Abstract
Calcium signaling in the diverse vascular structures is regulated by a wide range of mechanical and biochemical factors to maintain essential physiological functions of the vasculature. To properly transmit information, the intercellular calcium communication mechanism must be robust against various conditions in the cellular microenvironment. Using plasma lithography geometric confinement, we investigate mechanically induced calcium wave propagation in networks of human umbilical vein endothelial cells organized. Endothelial cell networks with confined architectures were stimulated at the single cell level, including using capacitive force probes. Calcium wave propagation in the network was observed using fluorescence calcium imaging. We show that mechanically induced calcium signaling in the endothelial networks is dynamically regulated against a wide range of probing forces and repeated stimulations. The calcium wave is able to propagate consistently in various dimensions from monolayers to individual cell chains, and in different topologies from linear patterns to cell junctions. Our results reveal that calcium signaling provides a robust mechanism for cell-cell communication in networks of endothelial cells despite the diversity of the microenvironmental inputs and complexity of vascular structures.
Keywords: plasma lithography, micropatterning, calcium, endothelial cell, cell signaling
1. Introduction
Many essential functions of the vasculature are known to be regulated by intracellular calcium signaling [1]. To allow proper physiological functions, cytosolic calcium is tightly controlled in endothelial cells by multiple intracellular and transplasmalemmal calcium regulatory mechanisms [2]. Under resting conditions, free calcium is maintained at a low concentration. The endoplasmic reticulum (ER), which contains numerous calcium binding proteins, is a major intracellular calcium store for endothelial cells [3]. The ER accounts for ~75% of the total intracellular calcium reserve while the majority of the remaining portion is stored in the mitochondria. The release of ER calcium to the cytoplasm can be controlled by calcium release channels, such as inositol 1,4,5- triphosphate (IP3) and ryanodine receptors, on the ER and can also be spontaneously released through luminal calcium leakage. Calcium mobilization can be triggered by agonists, e.g., IP-3 and ryanodine, which bind to their specific receptors and modulate the calcium release properties of these channels. Remarkably, calcium can trigger calcium release resulting in calcium induced calcium release in an autocatalytic manner. To avoid cytotoxicity due to high concentrations of calcium, the calcium release channels terminate after a short duration despite the presence of the agonists. At the same time, the cytosolic calcium is resequestered inside the ER as well as pumped outside of the cell through transmembrane ATPases, ATP-dependent calcium pumps, which continuously take up calcium from the cytosol. This resets the cytosolic calcium to a resting condition (~100 nM) and allows stimulation again after a refractory time period [1].
Physiologically, cells move calcium not only between cellular compartments and the exterior of a single cell, but also amongst neighboring cells [4–10]. These connections are made by gap junctions, which connect vascular as well as many other cell types and allow moving not only calcium ions but also transfer of other molecules and small proteins between cells. These junctions consist of connexin proteins which form pores between the cells allowing exchange of the various substances to pass through them. In the case of endothelial cells, several types of gap junction connexin proteins including connexin 40, 43 and 37 are relevant to calcium signaling [11]. Gap junctions directly link the cytoplasms of cells and allow exchange of ions and secondary messengers, including calcium and IP3. Furthermore, many cell types are known to communicate by releasing diffusible factors into the microenvironment. As a result, once calcium release in a cell has been stimulated, the signal can be transferred to neighboring cells via gap junction intercellular communication (GJIC), and extracellular diffusion, even though they are not affected by the stimulus themselves [12, 13]. The transfer of the calcium signal results in a spatiotemporal propagation of intercellular calcium wave communicating a signal between neighboring cells.
To serve as an effective cell-cell communication mechanism, intercellular calcium signaling must be robust against functional and operational conditions in vascular structures. These involve various topologies and continuous exposure to numerous biomechanical and biochemical stimuli in the cellular microenvironment. Despite the fact that extensive efforts have been devoted to elucidate the molecular mechanisms responsible for the regulation of cytosolic calcium, there is a lack of understanding in the implication of the local calcium regulation in the global characteristics of intercellular calcium communication. In particular, the functional properties of intercellular calcium signaling in the vascular systems, which have diverse dimensions (from individual cells to centimeters) and distinct architectures (e.g., linear chains and branching morphologies), are largely unknown [14–16]. Herein, we investigate the functional characteristics of intercellular calcium signaling of networks of mechanically stimulated human endothelial cells. The endothelial structures are confined using a plasma lithography cell patterning technique, which allows systematic control of the network topology and architecture [17–21]. Real-time intracellular calcium imaging is applied to observe the propagation of calcium wave in endothelial networks [22, 23]. To study the probing force, comb-drive capacitive force sensing probes are also applied to stimulate cells mechanically at the single cell level [24, 25].
2. Materials and methods
2.1 Plasma lithography cell patterning
In this study, geometric confinement of cells was achieved by plasma lithography, which creates spatial templates of cell adhesive surface chemistry on polystyrene substrates [17–21]. Plasma lithography applies selective shielding of plasma via a flexible rubber polydimethylsiloxane (PDMS) mold to produce a chemical template on the substrate (additional detail of plasma lithography can be found in supplementary information S1). The PDMS molds used to produce the selective plasma shielding were created via standard soft lithography [26], which replicated shapes by means of molding from master patterns. PDMS cast off of photoresist structures to make shielding molds was mixed in 8:1 ratio of polymer base to curing agent and degassed before being cured for 24 hours at room temperature (see also supplementary information S2 for mold fabrication method).
2.2 Cell culture
Human umbilical vein endothelial cells (HUVEC) were obtained from American Type Culture Collection (ATCC CRL-1730). HUVEC were cultured in F-12K Medium (ATCC) supplemented with 20% screened FBS (Gemini Bio-Products), 0.035 mg/ml endothelial cell growth supplement (Sigma-Aldrich), 0.1 mg/ml heparin (Sigma-Aldrich), and 0.1% gentamycin (GIBCO). HUVEC were used from passages three to six in the experiments.
2.3 Real-time intracellular calcium imaging
To perform real-time imaging of intercellular wave propagation, a calcium-sensitive dye (Fluro-3AM (Invitrogen) dissolved in DMSO (Fisher)) was first introduced inside the cells with 10 mg/ml of Pluronic® F-127 (Invitrogen). The dye was incubated inside the cells for 35 minutes for esterase cleavage activation. The dye then became fluorescent when bound to calcium thereby allowing visualization of intracellular calcium ion concentrations and movement. For fluorescence observation, endothelial cells were maintained on a microscope stage top hotplate at 37°C with Hank’s buffered salt solution (HyClone). The buffer normally contained calcium, except for experiments exploring the signaling mechanisms without extracellular calcium which it was removed from the medium. The microscope hotplate was placed onto an epi-fluorescence microscope (Nikon TE2000-U) equipped with a CCD camera (Cooke SensiCam) for real-time fluorescence imaging. The fluorescence intensity values are reported by normalizing the initial un-stimulated intensity for each individual cell.
2.4 Cell Stimulation
To mechanically stimulate calcium release at the single cell level, individual endothelial cells were probed with a comb-drive (capacitive) based force probe (FemtoTools Instruments, FT-S540) or a 30 gage syringe needle (Fisher). The comb-drive probe allows time-resolved measurement of force applied to the cell during stimulation, while the syringe needle allows improved observation of cells due to the size of the needle which is significantly smaller than the force probe. To control the location of mechanical stimulation, the probes were mounted to a custom three-axis translational stage. In our setup, two probes can be controlled simultaneously to provide mechanical stimulation to cells in the network independently. At the beginning of each experiment, the probes were brought close to the cells before stimulation and a bright field image was obtained to monitor the position of the cells. A fluorescence image was also gathered for background estimation in the image analysis. Real-time fluorescence imaging was then captured to study calcium wave propagation after mechanical stimulation. All images were captured within ~25 minutes of dye loading.
3. Results
3.1 Calcium wave propagation in HUVEC networks
HUVEC networks were organized via plasma lithography to create cell structures consisting of desired topologies (Figure 1a). Individual HUVECs could then be mechanically stimulated with a force probe or a needle (Figure 1b). Several structures, including monolayers, linear patterns, and cell junctions, were designed to explore the architecture dependence on calcium wave propagation in HUVECs (Figure 1c–e). Upon mechanical stimulation, the cells displayed an increase in calcium in the cytoplasm and the increase in calcium was observed to pass onto neighboring cells in monolayers and networks of HUVECs (Figure 1f; SI videos 1–3 supplementary information). Under the gentle probing conditions normally employed, cell membranes were mildly stretched and were not permanently damaged. The damage to the cells was monitored through several observations including no visible, permanent membrane deformation or cellular detachment after probing, and the ability of cells probed in such a manner to repeatedly release calcium when subsequently probed. Alternatively, if cells were probed more forcefully, visible damage and behavioral changes were observed in the cells. Mechanical wounding of the cells was also observed to initiate the propagation of calcium wave (data not shown) [27]. Cells probed in these manners, however, were not used for analysis of calcium wave transmission in this study.
Figure 1.
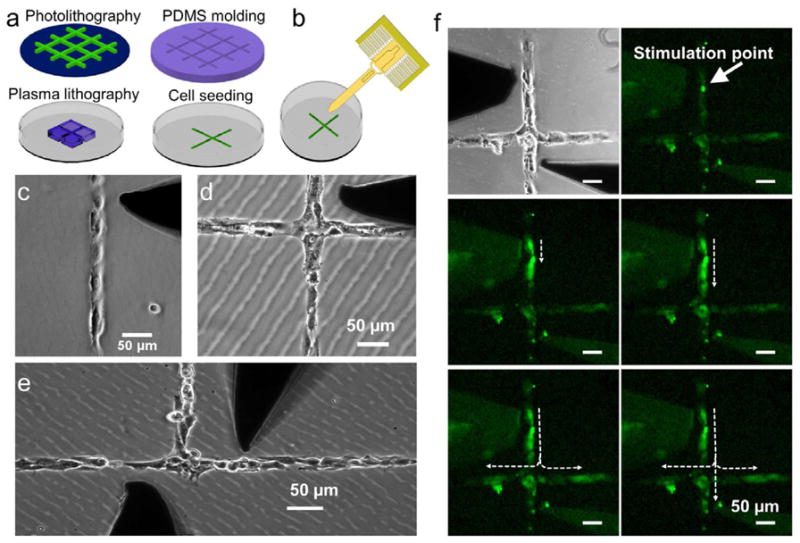
A mechanostimulation platform for studying the architecture dependence of intercellular calcium communication. (a) Plasma lithography for cell patterning. A PDMS mold is fabricated by molding photolithography patterned structures. The PDMS mold is used to physical shield selected areas of the substrate during plasma surface functionalization to create patterns for cell adhesion. (b) A comb-drive based force probe can be applied to physically stimulate the patterned cells with real-time force monitoring. (c–e) HUVEC structures patterned by plasma lithography. Dark shadows are the physical probe for single cell simulation. (f) A video time series showing a single cell (white arrow) was stimulated mechanically to create a calcium wave. The wave was then propagating along the branch and split at the junction. The duration of the experiment is 35 sec. Scale bars represent 50 μm.
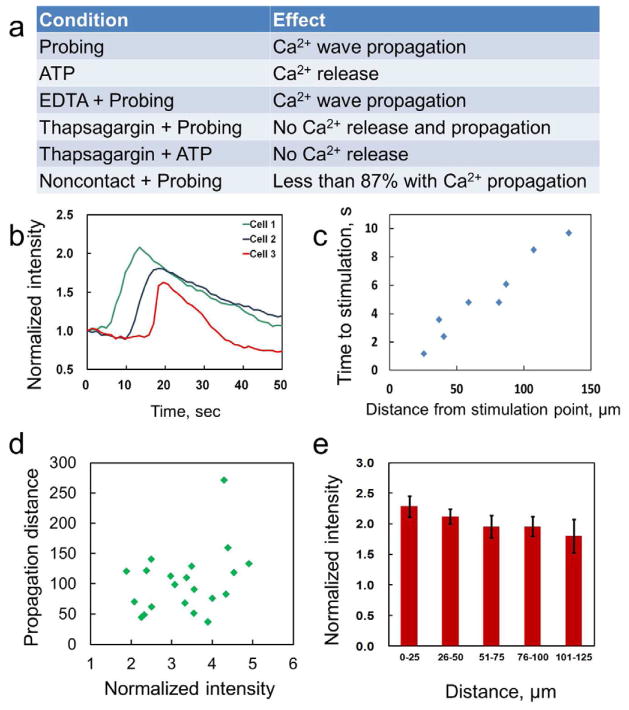
To study the nature of calcium communication in HUVEC networks, calcium release and propagation were observed under several experimental conditions (Figure 2a). Cellular calcium release can be triggered by both mechanical probing and ATP loading consistent with previous reports [28, 29]. To determine the calcium source, the endothelial cells were probed in the absence of calcium in the buffer by using a calcium free buffer (Calcium free HBSS (HyClone)) with additional calcium chelator, ethylene glycol tetraacetic acid (EGTA) (Boston Bioproducts). Under this condition, calcium wave propagation could still be observed suggesting that extracellular calcium is not a necessary condition in the mechanotransduciton of calcium wave in HUVEC. The same obsevation was also reported in monolayers of bovine aortic endothelial cells [28]. The increase in calcium is, therefore, likely contributed from intracellular stores, such as from the ER. The invovlment of the ER is further studied by blocking calcium uptake in the ER pharmacologically. With thapsigargin, an ER calcium pump inhibitor [30] that depletes intracellular calcium store, calcium signaling was not provoked either mechanical stimulation nor by addition of ATP. These observations further support that the primary source of calcium released in calcium wave propagation is from intracellular stores.
Figure 2.
Characteristics of calcium wave propagation in linear chains of HUVECs. (a) Behaviors of calcium release and propagation under different conditions. (b) Propagation of calcium wave in endothelial cells patterned in a linear cell chain. (c) A representative plot of the propagation distance and propagation time in a linear cell chain. (d) The dependence of the cell intensity on the propagation distance. (e) The average cell intensity as a function of distance from the stimulation point.
Intracellular calcium signaling can be mediated through extracellular diffusion and GJIC. Extracellular diffusion of messagers, such as ATP, could contribute to the intracellular calcium communication for several cell types [30–32]. To test the role ATP in calcium signaling of HUVECs, ATP was added to the extracellular space via a pipette. The addition of ATP gently, but not cell media, provokes calcium release in cells within the field of view of the image. This suggests calcium signaling in HUVEC can be initated by diffusible factors, such as ATP. This is consistent with previous studies that release of intercellular contents can induce calcium signaling and initiate injury response in pulmonary endothelial cells [33]. In fact, addition of extracellular ATP has also shown to be the dominant mode of comminication in networks of osteoblasts [31, 34, 35]. To examine the importance of GJIC in mechanically stimulated calcium signaling of the HUVEC network, individual cells were poked gently and the calcium levels of nearby, non-contacting cells were observed. In the majority cases (87%), nearby cells did not increase in the fluorescence intensity despite the calcium signal was provoked in the stimulated cells. These results indicate that direct cell-cell contact is required for calcium signaling in mechanically stimulated HUVEC. GJIC is concievably the primilary mechanism for mechanotransduction of HUVEC networks without injury, which is the focus of this study.
3.2 Physical characteristics of the calcium propagation
We further investigated the characteristics of calcium propagation in linear HUVEC structures. Figure 2b shows the calcium levels of three cells patterned in a linear chain. Examining the fluorescence intensity indicates that the free cytosolic calcium level of the stimulated cell undergoes a rapid increase followed by a slower decay, eventually returning to the resting level. A small duration (~1–6 seconds) is typically required to pass the signal between adjacent cells. A representative plot of the propagation distance versus arrival time behavior is shown in Figure 2c, which shows an approximately linear relationship with an average speed of ~12 μm/s. In our experiments, the calcium signal generally propagated for 4–6 cells (~120 μm). These observations are in good agreement with previous investigation in monolayers of bovine aortic endothelial cells [28]. Remarkably, the intensity of the stimulated cells shows only weak correlation with the propagation distance and the calcium wave propagates a similar distance independent of the initial signal amplitude (Figure 2d). We observed a large variation in the amplitude among cells in the networks; nevertheless, the average amplitude of the calcium wave appeared to decrease slightly along the propagation direction (Figure 2e). These observations further support that the calcium signaling in the HUVEC networks is originated by a local regenerative event, such as IP3 mediated calcium release and GJIC, in contrast to diffusive processes from the point of stimulation.
3.3 Mechanotransduction of calcium signaling in endothelial networks
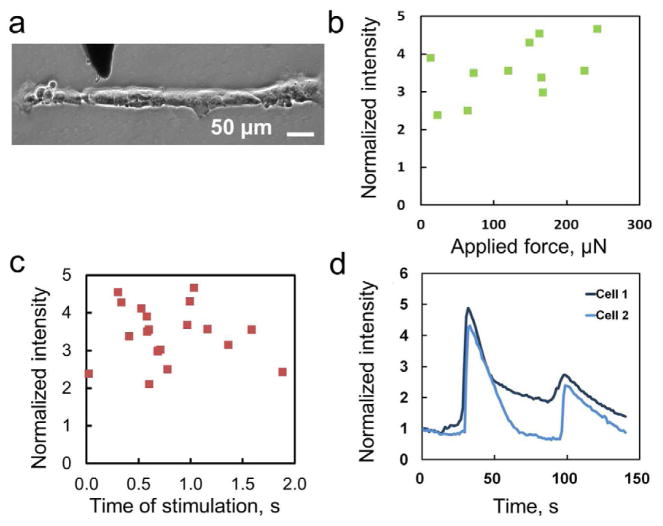
The effects of the applied mechanical stimulus itself were next investigated in order to determine the relationship between the imparted mechanical stimulus and the calcium release it provoked. Measurements of the force applied to cells during stimulation and the time that the force was applied were taken with the capacitive force sensing probe on a linear chain (Figure 3a). The force-intensity relationship is shown in Figure 3b. In the range of forces applied to stimulate cells (~2–300 μN), there is no correlation between increased force to changes in stimulation of the probed cell. Additionally, for the duration of stimulation which ranged from ~20 – 2000 milliseconds, the calcium release was also observed to be insensitive to the time of stimulation (Figure 3c). The mechanically induced calcium concentration in HUVECs is modulated to a constant level under different degrees of mechanical stimulation tested in the experiment. Furthermore, HUVECs are capable of responding to repeated mechanical stimulation. With multiple stimulations, it was observed that the wave propagation between cells typically takes a similar amount of time between multiple stimulations. The behaviors of two representative HUVECs under repeated mechanical stimulation are shown in Figure 3d. The propagation time between cells appears to be a cell-pair dependent property as well, as repeated stimulation of the same cell pair results in similar propagation time. These data suggest HUVECs are capable of responding to multiple mechanostimulations, which are in agreement with other calcium signaling behavior observed [36].
Figure 3.
Dependence of calcium wave under mechanical stimulation. (a) A bright field image of a linear cell chain. The dark shadow represents the probe. (b) The dependence of the applied force on the normalized intensity of the stimulated cell. (c) The dependence of the duration of the stimulation on the response of the cell. (d) A representative plot of two cells experienced repeated mechanical stimulations.
3.4 Architecture dependence in calcium wave propagation
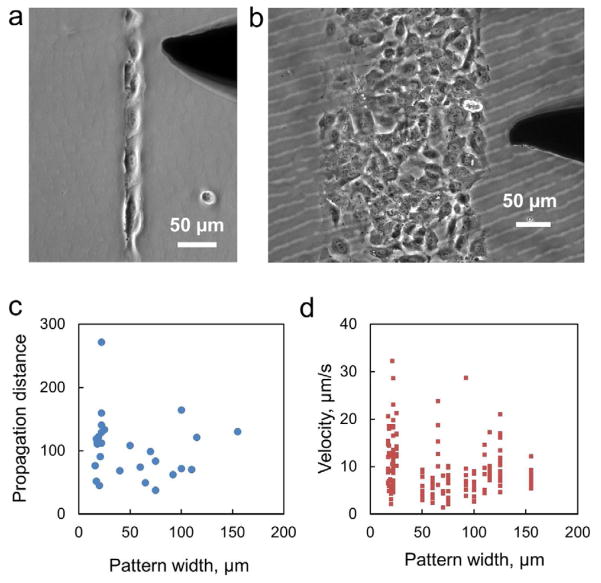
The dimensions of vascular structures span widely from single cell width in a capillary to the size of an artery. To investigate the effects of the dimension on the functional characteristics of calcium wave propagation, linear patterns of HUVECs were created with different widths. Figure 4a–b shows representative images of linear cell patterns with different widths (approximately one to ten cells). In a single cell chain, i.e., a linear pattern with single cell width, the calcium wave propagated for ~120 μm on average. When examining data for patterns with larger widths, the signal would propagate with a similar range of distance regardless of the width or number of neighboring cells. A correlation between the pattern width and propagation distance was not observed (R2 = 0.0216) and the propagation distance among the cell structures displayed a large variation (Figure 4c). The analysis of the speed of transmission in the constructs was likewise found to be insensitive to the width of the structure present. Different widths showed the same range of possible propagation speeds and there is no correlation (R2 = 0.0478) observed between the pattern width and the wave velocity (Figure 4d). Therefore, the propagation of calcium wave does not depend on the width of the structure or the number of neighboring cells in our experimental conditions.
Figure 4.
Width dependence of the cell structure on the propagation of calcium wave. (a–b) Representative images of cell structures with different widths applied in this study. (c) The wave propagation distance as a function of the pattern width of the linear structure. (d) The dependence of the pattern width on the velocity of the calcium wave.
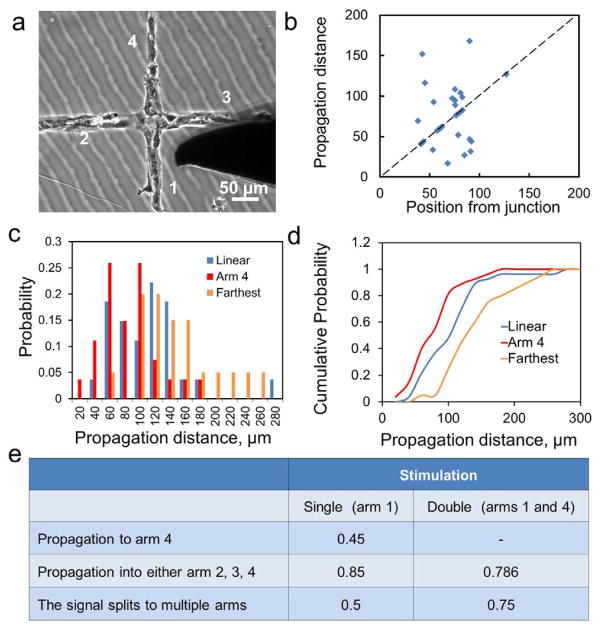
Another key feature of vascular networks is branching morphology and cell junctions (Figure 5a, see also supplementary information S3). To study the effect of a junction, individual cells in the branches (near the junction) were probed mechanically. The mechanical stimulation to a cell generally produced a calcium wave that traveled along the branch of the network and reached the junction. When the calcium wave reached the junction, the signal could split and continue to the arms present in the network downstream from the original stimulation point. The propagation distance to the opposite arm as a function of the distance of the stimulation position from the junction is shown in Figure 5b. We observed ~40% of the calcium waves stop before or at the junction (dotted line in Figure 5b). To study the effect of the cross junction on the propagation distance, the distributions of propagation distance of linear and cross patterns were compared (Figure 5c). The data generally follow Gaussian-like distributions and can be fitted by Poisson distributions with appropriate parameters. On average, the propagation distance to the opposite arm (i.e., arm 4 in Figure 5a) is ~25% lower than the propagation distance in a linear pattern. If the farthest distance in all arms (arm 2, 3, or 4) is considered, the propagation distance is ~35% higher than the value in linear chains. Figure 5d shows the cumulative probabilities for the three cases considered. These results suggest that a cross junction could reduce the chance of wave propagation if only a selected arm is considered while the overall chance of wave propagation is actually increased in a network.
Figure 5.
Effects of cell junctions on the propagation of calcium wave. (a) A bright field image of a cross junction. (b) The propagation distance as a function of the stimulation distance from the junction. The dotted line represents the location of the junction. (c) Probability distribution of the propagation distance in linear patterns (blue) and cross junctions to the opposite arm (red) or farthest location in all arms (orange). (d) Cumulative probability distribution of the three cases considered in (c). (e) Probability of wave propagation over a junction under single and double conditions.
For network structures, such as capillaries, waves could be split and merged during wave propagation. To investigate signal splitting and merging, the probabilities of wave propagation to the arms of the junction were examined (Figure 5c). With a single stimulation (i.e., only one cell in arm 1 is stimulated), 45% of the waves were able to propagate to the opposite arm (arm 4). The value increased to 85% if all arms (2, 3, and 4) are considered. Signal splitting (i.e., the signal splits to two or three arms) was observed in 50% of the experiment. We have also performed double stimulation, i.e., cells in the opposite arms (arms 1 and 4) were stimulated simultaneously (Fig. 5e). With double stimulation, signal splitting to arms 2 and 3 was observed in the majority of the experiment (~75%). If double stimulation is performed at two ends of a linear cell chain, the calcium waves meet and stop (data not shown). In other words, there is no wave crossing. These observations suggest signal merging can increase the chance of signal propagation at the junction. Furthermore, the intensities of cells at the junction and in the arms are similar between single and double stimulation (i.e., no adding effect) while the chance of wave splitting and propagation increases. Overall, wave splitting and merging could potentially provide an effective mechanism for calcium wave propagation in a cell network globally.
4. Discussion
In this study, the behavior of calcium released by mechanical stimulation and transmitted through a confined network of endothelial cells is examined in order to study the influences of several microenvironmental factors on intercellular calcium signaling. These behaviors include signal conveyance amongst a microscale network which is observed to be able to transfer a calcium signal between cells, and signal splitting and merging based upon the configuration of the network. The signaling observed is repeatable. Other characteristics of the cell calcium release and transfer behavior including distance of signal transmission, speed of signal transmission, and amount of calcium released are seen to be insensitive to the physical factors studied. These factors consisted of cell neighborhoods with differing numbers of adjacent cells or network structure which could provide for different paths for the calcium signal to propagate, probing cells with different forces and times of stimulation, and controlling the presence of multiple waves meeting at a cell.
The overall characteristic which emerges from these observations is that a robust signaling method is employed in the HUVEC network which is able to be initiated mechanically, but is not overly sensitive to many microenvironmental factors. This robust intercellular communication method can be understood by the combination of IP3 mediated calcium release and GJIC [1]. Similar to osteocytes, calcium signaling of endothelial cells can be triggered by ATP in the cellular microenvironment [37, 38]. Nevertheless, HUVECs appear to adopt GJIC as the primary mechanism in mechanotransduction of calcium signaling. GJIC appears to ensure that a calcium signal propagates a consistent range of distances and with a consistent speed, irrespective of several influences in the local microenvironment. Additionally, this signaling mechanism allows a consistent range of calcium to be released. The constant level in calcium implies that this type of transient, mechanically induced calcium release is governed by a digital mechanism which functions somewhat like a switch in that it may be activated, or not, but the result does not vary with the strength of activation above a threshold value. This behavior is conceivably due to the autoregulation of the calcium level by IP3 mediated calcium release and cytosolic calcium uptake, which maintains the amplitude of the calcium wave. In other words, the mechanical information propagating in the HUVEC networks is not encoded by the amplitude and is shown to allow robust propagation of the signal. Previous reports have suggested that cells may adopt frequency modulation of calcium in the regulation of essential cell functions [39–41]. The exact mechanisms that endothelial cells utilize to encode the mechanical signal and to regulate their responses within a mechanotransduction context should be furthered studied in the future.
The lack of signal adding, and wave crossing, observed in the cell networks also supports the robustness and consistency of the signaling which is possible. It additionally suggests a possible mechanism where multiple signals could influence signal transmission depending upon whether or not they meet at a certain location. In a scenario where two nearby signals meet and annihilate each other, this would mean that signals which would have been transferred to different locations would not proceed once annihilated. If this happened near a junction point, however, the normal signal propagation would still likely progress down the branch or branches, as the cells in those locations would not be refractory to stimulation as the cells upstream of the meeting cell. This further supports the idea that the calcium signaling is governed by internal cell control to ensure a consistent and stable means of signal propagation among endothelial cells in the vascular structures.
5. Conclusions
The signaling behavior which was observed implies that the body incorporates a calcium release mechanism which provides for fairly regular behavior in terms of the amount of calcium released as well as signal transmission distance and speed in relation to mechanical inputs. This is in line with the tight control that is usually observed for cellular calcium handling. This in turn is usually believed to be related to the many possible actions of free calcium which require tight control for proper cell function. Furthermore, our study demonstrates the technological platform combining the cell patterning and mechanostimulation technologies for systematic investigation of the architecture dependence of intercellular calcium communication. The platform is envisioned to be applicable in studying other collective cell behaviors.
Supplementary Material
Acknowledgments
This work is supported by the James S. McDonnell Foundation and NIH Director’s New Innovator Award (1DP2OD007161-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Trana QK, Ohashib K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000;48:13–22. doi: 10.1016/s0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 3.Booth C, Koch GL. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989;59:729–37. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- 4.Sun B, Lembong J, Normand V, Rogers M, Stone HA. Spatial-temporal dynamics of collective chemosensing. Proc Natl Acad Sci U S A. 2012;109:7753–8. doi: 10.1073/pnas.1121338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J, Zheng Y, Feng XJ, Du W, Liu BF. Analysis of intercellular calcium signaling using microfluidic adjustable laminar flow for localized chemical stimulation. Analytica Chimica Acta. 2012;721:104–9. doi: 10.1016/j.aca.2012.01.053. [DOI] [PubMed] [Google Scholar]
- 6.Huo B, Lu XL, Costa KD, Xu Q, Guo XE. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium. 2010;47:234–41. doi: 10.1016/j.ceca.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covian-Nares JF, Koushik SV, Puhl HL, 3rd, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci. 2010;123:1884–93. doi: 10.1242/jcs.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumacher JA, Hsieh YW, Chen S, Pirri JK, Alkema MJ, Li WH, et al. Intercellular calcium signaling in a gap junction-coupled cell network establishes asymmetric neuronal fates in C. elegans. Development. 2012;139:4191–201. doi: 10.1242/dev.083428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular Calcium Signaling Induced by Extracellular Adenosine 5′-Triphosphate and Mechanical Stimulation in Airway Epithelial-Cells. Journal of Cell Science. 1993;106:995–1004. doi: 10.1242/jcs.106.4.995. [DOI] [PubMed] [Google Scholar]
- 10.Suadicani SO, Vink MJ, Spray DC. Slow intercellular Ca2+ signaling in wild-type and Cx43-null neonatal mouse cardiac myocytes. American Journal of Physiology-Heart and Circulatory Physiology. 2000;279:H3076–H88. doi: 10.1152/ajpheart.2000.279.6.H3076. [DOI] [PubMed] [Google Scholar]
- 11.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–32. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Faucheux N, Zahm JM, Bonnet N, Legeay G, Nagel MD. Gap junction communication between cells aggregated on a cellulosecoated polystyrene: influence of connexin 43 phosphorylation. Biomaterials. 2004;25:2501–6. doi: 10.1016/j.biomaterials.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Li YP, Nagira T, Tsuchiya T. The effect of hyaluronic acid on insulin secretion in HIT-T15 cells through the enhancement of gap-junctional intercellular communications. Biomaterials. 2006;27:1437–43. doi: 10.1016/j.biomaterials.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Dokukina IV, Gracheva ME, Grachev EA, Gunton JD. Role of network connectivity in intercellular calcium signaling. Physica D. 2008;237:745–54. [Google Scholar]
- 15.Deymier PA, Eray M, Deymier MJ, Runge K, Hoying JB, Vasseur JO. Architecture-dependent signal conduction in model networks of endothelial cells. Phys Rev E. 2010:81. doi: 10.1103/PhysRevE.81.041915. [DOI] [PubMed] [Google Scholar]
- 16.Eray M, Deymier PA, Hoying JB, Runge K, Vasseur JO. Resonant filtering of compositional waves in multicellular networks. Physica D. 2008;237:2777–86. [Google Scholar]
- 17.Yang YL, Volmering J, Junkin M, Wong PK. Comparative assembly of colloidal quantum dots on surface templates patterned by plasma lithography. Soft Matter. 2011;7:10085–90. [Google Scholar]
- 18.Junkin M, Wong PK. Probing cell migration in confined enivironments by plasma lithography. Biomaterials. 2011;32:1848–55. doi: 10.1016/j.biomaterials.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junkin M, Leung SL, Yang Y, Lu Y, Volmering J, Wong PK. Plasma lithography surface patterning for creation of cell networks. J Vis Exp. 2011:52. doi: 10.3791/3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Junkin M, Leung SL, Whitman S, Gregorio CC, Wong PK. Cellular self-organization by autocatalytic alignment feedback. J Cell Sci. 2011;124:4213–20. doi: 10.1242/jcs.088898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junkin M, Watson J, Geest JPV, Wong PK. Template-guided self-assembly of colloidal quantum dots using plasma lithography. Adv Mater. 2009;21:1247–51. [Google Scholar]
- 22.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, et al. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A. 2002;99:9840–5. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard TJ, Bowman PS, Jefferson A, Tosun M, Lynch RM, Paul RJ. Na+-K+-ATPase and Ca2+ clearance proteins in smooth muscle: a functional unit. American journal of physiology. 2010;299:H548–H56. doi: 10.1152/ajpheart.00527.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang WC, Nguyen TCH, Howe RT. Laterally driven polysilicon resonant microstructures. Sensor Actuator. 1989;20:25–32. [Google Scholar]
- 25.Sun Y, Nelson BJ, Potasek DP, Enikov E. A bulk microfabricated multi-axis capacitive cellular force sensor using transverse comb drives. J Micromech Microeng. 2002;12:832–40. [Google Scholar]
- 26.Whitesides GM, Gates B, Mayers B, Xu QB. Soft lithography and nanofabrication. Abstr Pap Am Chem S. 2005;229:U936–U. [Google Scholar]
- 27.Riahi R, Yang Y, Zhang DD, Wong PK. Advances in wound-healing assays for probing collective cell migration. Journal of laboratory automation. 2012;17:59–65. doi: 10.1177/2211068211426550. [DOI] [PubMed] [Google Scholar]
- 28.Demer LL, Wortham CM, Dirksen ER, Sanderson MJ. Mechanical stimulation induces intercellular calcium signaling in bovine aortic endothelial cells. American journal of physiology. 1993;264:H2094–102. doi: 10.1152/ajpheart.1993.264.6.H2094. [DOI] [PubMed] [Google Scholar]
- 29.Pubill D, Dayanithi G, Siatka C, Andres M, Dufour MN, Guillon G, et al. ATP induces intracellular calcium increases and actin cytoskeleton disaggregation via P2x receptors. Cell Calcium. 2001;29:299–309. doi: 10.1054/ceca.2000.0194. [DOI] [PubMed] [Google Scholar]
- 30.Nejime N, Tanaka N, Yoshihara R, Kagota S, Yoshikawa N, Nakamura K, et al. Effect of P2 receptor on the intracellular calcium increase by cancer cells in human umbilical vein endothelial cells. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:429–36. doi: 10.1007/s00210-007-0259-2. [DOI] [PubMed] [Google Scholar]
- 31.Henriksen Z, Hiken JF, Steinberg TH, Jorgensen NR. The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells. Cell Calcium. 2006;39:435–44. doi: 10.1016/j.ceca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Klepeis VE, Weinger I, Kaczmarek E, Trinkaus-Randall V. P2Y receptors play a critical role in epithelial cell communication and migration. J Cell Biochem. 2004;93:1115–33. doi: 10.1002/jcb.20258. [DOI] [PubMed] [Google Scholar]
- 33.Sammak PJ, Hinman LE, Tran POT, Sjaastad MD, Machen TE. How do injured cells communicate with the surviving cell monolayer? J Cell Sci. 1997;110:465–75. doi: 10.1242/jcs.110.4.465. [DOI] [PubMed] [Google Scholar]
- 34.Godin LM, Suzuki S, Jacobs CR, Donahue HJ, Donahue SW. Mechanically induced intracellular calcium waves in osteoblasts demonstrate calcium fingerprints in bone cell mechanotransduction. Biomech Model Mechan. 2007;6:391–8. doi: 10.1007/s10237-006-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huo B, Lu XL, Hung CT, Costa KD, Xu QB, Whitesides GM, et al. Fluid flow induced calcium response in bone cell network. Cell Mol Bioeng. 2008;1:58–66. doi: 10.1007/s12195-008-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones TJ, Nauli SM. Mechanosensory calcium signaling. Advances in experimental medicine and biology. 2012;740:1001–15. doi: 10.1007/978-94-007-2888-2_46. [DOI] [PubMed] [Google Scholar]
- 37.Evans JH, Sanderson MJ. Intracellular calcium oscillations induced by ATP in airway epithelial cells. Am J Physiol. 1999;277:L30–41. doi: 10.1152/ajplung.1999.277.1.L30. [DOI] [PubMed] [Google Scholar]
- 38.Barradas AMC, Fernandes HAM, Groen N, Chai YC, Schrooten J, van de Peppel J, et al. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials. 2012;33:3205–15. doi: 10.1016/j.biomaterials.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Cai L, Dalal CK, Elowitz MB. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature. 2008;455:485–90. doi: 10.1038/nature07292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends in biochemical sciences. 2011;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg M, De Pitta M, Volman V, Berry H, Ben-Jacob E. Nonlinear gap junctions enable long-distance propagation of pulsating calcium waves in astrocyte networks. Plos Comput Biol. 2010:6. doi: 10.1371/journal.pcbi.1000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.