Prognosis in relapsed/refractory acute myeloid leukemia (AML) is poor due to drug resistance. Gemtuzumab ozogamicin (GO) is a molecularly targeted drug, which consists of anti-CD33 antibody and calicheamicin. The high expression of CD33 antigen on most AML cells and exceptionally marked cytotoxicity of calicheamicin have attracted strong interest in GO, but clinical results on GO monotherapy have been disappointing, and methods of overcoming resistance to GO have accordingly been sought.
GO requires several cellular steps to exert its effect, including expression of the CD33 antigen, internalization of the CD33–GO complex into the cell, linker digestion in lysosomes and intercalation of calicheamicin into DNA (Supplementary Figure S1).1, 2 The exertion of cytotoxicity thus requires that these target cell functions remain intact. Some of these cellular functions may be characteristically suppressed in refractory leukemic cells, or downgraded by the combined chemotherapy. We therefore speculated that the use of an agent that amplifies the cellular functions necessary for GO's action would extract its essential efficacy.
In AML or myelodysplastic syndrome, epigenetic alterations of methylation or acetylation are considered to be associated with disease cause.3, 4, 5 In low concentrations, the purine analogs decitabine (DAC) and azacitidine (AZA) act mainly to inhibit DNA methyltransferase rather than to induce cell death,3, 4 and have therefore been speculated to improve epigenetic deterioration.3, 4, 6, 7 The histone deacetylase inhibitor valproic acid (VPA) is also expected to improve epigenetic deterioration,8, 9 and has been reported to intensify the cytotoxic effect of GO.10
Accordingly, we considered that strengthening the activity of GO might be better achieved by taking advantage of epigenetic-modulating agents such as DAC, VPA and AZA, rather than by the simple combination of strongly cytotoxic drugs. Here, we report the use of pretreatment with DAC to augment the efficacy of GO.
We first evaluated the cytotoxic effect of GO, DAC, VPA and AZA as single agents in various leukemic cell lines (Supplementary Figure S2). GO induced strong apoptosis in NB4 cells derived from an acute promyelocytic leukemia patient. The other cell lines, most of them were derived from refractory patients, were less sensitive to GO, including AML/MRC-derived lines (SKK-1 cells and SKM-1 cells), complex karyotype AML cells (SKNO-1, K052 and Kasumi-3) and a myelomonocytic leukemia line (U-937) (Supplementary Figure S2). DAC, VPA or AZA revealed scant cytotoxicity in any of these cells.
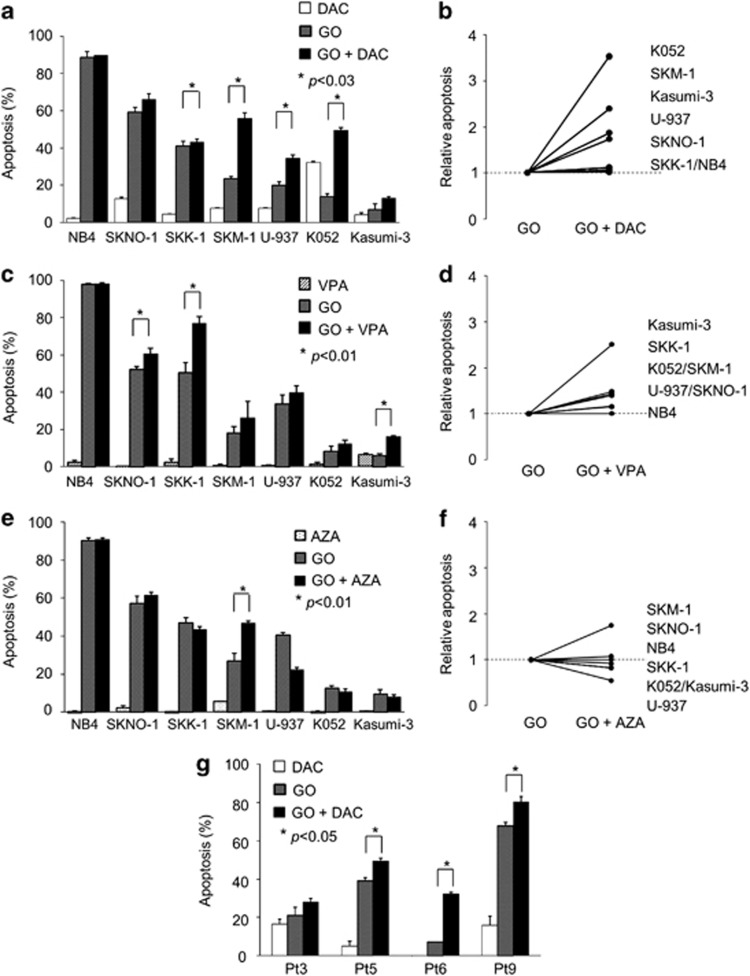
We next investigated whether the cytotoxic effect of GO was enhanced on combination with these three agents. We first combined GO and DAC. As no combination effect was seen when GO and DAC were administered simultaneously (data not shown), we evaluated a change in timing of DAC administration from 12, 24, 48 and 72 h before the administration of GO and 12 h after. A degree of combination effect was observed at every point that DAC was administered before GO, which was maximal at 48 h before GO (data not shown). Pretreatment with DAC enhanced sensitivity to GO in all GO-resistant AML cells (Figure 1a). The degree of combination effect varied among cell lines (Figure 1b); in particular, pretreatment in K052 and SKM-1 cells demonstrated a greater than twofold increase in cytotoxic effect compared with GO alone (Figure 1b). We next analyzed the combinational effects of VPA (Figures 1c and d) and AZA (Figures 1e and f) with GO. Similar to DAC, AZA produced a combination effect only when administered before GO, whereas concurrent administration of VPA and GO did show a combination effect. Among the seven cell lines analyzed, a combination effect was observed in four cell lines with DAC, three with VPA and one with AZA, with mean relative increases in apoptosis of 2.02, 1.87 and 1.27, respectively. These findings clearly show that pretreatment with DAC provided the greatest increase in effect of GO, and suggest that DAC may have some effect in overcoming resistance to GO.
Figure 1.
Combination effect of GO with DAC, VPA or AZA in CD33-positive leukemic cells. Cells were treated with 100 nℳ DAC (a, b, g), 1 mℳ VPA (c, d) or 2 μℳ AZA (e, f) for 48 h before GO (2.5 μg/ml) administration, cultured for an additional 24 h, and then apoptosis was measured. Apoptotic cells (%) increased in SKNO-1, SKK-1, SKM-1, U-937 and K052 cells when combined with DAC (a), in SKNO-1, SKK-1 and Kasumi-3 cells when combined with VPA (c) and in SKM-1 cells when combined with AZA (e). (b, d, f) Cell lines show relative apoptosis, namely apoptotic cells (%) in combination/apoptotic cells (%) in single GO administration, with any number >1 indicating a combination effect versus any number <1 meaning no combination effect. Combination of GO and DAC was superior in both the number of cell lines impacted and mean relative apoptosis. (g) Combination effect of GO with DAC was seen in four cells among 14 cells from patients with refractory AML. The degree of effect varied widely.
To confirm the enhancement in freshly-isolated cells, primary leukemic cells obtained from patients with refractory AML were treated in vitro with DAC following GO, and the degree of apoptosis was measured (Figure 1g). A combination effect of DAC and GO on apoptosis was observed in four of 14 patients (29%). The degree of combination effect differed widely. This result suggested that ∼30% of patients with poor outcomes under conventional therapy might expect some effect by this combination.
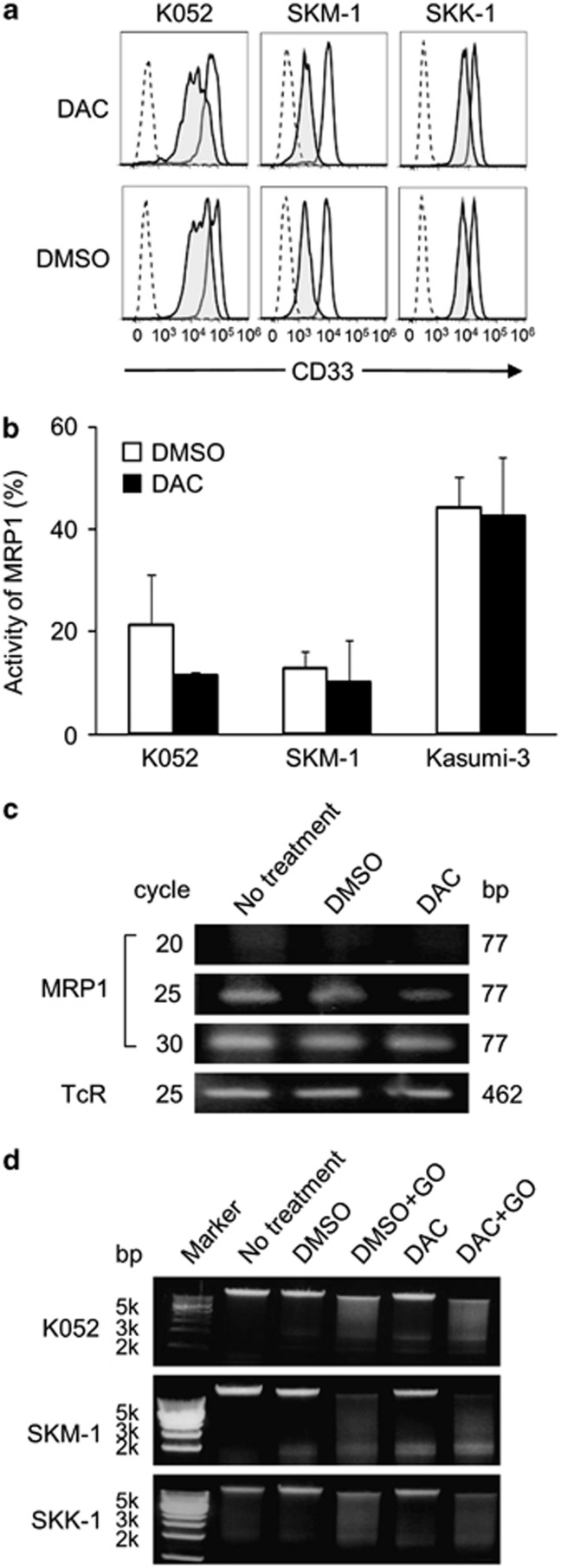
We then investigated the mechanism by which DAC overcomes resistance to GO, along with the putative steps of action of GO (Supplementary Figure S1). We first measured the expression level of CD33 antigen in each cell line, and then assessed the degree of internalization. Cell surface expression of CD 33 antigen was seen in all cell lines (Figure 2a, solid lines; only data of K052, SKM-1 and SKK-1 are indicated). No difference in CD33 expression was seen before and after pretreatment with DAC (Figure 2a, solid lines). To determine the degree of internalization, we used calicheamicin-unbound anti-CD33 antibody instead of GO, as described previously.2 Four hours after the addition of the antibody, the amount of CD33 antigen on the cell surface decreased in all cell lines (Figure 2a, shaded curves), indicating that anti-CD33 antibody was internalized by all cell lines. Pretreatment with DAC did not induce any change in the degree of internalization of anti-CD33 antibody (Figure 2a), indicating that DAC has no promotive effect on the internalization of GO.
Figure 2.
Points of action of the pretreatment effect of DAC. (a) Change in the expression of CD33 antigen was measured with a flow cytometer. CD33 antigen expression (MFI) was decreased after 4-h incubation with anti-CD33 antibody (shaded curves). Dotted curves represent isotype controls. (b, c) Activity and mRNA expression of MRP1 with or without DAC treatment. MRP1 activity was reduced by DAC treatment in K052 cells, whereas no change was seen in SKM-1 or Kasumi-3 cells (b). mRNA expression of MRP1 was reduced by DAC treatment in K052 cells (c). (d) DNA fragmentation by GO was enhanced by DAC pretreatment in K052 and SKM-1 cells. DAC pretreatment produced no increase in DNA fragmentation by GO in SKK-1 cells.
Next, given that free calicheamicin molecule number in cytoplasm is influenced by efflux pumps,11 we then investigated whether DAC had any effect on one of these efflux pumps, multidrug resistance-associated protein 1 (MRP1). For this, MRP1 activity and mRNA expression were evaluated in the presence or absence of DAC treatment in K052, SKM-1 and Kasumi-3 cells. DAC treatment decreased the activity (Figure 2b) and the mRNA expression (Figure 2c) of MRP1 in K052 cells. This inhibition of MRP1 activity appears to be one of the causes of the enhancement of the effect of GO.
The last step in the activity of GO, namely the degradation of DNA by calicheamicin, was examined using the DNA ladder method (Figure 2d). GO was added to genomic DNA extracted from K052, SKM-1 and SKK-1 cells before and after DAC treatment.10 In K052 and SKM-1 cells, the degree of fragmentation was more prominent in DAC pretreatment plus GO than in the control (DMSO plus GO), whereas no difference was seen in SKK-1 cells, indicating that DAC's enhancement of the effect of GO was partly due to the augmentation of calicheamicin's intercalation with DNA (Figure 2d).
Our present results clearly contrast with those of a previous phase III trial, the SWOG S0601 trial.12 The major difference between studies is our use of pretreatment drugs, which retain cellular functions, rather than simultaneous use of strongly cytotoxic drugs. Most previous clinical trials of GO have aimed to escalate the cytotoxic intensity of treatment by simultaneous treatment of GO with conventional chemotherapy. The induction course in the SWOG S0106 trial for de novo AML used GO (6 mg/m2) on the middle day (day 4) of a seven-day course of cytotoxic chemotherapy. Results showed no significant improvement in either complete remission rate or leukemia free survival, but rather a significant increase in deaths from early complications.12 These findings might be interpreted two ways. First, the effect of GO might not have been sufficiently exerted owing to the inhibition of one or more cellular functions necessary for GO's action (Supplementary Figure S1) in leukemic cells by the preceding chemotherapy. Second, concurrent use of GO and chemotherapy might exacerbate bone marrow suppression, leading to the increase in death from complications.
Although GO was withdrawn from the US market based on the results of the SWOG S0106 trial, the MRC15 and ALFA studies recently reported that GO was beneficial for at least one or two subsets of de novo AML patients when used in low (3 mg/m2 on day 1 in the MRC15 study) or fractionated doses (3 mg/m2 three times on days 1, 4 and 7 in the ALFA study) in combination with chemotherapy.13, 14 These recent and the present findings argue strongly against the abandonment of GO, but rather for its re-evaluation for use in relapsed/refractory cases under a more sophisticated administration protocol.
In this study, we demonstrated that the combination of low-dose DAC followed by GO was effective in a part of relapsed/refractory AML cells. Our three major findings, namely that DAC showed the best compatibility with GO among the three epigenetic-modulating agents studied, that efficacy was optimized by pretreatment with DAC, and that DAC exerted this effect via an effect on efflux pumps and intercalation, will significantly assist the development of new and innovative protocols for the use of GO in patients with relapsed/refractory AML. Confirmation of the efficacy of this combination awaits validation in a large clinical trial.
Acknowledgments
We thank Dr M Ito for his critical reading of the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Giles F, Estey E, O'Brien S. Gemtuzumab ozogamicin in the treatment of acute myeloid leukemia. Cancer. 2003;98:2095–2104. doi: 10.1002/cncr.11791. [DOI] [PubMed] [Google Scholar]
- van Der Velden VH, te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS, et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood. 2001;97:3197–3204. doi: 10.1182/blood.v97.10.3197. [DOI] [PubMed] [Google Scholar]
- Leone G, Teofili L, Voso MT, Lubbert M. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica. 2002;87:1324–1341. [PubMed] [Google Scholar]
- Lehmann U, Brakensiek K, Kreipe H. Role of epigenetic changes in hematological malignancies. Ann Hematol. 2004;83:137–152. doi: 10.1007/s00277-003-0798-7. [DOI] [PubMed] [Google Scholar]
- Graubert T, Walter MJ. Genetics of myelodysplastic syndromes: new insights. Hematology Am Soc Hematol Educ Program. 2011;2011:543–549. doi: 10.1182/asheducation-2011.1.543. [DOI] [PubMed] [Google Scholar]
- Daskalakis M, Nguyen TT, Nguyen C, Guldberg P, Kohler G, Wijermans P, et al. Demethylation of a hypermethylated P15/INK4B gene in patients with myelodysplastic syndrome by 5-Aza-2′-deoxycytidine (decitabine) treatment. Blood. 2002;100:2957–2964. doi: 10.1182/blood.V100.8.2957. [DOI] [PubMed] [Google Scholar]
- Issa JP, Kantarjian HM. Targeting DNA methylation. Clin Cancer Res. 2009;15:3938–3946. doi: 10.1158/1078-0432.CCR-08-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Faussat AM, Majdak P, Perrot JY, Chaoui D, Legrand O, et al. Valproic acid inhibits proliferation and induces apoptosis in acute myeloid leukemia cells expressing P-gp and MRP1. Leukemia. 2004;18:1246–1251. doi: 10.1038/sj.leu.2403390. [DOI] [PubMed] [Google Scholar]
- Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, et al. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med. 2005;11:71–76. doi: 10.1038/nm1160. [DOI] [PubMed] [Google Scholar]
- ten Cate B, Samplonius DF, Bijma T, de Leij LF, Helfrich W, Bremer E. The histone deacetylase inhibitor valproic acid potently augments gemtuzumab ozogamicin-induced apoptosis in acute myeloid leukemic cells. Leukemia. 2007;21:248–252. doi: 10.1038/sj.leu.2404477. [DOI] [PubMed] [Google Scholar]
- Walter RB, Raden BW, Hong TC, Flowers DA, Bernstein ID, Linenberger ML. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood. 2003;102:1466–1473. doi: 10.1182/blood-2003-02-0396. [DOI] [PubMed] [Google Scholar]
- Petersdorf S, Kopecky K, Stuart RK, Larson RA, Nevill TJ, Stenke L, et al. Preliminary results of southwest oncology group study S0106: an international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia Blood 2009114(ASH Abstract, 790). [Google Scholar]
- Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie J-N. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.