Abstract
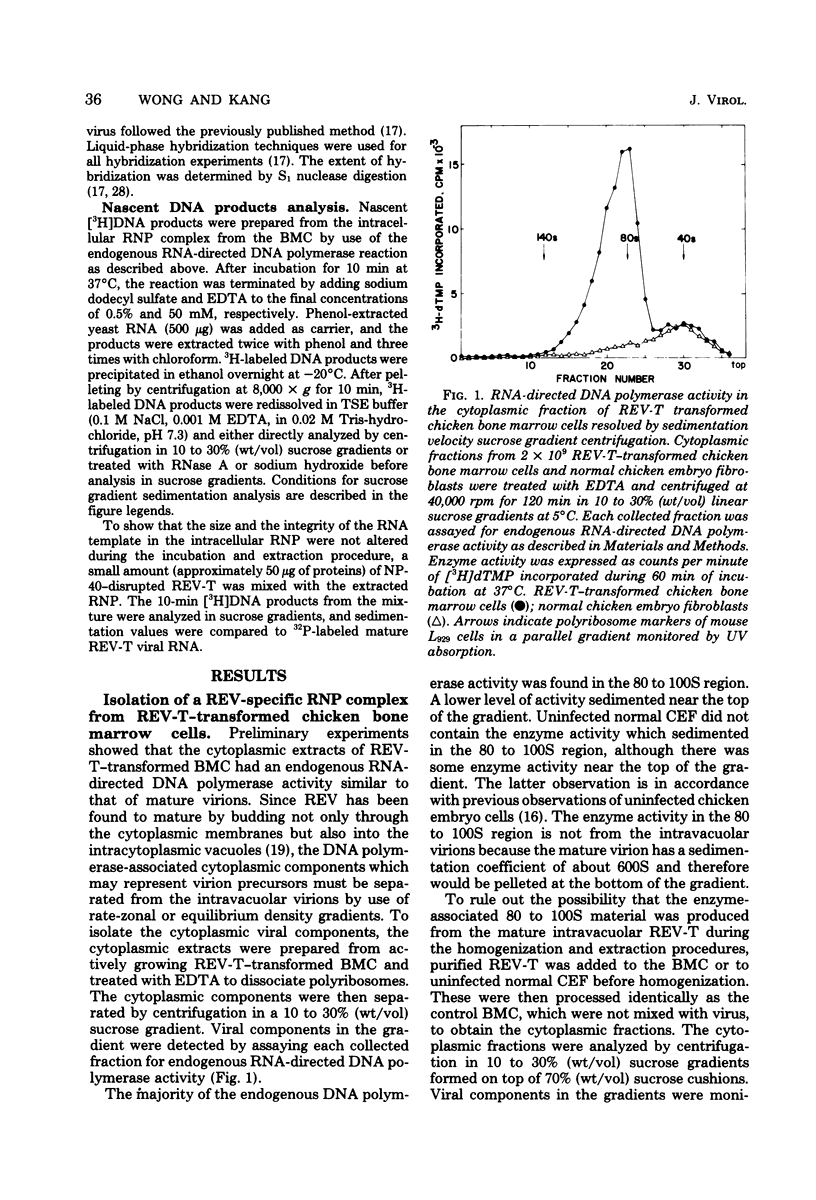
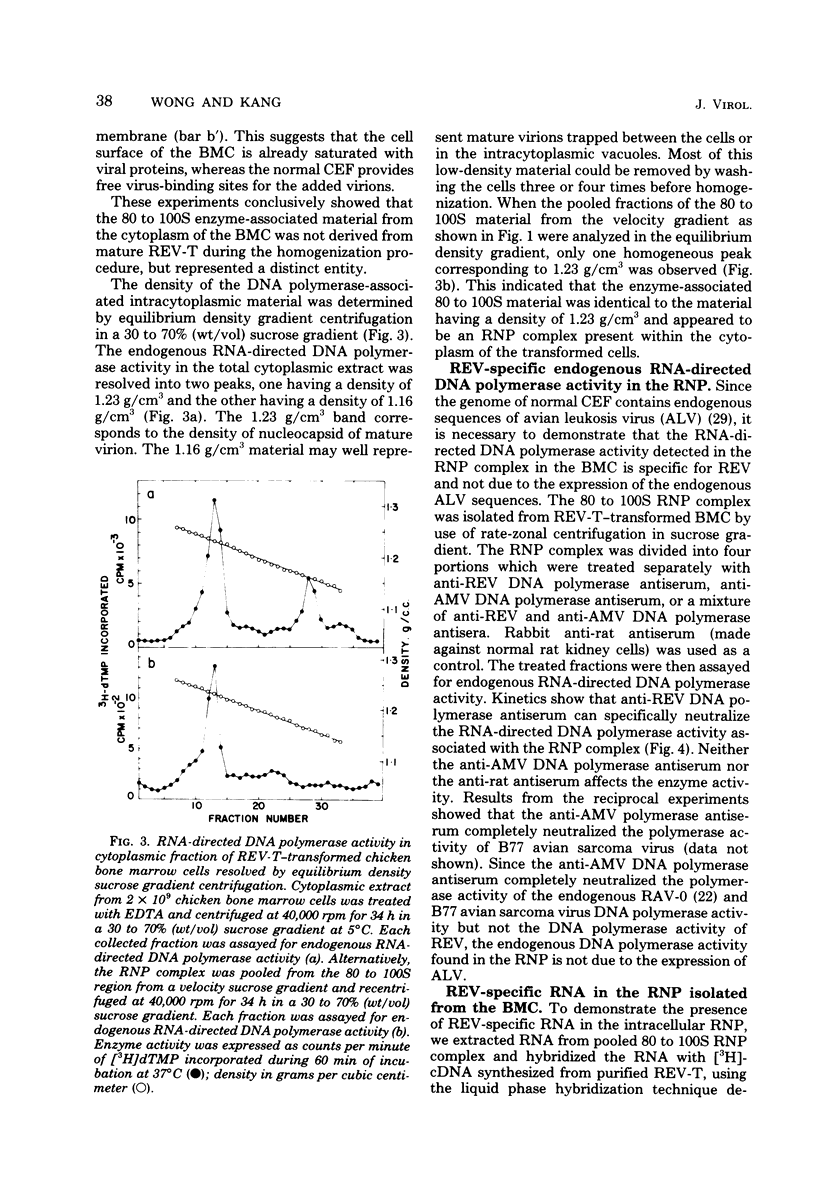
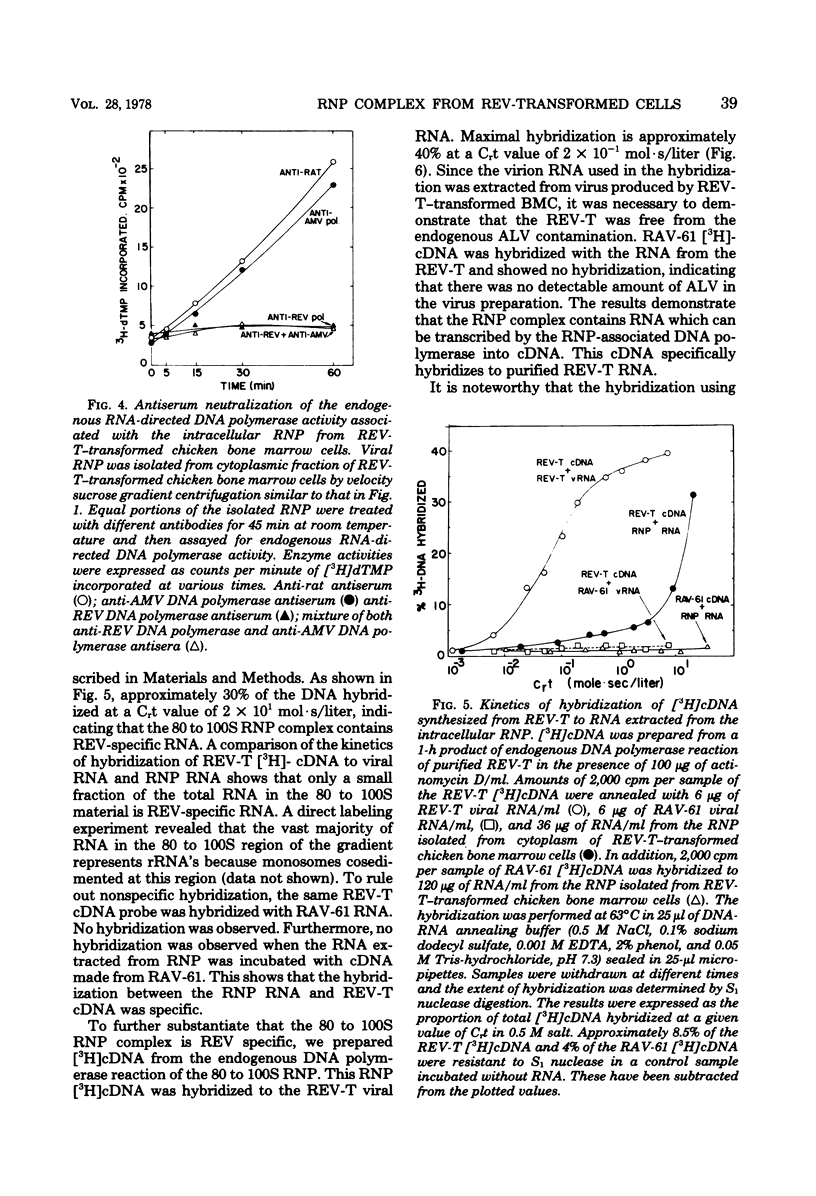
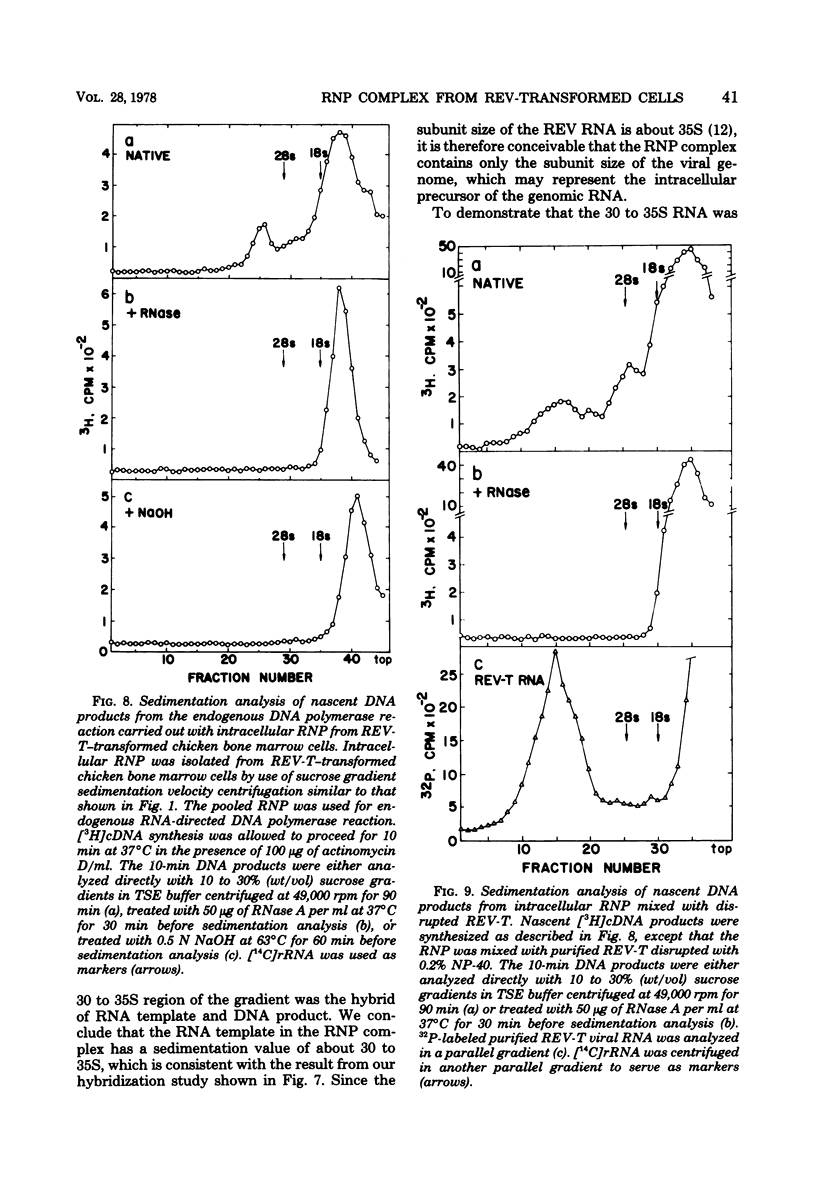
Chicken bone marrow cells transformed by reticuloendotheliosis virus (REV) produce in the cytoplasm a ribonucleoprotein (RNP) complex which has a sedimentation value of approximately 80 to 100S and a density of 1.23 g/cm3. This RNP complex is not derived from the mature virion. An endogenous RNA-directed DNA polymerase activity is associated with the RNP complex. The enzyme activity was completely neutralized by anti-REV DNA polymerase antibody but not by anti-avian myeloblastosis virus DNA polymerase antibody. The DNA product from the endogenous RNA-directed DNA polymerase reaction of the RNP complex hybridized to REV RNA but not to avian leukosis virus RNA. The RNA extracted from the RNP hybridized only to REV-specific complementary DNA synthesized from an endogenous DNA polymerase reaction of purified REV. The size of the RNA in the RNP is 30 to 35S, which represents the subunit size of the genomic RNA. No 60S mature genomic RNA was found within the RNP complex. The significance of finding the endogenous DNA polymerase activity in the viral RNP in infected cells and the maturation process of 60S virion RNA of REV are discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altaner C., Temin H. M. Carcinogenesis by RNA sarcoma viruses. XII. A quantitative study of infection of rat cells in vitro by avian sarcoma viruses. Virology. 1970 Jan;40(1):118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Franklin R. B., Maldonado R. L., Bose H. R. Isolation and characterization of reticuloendotheliosis virus transformed bone marrow cells. Intervirology. 1974;3(5-6):342–352. doi: 10.1159/000149771. [DOI] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Wade E., Rucker E., Baxter-Gabbard K. L., Levine A. S., Friis R. R. A study of the relationship of reticuloendotheliosis virus to the avian leukosis-sarcoma complex of viruses. Virology. 1973 Jun;53(2):287–299. doi: 10.1016/0042-6822(73)90206-7. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Baltimore D. Size of murine RNA tumor virus-specific nuclear RNA molecules. J Virol. 1976 Aug;19(2):331–337. doi: 10.1128/jvi.19.2.331-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y. Characterization of endogenous RNA-directed DNA polymerase activity of reticuloendotheliosis viruses. J Virol. 1975 Oct;16(4):880–886. doi: 10.1128/jvi.16.4.880-886.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Endogenous RNA-directed DNA polymerase activity in uninfected chicken embryos. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1550–1554. doi: 10.1073/pnas.69.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Reticuloendotheliosis virus nucleic acid sequences in cellular DNA. J Virol. 1974 Nov;14(5):1179–1188. doi: 10.1128/jvi.14.5.1179-1188.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Wong T. C., Holmes K. V. Comparative ultrastructural study of four reticuloendothelias viruses. J Virol. 1975 Oct;16(4):1027–1038. doi: 10.1128/jvi.16.4.1027-1038.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R., Jr Group-specific antigen shared by the members of the reticuloendotheliosis virus complex. J Virol. 1976 Mar;17(3):983–990. doi: 10.1128/jvi.17.3.983-990.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R. L., Bose H. R. Relationship of reticuloendotheliosis virus to the avian tumor viruses: nucleic acid and polypeptide composition. J Virol. 1973 May;11(5):741–747. doi: 10.1128/jvi.11.5.741-747.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Lack of serological relationship among DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken cells. J Virol. 1973 Sep;12(3):440–448. doi: 10.1128/jvi.12.3.440-448.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S., Temin H. M. Specific serological relationships among partially purified DNA polymerases of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and avian cells. J Virol. 1974 May;13(5):1020–1029. doi: 10.1128/jvi.13.5.1020-1029.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. A., Baxter-Gabbard K. L., Levine A. S. Avian reticuloendotheliosis virus (strain T): V. DNA polymerase. Virology. 1972 Jan;47(1):251–254. doi: 10.1016/0042-6822(72)90259-0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Ludford C., Nazerian K., Cox H. W. A new group of oncogenic viruses: reticuloendotheliosis, chick syncytial, duck infectious anemia, and spleen necrosis viruses. J Natl Cancer Inst. 1973 Aug;51(2):489–499. [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Shank P. R., Varmus H. E. Mouse mammary tumor virus DNA in infected rat cells: characterization of unintegrated forms. Cell. 1977 Jan;10(1):19–26. doi: 10.1016/0092-8674(77)90135-0. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Temin H. M. The cellular and molecular biology of RNA tumor viruses, especially avian leukosis-sarcoma viruses, and their relatives. Adv Cancer Res. 1974;19(0):47–104. doi: 10.1016/s0065-230x(08)60052-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Green M. Intracellular and virion 35 S RNA species of murine sarcoma and leukemia viruses. Virology. 1974 May;59(1):258–265. doi: 10.1016/0042-6822(74)90221-9. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Shank P. R. Unintegrated viral DNA is synthesized in the cytoplasm of avian sarcoma virus-transformed duck cells by viral DNA polymerase. J Virol. 1976 May;18(2):567–573. doi: 10.1128/jvi.18.2.567-573.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite M. R., Allen P. T. RNA-directed DNA polymerase activity of reticuloendotheliosis virus: characterization of the endogenous and exogenous reactions. J Virol. 1975 Oct;16(4):872–879. doi: 10.1128/jvi.16.4.872-879.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


