (62.9) | 47,967 (75.0) | 19,756 (45.1) | |
| 21-30 | 22,868 (21.2) | 10,617 (16.6) | 12,251 (28.0) | |
| 31-40 | 8402 (7.8) | 3042 (4.8) | 5360 (12.2) | |
| 41-50 | 3835 (3.6) | 1189 (1.9) | 2646 (6.0) | |
| 51-60 | 2,102 (2.0) | 511 (0.8) | 1591 (3.6) | |
| 61-80 | 1798 (1.7) | 405 (0.6) | 1393 (3.2) | |
| >80 | 977 (0.9) | 213 (0.3) | 764 (1.7) | |
| Grade | <.001 | |||
| I | 14,029 (13.0) | 10,652 (16.7) | 3377 (7.7) | |
| II | 40,692 (37.8) | 25,113 (39.3) | 15,579 (35.6) | |
| III and UD | 45,391 (42.1) | 23,110 (36.1) | 22,281 (50.9) | |
| Unknown | 7593 (7.0) | 5069 (7.9) | 2524 (5.8) | |
| ER status | <.001 | |||
| Negative | 29,679 (27.6) | 16,973 (26.5) | 12,706 (29.0) | |
| Positive | 78,026 (72.4) | 46,971 (73.5) | 31,055 (71.0) | |
| PR status | <.001 | |||
| Negative | 38,748 (36.0) | 22,276 (34.8) | 16,472 (37.6) | |
| Positive | 68,957(64.0) | 41,668 (65.2) | 27,289 (62.4) | |
| No. of LNs dissected | <.001 | |||
| 4-10 | 32,292 (30.0) | 21,503 (33.6) | 10,789 (24.7) | |
| 11-20 | 57,534 (53.4) | 33,324 (52.1) | 24,210 (55.3) | |
| ≥21 | 17,879 (16.6) | 9117 (14.3) | 8762 (20.0) | |
ER = estrogen receptor; IQR = interquartile range; LN = lymph node; PR = progesterone receptor; SEER = Surveillance, Epidemiology and End Results; UD = undifferentiated.
Data are presented as No. (percentage) of patients unless otherwise indicated.
Including American Indian, Alaska Native, Asian, and Pacific Islander.
For the FDSCC data, study variables are shown in Supplemental Table 1 (available online at http://www.mayoclinicproceedings.org). Because the SEER results indicated a more favorable survival in patients with 61- to 80-mm tumors when compared with those having 41- to 50-mm tumors or tumors larger than 80 mm in the context of LN-negative disease, we confined our analysis to the 40- to 80-mm tumors. Few LN-negative tumors were larger than 80 mm in size and were thus excluded from analysis. With a median follow-up of 49 months, 4-year rates for RFS and DRFS rather than overall survival were reported.
Comparisons of patient and tumor characteristics by LN involvement were performed using χ2 tests. Survival curves were constructed using the Kaplan-Meier method, and univariate survival difference was determined with the log-rank test. Time point survival was estimated using the life-table method. Adjusted hazard ratios (HRs) with 95% CIs were calculated using Cox proportional hazards models. All the statistical analysis was performed using Stata statistical software, version 10.0 (StataCorp). Two-sided P<.05 was considered statistically significant.
Results
Interaction Between Tumor Size and LN Status in BCSM in the SEER Set
We identified 107,705 eligible patients, 16,079 of whom died of breast cancer. The patient and tumor characteristics by LN status are summarized in Table 1. Lymph node negativity was correlated with older age, white race, smaller tumor size, lower grade, ER and PR positivity, and limited LN dissection.
In the univariate analysis, year of diagnosis, patient age, race, tumor size, LN status, tumor grade, ER and PR statuses, and number of LNs dissected were significantly associated with breast cancer–specific survival (Supplemental Table 2; available online at http://www.mayoclinicproceedings.org). In the multivariate analysis, a significant interaction was found between tumor size and LN status in determining BCSM (P<.001; Table 2). Separate survival curves for LN-negative and LN-positive patients stratified by categorical tumor size are shown in Figure 1 (predicted by Cox regression model) and Supplemental Figure 1 (available online at http://www.mayoclinicproceedings.org) (crude Kaplan-Meier curves). Among LN-negative patients, those with 61- to 80-mm tumors experienced a significantly lower BCSM compared with those with 40- to 50-mm tumors (HR, 1.41; 95% CI, 1.04-1.90; P=.02; 61- to 80-mm tumors as the reference), but they experienced a similar BCSM to those with 21- to 30-mm tumors (HR, 0.95; 95% CI, 0.72-1.25; P=.70; 61- to 80-mm tumors as the reference). In contrast, in either overall or LN-positive patients, a straightforward dose-effect relationship of larger tumor size with increasing BCSM was observed. To determine whether there was a confounding effect of year of diagnosis, we stratified our Cox model by year of diagnosis. The stratified and unstratified analyses gave essentially identical results.
TABLE 2.
Multivariate Analysis of Breast Cancer–Specific Mortalitya
| Variable | Overall |
Pairwise |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Year of diagnosis | <.001 | … | … | … | … | |
| 1990-1994 | 1.00 [Reference] | |||||
| 1995-1999 | 0.76 (0.73-0.79) | |||||
| 2000-2003 | 0.60 (0.57-0.63) | |||||
| Patient age (y) | <.001 | … | … | … | … | |
| ≤50 | 1.00 [Reference] | |||||
| >50 | 1.14 (1.10-1.18) | |||||
| Race | <.001 | … | … | … | … | |
| White | 1.00 [Reference] | |||||
| Black | 1.41 (1.35-1.48) | |||||
| Othersb | 0.89 (0.84-0.95) | |||||
| Unknown | UC | |||||
| Grade | <.001 | … | … | … | … | |
| I | 1.00 [Reference] | |||||
| II | 2.59 (2.34-2.88) | |||||
| III and UD | 4.07 (3.67-4.52) | |||||
| Unknown | UC | |||||
| ER status | <.001 | … | … | … | … | |
| Negative | 1.00 [Reference] | |||||
| Positive | 0.74 (0.71-0.78) | |||||
| PR status | <.001 | … | … | … | … | |
| Negative | 1.00 [Reference] | |||||
| Positive | 0.78 (0.75-0.82) | |||||
| No. of LNs dissected | .06 | … | … | … | … | |
| 4-10 | 1.00 [Reference] | |||||
| 11-20 | 0.96 (0.93-1.00) | |||||
| ≥21 | 0.94 (0.90-0.99) | |||||
| Tumor size | <.001 | … | … | … | … | |
| LN status | <.001 | … | … | … | … | |
| Size × nodesc | <.001 | … | … | … | … | |
| Size and nodesd | <.001 | … | … | … | … | |
| 0-20 mm N0 | 1.00 [Reference] | 0.47 (0.44-0.50) | <.001e | 0.44 (0.34-0.58) | <.001f | |
| 21-30 mm N0 | 2.13 (1.99-2.28) | 1.00 [Reference] | 0.95 (0.72-1.25) | .70g | ||
| 31-40 mm N0 | 2.76 (2.51-3.04) | 1.30 (1.17-1.44) | <.001e | 1.23 (0.93-1.63) | .15f | |
| 41-50 mm N0 | 3.16 (2.75-3.63) | 1.49 (1.29-1.71) | <.001e | 1.41 (1.04-1.90) | .02f | |
| 51-60 mm N0 | 2.87 (2.33-3.54) | 1.35 (1.09-1.67) | .006e | 1.28 (0.91-1.79) | .16f | |
| 61-80 mm N0 | 2.25 (1.71-2.95) | 1.06 (0.80-1.39) | .70e | 1.00 [Reference] | ||
| >80 mm N0 | 3.51 (2.56-4.80) | 1.65 (1.20-2.26) | .002e | 1.56 (1.03-2.35) | .03f | |
| 0-20 mm N1+ | 3.22 (3.04-3.40) | 0.62 (0.59-0.65) | <.001g | 0.31 (0.29-0.34) | <.001h | |
| 21-30 mm N1+ | 5.20 (4.92-5.49) | 1.00 [Reference] | 0.51 (0.46-0.55) | <.001h | ||
| 31-40 mm N1+ | 6.74 (6.32-7.18) | 1.30 (1.22-1.37) | <.001g | 0.66 (0.60-0.72) | <.001h | |
| 41-50 mm N1+ | 7.68 (7.11-8.30) | 1.48 (1.37-1.59) | <.001g | 0.75 (0.68-0.83) | <.001h | |
| 51-60 mm N1+ | 8.47 (7.73-9.28) | 1.63 (1.49-1.78) | <.001g | 0.83 (0.74-0.93) | .001h | |
| 61-80 mm N1+ | 10.25 (9.35-11.24) | 1.97 (1.80-2.16) | <.001g | 1.00 [Reference] | ||
| >80 mm N1+ | 12.61 (11.27-14.10) | 2.43 (2.18-2.71) | <.001g | 1.23 (1.08-1.40) | .002h | |
CI = confidence interval; ER = estrogen receptor; HR = hazard ratio; LN = lymph node; PR = progesterone receptor; UC = uncalculated; UD = undifferentiated.
Including American Indian, Alaska Native, Asian, and Pacific Islander.
To determine whether there was significant interaction between degree of tumor size and nodes involvement in predicting breast cancer–specific mortality, we defined an interaction term (ie, size × nodes).
When the variable size and nodes was included, the variables tumor size, LN status, and their interaction term size × nodes were removed from the Cox model.
Reference group: 21 to 30 mm N0.
Reference group: 61 to 80 mm N0.
Reference group: 21 to 30 mm N1+.
Reference group: 61 to 80 mm N1+.
FIGURE 1.
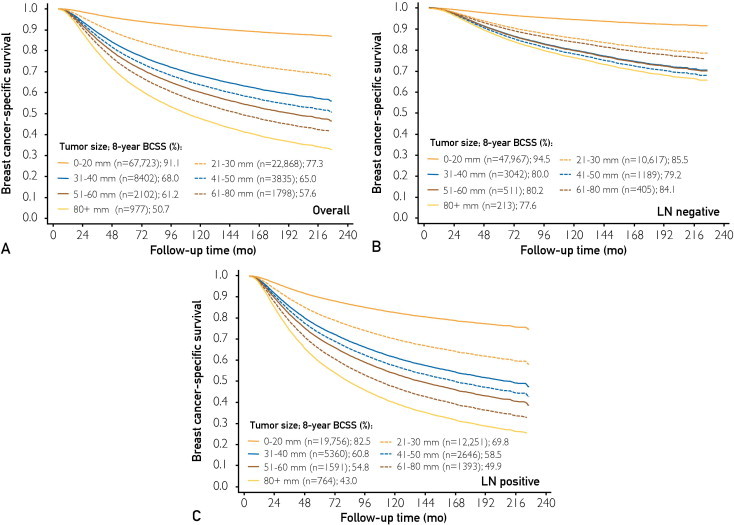
Survival curves stratified by categorical tumor size using estimates predicted by Cox regression model survival. Overall (A), lymph node (LN)–negative (B), and LN-positive (C) patients from the Surveillance, Epidemiology and End Results database were stratified into 7 categories of tumor size: 0 to 20 mm, 21 to 30 mm, 31 to 40 mm, 41 to 50 mm, 51 to 60 mm, 61 to 80 mm, and larger than 80 mm. Among patients with negative LNs (B), those with 61- to 80-mm tumors experienced a breast cancer–specific survival (BCSS) similar to those with 21- to 30-mm tumors and higher than patients with 31- to 60-mm or larger than 80-mm tumors. Survival curves are plotted using estimates predicted by Cox regression model survival. The 8-year survival rates were calculated by the life-table method.
The relationship between continuous tumor size and 8-year BCSM stratified by LN status is illustrated in Supplemental Figure 2 (available online at http://www.mayoclinicproceedings.org). Supplemental Figure 2, A reveals a pattern of increasing 8-year BCSM with increasing tumor size at moderate to large tumor sizes until a threshold size (approximately 50 mm) is reached, after which point increasing tumor size is unexpectedly related to decreasing 8-year BCSM; the decreasing BCSM reached a nadir (61-80 mm), followed by a gradual increase with increasing tumor size. The 8-year BCSM in patients with tumors larger than 80 mm had wide 95% CIs because of the small sample size. Supplemental Figure 2, C displays the hazard of BCSM in LN-negative disease vs LN-positive disease at each tumor size category, which implies that larger tumors (61-80 mm) coupled with negative LNs represents a unique subtype with a better prognosis.
Interaction by ER Status in the SEER Set
Among both ER-negative (n=29,679) and ER-positive patients (n=78,026), a significant interaction between tumor size and LN involvement was also identified. Among the ER-negative patients with LN-negative disease, those with 21- to 30-mm tumors experienced a significantly lower BCSM relative to other patients with larger tumors (HRs from 1.29 to 1.68; with P values from <.001 to .07; 21- to 30-mm tumors as the reference) except for those with 61- to 80-mm tumors (HR, 1.02; 95% CI, 0.72-1.44; P=.91; 21- to 30-mm tumors as the reference) (Table 3). Similar results were observed in ER-positive patients with LN-negative disease. A comparable BCSM between 21- to 30-mm tumors and 61- to 80-mm tumors in either ER-negative or ER-positive patients was unlikely because of the underpowered sample size after ER stratification, which might cause a failure in detecting an existing difference because 61- to 80-mm tumors in both ER-negative and ER-positive groups consistently revealed a significant BCSM difference with 41- to 50-mm tumors, which had much fewer samples than 21- to 30-mm tumors.
TABLE 3.
Breast Cancer–Specific Mortality With Interaction Term by ER Statusa
| Size and nodesb | ER negative |
ER positive |
||||||
|---|---|---|---|---|---|---|---|---|
| Overall |
Pairwise |
Overall |
Pairwise |
|||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| 0-20 mm N0 | 1.00 [Reference] | <.001 | 0.56 (0.50-0.62) | <.001c | 1.00 [Reference] | <.001 | 0.41 (0.37-0.44) | <.001c |
| 21-30 mm N0 | 1.80 (1.62-1.99) | 1.00 [Reference] | 2.47 (2.25-2.71) | 1.00 [Reference] | ||||
| 31-40 mm N0 | 2.32 (2.03-2.64) | 1.29 (1.12-1.48) | <.001c | 3.34 (2.90-3.84) | 1.35 (1.17-1.57) | <.001c | ||
| 41-50 mm N0 | 2.91 (2.43-3.47) | 1.62 (1.35-1.94) | <.001c | 3.15 (2.51-3.94) | 1.28 (1.01-1.61) | .04c | ||
| 51-60 mm N0 | 2.34 (1.77-3.08) | 1.30 (0.98-1.72) | .07c | 3.84 (2.78-5.30) | 1.55 (1.12-2.16) | .008c | ||
| 60-80 mm N0 | 1.83 (1.30-2.58) | 1.02 (0.72-1.44) | .91c | 3.14 (1.99-4.94) | 1.27 (0.81-2.01) | .30c | ||
| >80 mm N0 | 3.03 (2.06-4.44) | 1.68 (1.15-2.48) | .008c | 4.14 (2.40-7.15) | 1.68 (0.97-2.91) | .06c | ||
| 0-20 mm N1+ | 3.10 (2.84-3.38) | 0.71 (0.65-0.77) | <.001d | 3.29 (3.06-3.54) | 0.57 (0.53-0.61) | <.001d | ||
| 21-30 mm N1+ | 4.38 (4.01-4.77) | 1.00 [Referent] | 5.81 (5.41-6.25) | 1.00 [Referent] | ||||
| 31-40 mm N1+ | 5.40 (4.90-5.96) | 1.23 (1.13-1.35) | <.001d | 7.91 (7.27-8.61) | 1.36 (1.26-1.47) | <.001d | ||
| 41-50 mm N1+ | 6.54 (5.83-7.34) | 1.49 (1.34-1.67) | <.001d | 8.47 (7.63-9.41) | 1.46 (1.32-1.61) | <.001d | ||
| 51-60 mm N1+ | 6.95 (6.07-7.95) | 1.59 (1.39-1.81) | <.001d | 9.70 (8.56-10.99) | 1.67 (1.48-1.88) | <.001d | ||
| 61-80 mm N1+ | 8.46 (7.38-9.70) | 1.93 (1.69-2.21) | <.001d | 11.23 (9.91-12.74) | 1.93 (1.71-2.18) | <.001d | ||
| >80 mm N1+ | 12.33 (10.58-14.38) | 2.82 (2.43-3.27) | <.001d | 11.38 (9.64-13.44) | 1.96 (1.67-2.30) | <.001d | ||
CI = confidence interval; ER = estrogen receptor; HR = hazard ratio.
Adjusted for year of diagnosis, patient age, race, grade, progesterone receptor status, and number of lymph nodes dissected.
Referent group: 21 to 30 mm N0.
Referent group: 21 to 30 mm N1+.
Evaluating the SEER Outcomes in the FDSCC Set
These findings should be treated with caution because the results could be biased by confounding factors, such as NCT response and adjuvant therapies. Clinically, patients with tumor larger than 40 mm had probably received NCT, but the SEER database failed to record this information. To evaluate the reliability of SEER results, we studied relevant issues in 335 patients with locally advanced breast cancer who were treated with NCT from the FDSCC database. The patients were divided into 2 tumor size categories (40-60 mm and 61-80 mm) according to the SEER results.
First, we studied the effect of NCT on tumors with different size and LN categories. The rates of pCR in the breast after NCT were comparable between the 40- to 60-mm tumors and the 61- to 80-mm tumors in LN-negative (P=.65) or LN-positive disease (P=.52).
Second, we evaluated the influence of adjuvant treatments. Adjuvant chemotherapy, adjuvant radiotherapy, or adjuvant endocrine therapy did not specifically reduce the relapse risk of 61- to 80-mm tumors compared with the 40- to 60-mm group in either LN-negative or LN-positive disease (data not shown).
Third, we estimated the proportion of NCT-induced LN-negative disease after surgery (ie, LN-positive before NCT but LN-negative after surgery) in the SEER database. In our study, 17.9% of cases with LN-positive disease, confirmed by fine-needle aspiration of palpable LNs before NCT, were proven to be LN negative after NCT. Of note, early patients in the SEER database treated with NCT using cyclophosphamide, methotrexate, and fluorouracil might achieve a limited pCR rate in contrast to that in the anthracycline and taxane era. We thus speculated that more than 80% of the patients with T3 disease recorded in the SEER database had the same LN status as their pre-NCT LN results.
Finally, we investigated the relapse pattern of large tumors. In the univariate analysis, patient age, pCR, and tumor size and LN category were significantly associated with RFS and DRFS. The LN-negative patients with larger tumors (61-80 mm) had similar RFS rates to those with 40- to 60-mm tumors (log-rank P=.82; Figure 2, A). When the analysis was confined to distant metastatic events (DRFS), 61- to 80-mm tumors had a relatively lower risk of distant failure compared with 40- to 60-mm tumors, but the difference was not significant (log-rank P=.24; Figure 2, B), which was probably because of the small sample size (n=73) and limited events (n=9). Similar multivariate results are given in Supplemental Table 3 (available online at http://www.mayoclinicproceedings.org).
FIGURE 2.
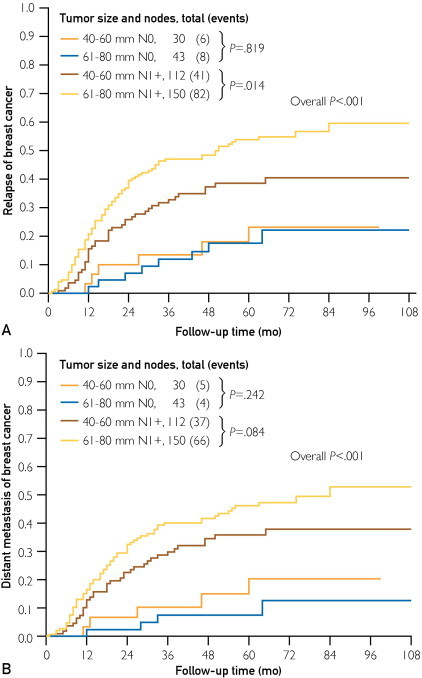
Cumulative breast cancer–specific mortality curves stratified by tumor size and lymph node (LN) status. Relapse curves (A) or distant relapse curves (B) using the Kaplan-Meier method in patients with locally advanced breast cancer from the Fudan University Shanghai Cancer Center are shown. N0 cases represent patients who had both clinically detected LN-negative status before neoadjuvant chemotherapy (NCT) and pathologically confirmed LN-negative status after surgery; N1+ cases represent patients who were either clinically detected LN positive before NCT or pathologically confirmed LN positive after surgery. Clinically detected is defined as detected by imaging studies or clinical examination and having characteristics highly suggestive of involved LNs or a presumed pathologic macrometastasis on the basis of fine-needle aspiration biopsy with cytologic examination.
Discussion
We hypothesized that for primary breast cancer with a relatively large tumor size concurrent with negative LN involvement, tumor size may be a surrogate for biologically indolent disease of regional/distant dissemination and consequently result in a lower BCSM. After adjustment for known breast cancer prognostic factors, we observed that patients with 61- to 80-mm tumors had a significantly lower BCSM compared with those with 41- to 50-mm tumors or tumor larger than 80 mm, whereas the BCSM associated with 61- to 80-mm tumors was comparable with that of 21- to 30-mm tumors. In contrast, such a piecewise relationship between tumor size and BCSM was not observed in patients with LN-positive disease. The observed relationships remained after ER stratification. We further analyzed a cohort of patients with locally advanced breast cancer treated with NCT and found a tendency toward a lower risk of developing distant metastasis in LN-negative, 61- to 80-mm tumors, which might account for the paradoxical observation of decreased mortality in this subgroup.
Although the conventional view of cancer spread is that cancer gains metastatic ability through an accumulation of mutations as they grow to a large size,9 recent studies have suggested that for some tumors, the acquisition of metastatic potential may occur early in cancer development, even in the absence of detectable primary tumors.5,10,11 Wo et al6 determined that in extensive LN-positive disease, a very small tumor size may be a surrogate for biologically aggressive disease, whereas in LN-negative disease, as the present study suggested, larger tumor size may be a surrogate for disease biologically indolent for distant metastasis. Our findings and those of Wo et al consistently reinforce the idea that the initial biological feature rather than the accumulated metastatic ability during tumor evolution likely determines the potential of distant dissemination. Some primary cancers are excellent at distant seeding per se, whereas some cancers are self-seeders. The distant seeder is good at dissemination even when the primary tumor is very small, and a pure self-seeder reveals a pronounced mitotic activity and local proliferation but fails to migrate to or blossom in regional LNs or distant organs.12
In our study, a piecewise relationship between large tumor size and BCSM was observed. The 61- to 80-mm group had the lowest BCSM compared with the 31- to 40-mm, 41- to 50-mm, 51- to 60-mm, and greater than 80-mm group. A potential explanation is that, as tumor grows, distant or regional seeders have a high chance to disseminate to LN; only the stenoplastic type of self-seeder develops to a large local tumor without evident regional or distant dissemination. The 61- to 80-mm tumors concurrent with LN-negative disease represent a highly self-seeding subtype, which harbors a low potential of metastasis, a major cause of breast cancer mortality.13 In contrast, LN-negative cancer in moderately sized tumors (31-50 mm) did not guarantee an indolent nature. In our study, 35% of 31- to 50-mm tumors were LN negative, whereas 22% of 61- to 80-mm tumors were LN negative, indicating that approximately one-third (37%) of the present LN-negative 31- to 50-mm tumors would become LN-positive disease if they continued to grow. Furthermore, tumors larger than 80 mm might represent another aggressive subtype, which proliferates precipitately and probably exhibits T4 tumor features. The SEER database failed to discriminate between T3 and T4 tumors.
The advantage of 61- to 80-mm tumors in survival was seen in both ER-negative and ER-positive patients, strongly suggesting that even in ER-negative tumors (including those that are HER2 positive and triple negative) there could be certain subsets of breast cancer displaying favorable biological features14 and that additional biological markers to define this indolent subgroup that are not yet identified may aid in the prognostic determination.
Our findings might have an effect on clinical practice. Because those larger tumors with negative LNs probably have a low chance of distant dissemination, the local-regional treatments might be more crucial. The extension of surgery, systemic treatment options, and timing of adjuvant chemotherapy (before or after radiotherapy) should be individualized.
Our study had several limitations. First, the SEER database lacks several important tumor characteristics (eg, HER2), cancer therapy (neoadjuvant and adjuvant), and patient outcome (recurrence and metastasis) variables. Thus, our analyses could not adjust for these potential confounding factors. Second, despite a large initial study population, individual subgroups became small after stratifying by tumor size, LN involvement, and ER status, yielding limited statistical power. Similarly, in the FDSCC set, a trend of a lower distant relapse rate without statistical significance was observed in the group of 61- to 80-mm tumors with negative LN, which was probably due to the limited sample size, short follow-up time, and rare events. Third, our study was performed using 2 retrospective databases rather than prospective cohorts; this approach might introduce unaccounted biases. Fourth, although we observed that the behavior of LN-negative, 61- to 80-mm tumors is similar to the 21- to 30-mm tumors, almost all the tumor sizes between 20 and 80 mm have overlapping 95% CIs to different extents. A real and precise association between tumor size and breast cancer survival in node-negative tumors needs to be further validated.
Conclusion
Our study reveals that patients with larger tumors (61-80 mm) may have decreased BCSM compared with moderate to large (41-50 mm) tumors but have a survival comparable with small (21-30 mm) tumors in the context of LN-negative disease, suggesting that larger tumor size with negative LNs may be a surrogate for biologically indolent disease of distant dissemination. Such a subtype is better at seeding itself but less proficient at seeding regional LNs or distant sites. Although the current study is the most robust evaluation of the interaction between large tumor size and LN involvement in breast cancer thus far, our findings should be interpreted with caution and validated within other large databases because of the relatively small sample size after stratification. If our findings and those of Wo et al6 are successfully validated in other databases, further correlative studies should focus on the subsets of cancer (eg, distant-seeding tumors or locally self-seeding tumors) to help elucidate genetic, genomic, or molecular differences that specifically contribute to local proliferation or distant metastasis.
Acknowledgment
Drs Yu, Jiang, and Chen have contributed equally to this work.
Footnotes
Grant Support: This research was supported by grants 30971143, 30972936, and 81001169 from the National Natural Science Foundation of China, grant SHDC12010116 from the Shanghai United Developing Technology Project of Municipal Hospitals, the Key Clinical Program of the Ministry of Health (2010-2012), the Zhuo-Xue Project of Fudan University (K.-D.Y.), and grant 11QA1401400 from the Shanghai Committee of Science and Technology Fund for the 2011 Qimingxing Project (K.-D.Y.).
Role of the Sponsors: The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Ke-Da Yu, Email: yukd@shca.org.cn.
Zhi-Ming Shao, Email: zhimingshao@yahoo.com.
Supplemental Online Material
References
- 1.Goldhirsch A., Glick J.H., Gelber R.D., Coates A.S., Thurlimann B., Senn H.J. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16(10):1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 2.Singletary S.E., Allred C., Ashley P. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20(17):3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Carter C.L., Allen C., Henson D.E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63(1):181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Chia S.K., Speers C.H., Bryce C.J., Hayes M.M., Olivotto I.A. Ten-year outcomes in a population-based cohort of node-negative, lymphatic, and vascular invasion-negative early breast cancers without adjuvant systemic therapies. J Clin Oncol. 2004;22(9):1630–1637. doi: 10.1200/JCO.2004.09.070. [DOI] [PubMed] [Google Scholar]
- 5.Husemann Y., Geigl J.B., Schubert F. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Wo J.Y., Chen K., Neville B.A., Lin N.U., Punglia R.S. Effect of very small tumor size on cancer-specific mortality in node-positive breast cancer. J Clin Oncol. 2011;29(19):2619–2627. doi: 10.1200/JCO.2010.29.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hankey B.F., Ries L.A., Edwards B.K. The Surveillance, Epidemiology, and End Results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–1121. [PubMed] [Google Scholar]
- 8.Chen S., Chen C.M., Yu K.D., Yang W.T., Shao Z.M. A prognostic model to predict outcome of patients failing to achieve pathological complete response after anthracycline-containing neoadjuvant chemotherapy for breast cancer. J Surg Oncol. 2012;105(6):577–585. doi: 10.1002/jso.22140. [DOI] [PubMed] [Google Scholar]
- 9.Norton L., Massague J. Is cancer a disease of self-seeding? Nat Med. 2006;12(8):875–878. doi: 10.1038/nm0806-875. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Kittler O., Ragg T., Daskalakis A. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003;100(13):7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altan E., Altundag K. Clinical and pathological characteristics of occult breast cancer and review of the literature. J BUON. 2011;16(3):434–436. [PubMed] [Google Scholar]
- 12.Comen E.A., Norton L., Massague J. Breast cancer tumor size, nodal status, and prognosis: biology trumps anatomy. J Clin Oncol. 2011;29(19):2610–2612. doi: 10.1200/JCO.2011.36.1873. [DOI] [PubMed] [Google Scholar]
- 13.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 14.Doane A.S., Danso M., Lal P. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


