Abstract
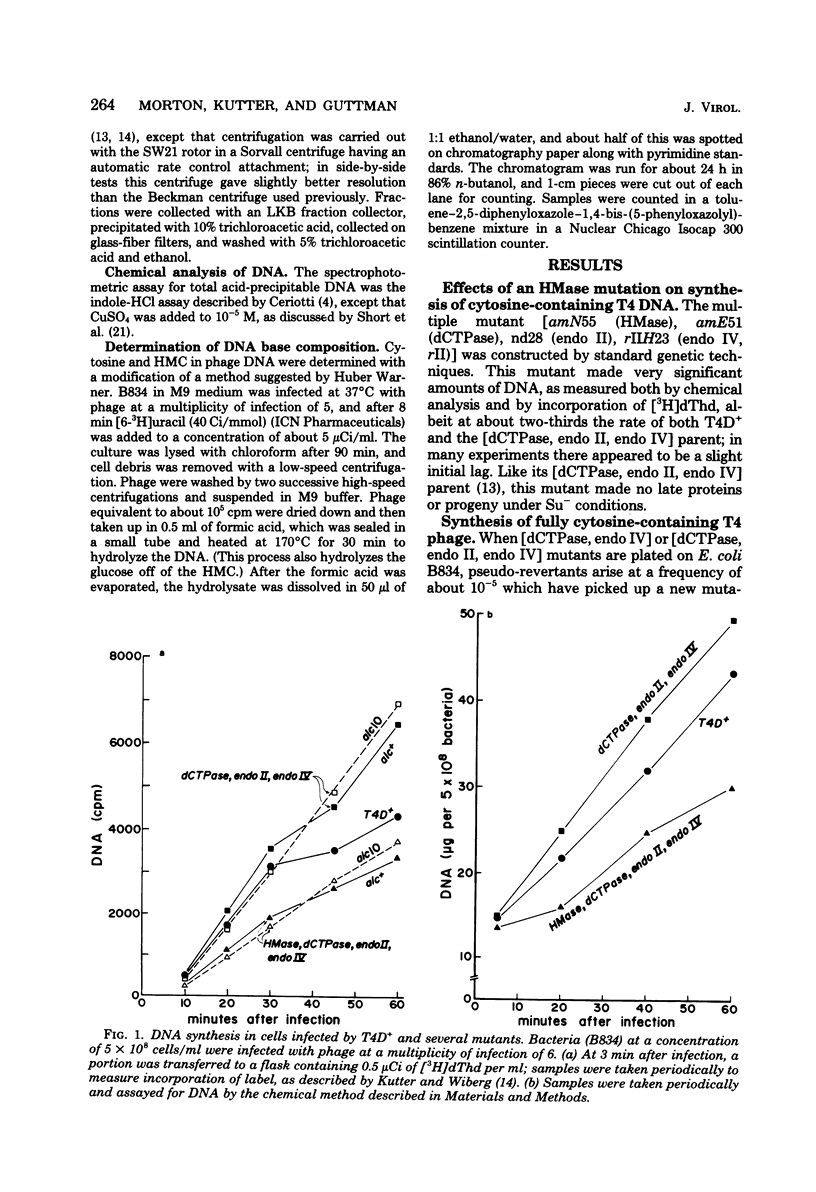
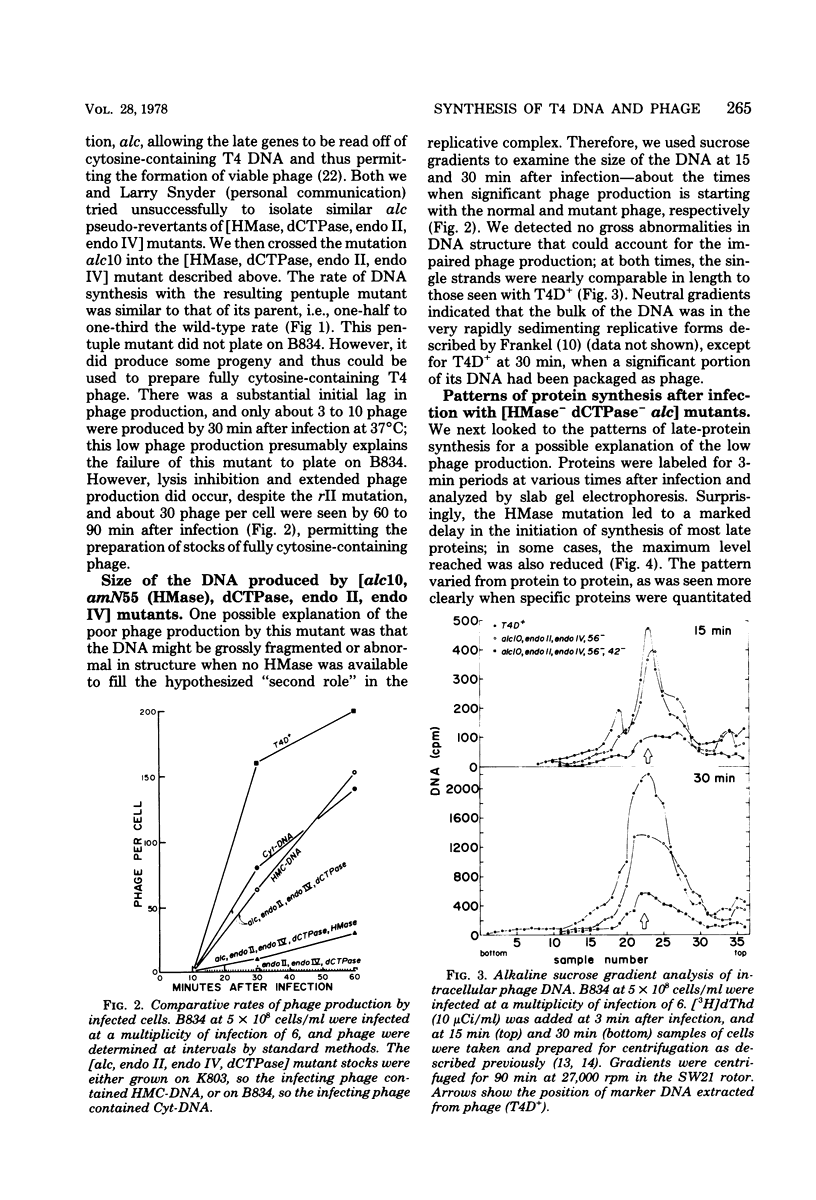
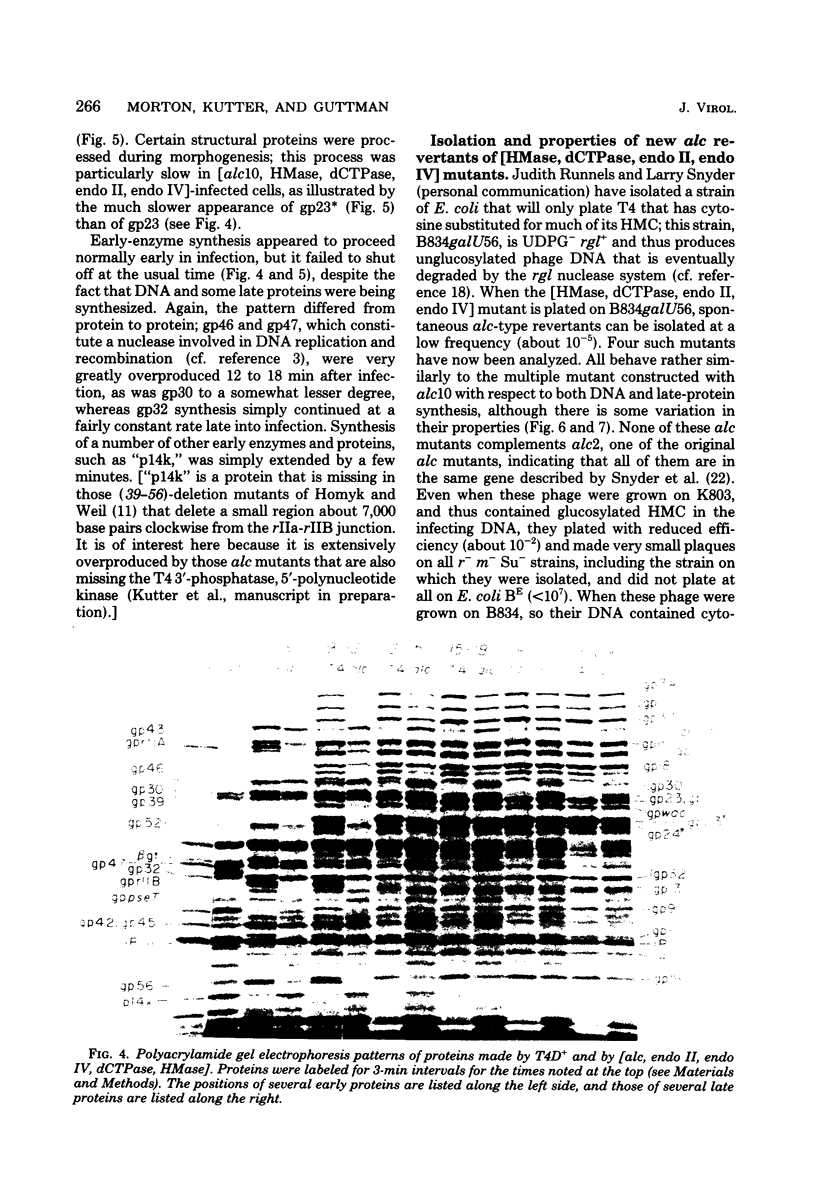
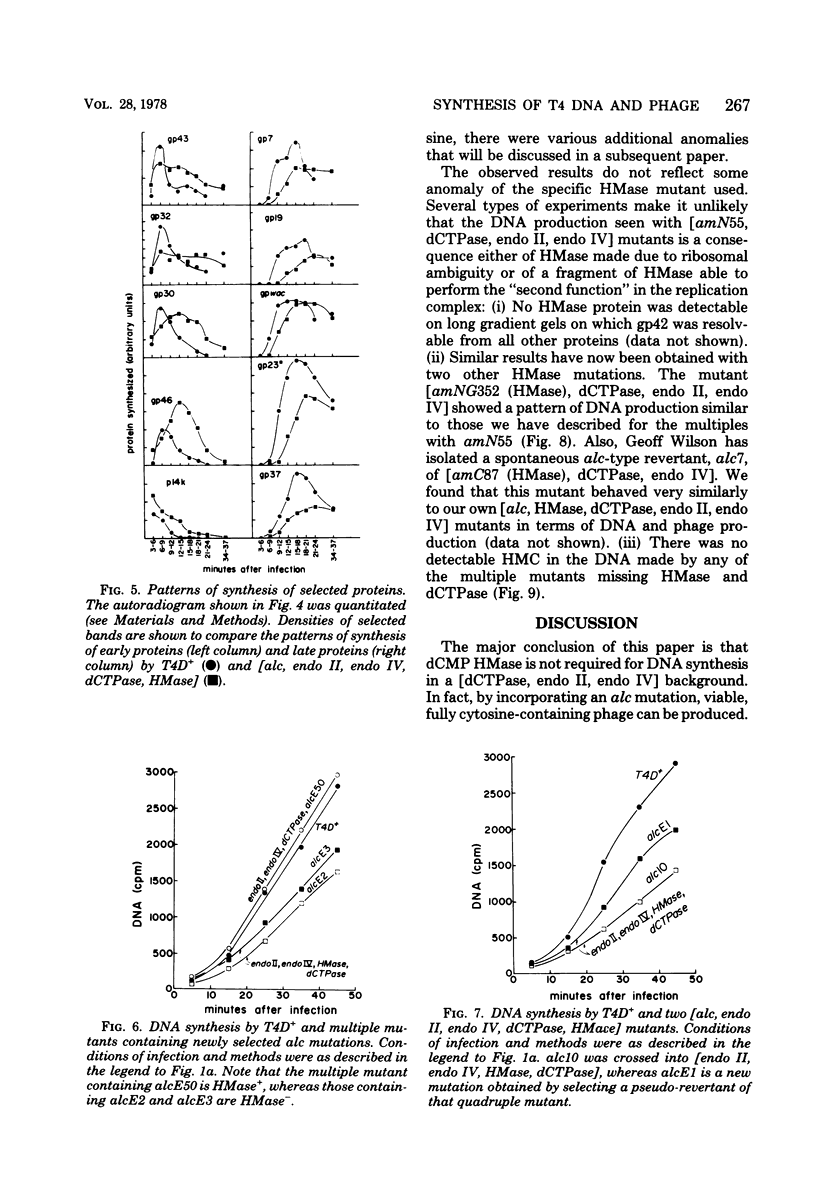
Several lines of research have suggested that the dCMP hydroxymethylase (HMase) coded by bacteriophage T4 is an essential protein in a DNA replication complex, as well as a supplier of hydroxymethyl dCMP for phage DNA synthesis. We show that a mutant [HMase, dCTPase, endonuclease II, endonuclease IV] which lacked this enzyme made cytosine-containing DNA at about two-thirds of the normal rate. When coupled with an alc mutation which permitted synthesis of late proteins, a small burst of phage was produced whose DNA contained no hydroxymethylcytosine. This pentuple mutant made both early and late proteins with abnormal kinetics, whereas the HMase+ parent showed normal kinetics. However, intracellular phage DNA showed no gross abnormalities in alkaline sucrose gradients. We conclude that HMase is not required for DNA synthesis when hydroxymethyl dCMP is not needed as a substrate; however, its absence still impairs both replication and transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry J., Alberts B. In vitro complementation as an assay for new proteins required for bacteriophage T4 DNA replication: purification of the complex specified by T4 genes 44 and 62. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2717–2721. doi: 10.1073/pnas.69.9.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolle A., Epstein R. H., Salser W., Geiduschek E. P. Transcription during bacteriophage T4 development: requirements for late messenger synthesis. J Mol Biol. 1968 Apr 28;33(2):339–362. doi: 10.1016/0022-2836(68)90193-9. [DOI] [PubMed] [Google Scholar]
- Broker T. R., Doermann A. H. Molecular and genetic recombination of bacteriophage T4. Annu Rev Genet. 1975;9:213–244. doi: 10.1146/annurev.ge.09.120175.001241. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- Chao J., Leach M., Karam J. In vivo functional interaction between DNA polymerase and dCMP-hydroxymethylase of bacteriophage T4. J Virol. 1977 Nov;24(2):557–563. doi: 10.1128/jvi.24.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. S., Greenberg G. R. Evidence for a possible direct role of dCMP hydroxymethylase in T4 phage DNA synthesis. Cold Spring Harb Symp Quant Biol. 1968;33:351–359. doi: 10.1101/sqb.1968.033.01.041. [DOI] [PubMed] [Google Scholar]
- Collinsworth W. L., Mathews C. K. Biochemistry of DNA-defective amber mutants of bacteriophage T4. IV. DNA synthesis in plasmolyzed cells. J Virol. 1974 Apr;13(4):908–915. doi: 10.1128/jvi.13.4.908-915.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicou L., Cozzarelli N. R. Bacteriophage T4-directed DNA synthesis in toluene-treated cells. J Virol. 1973 Dec;12(6):1293–1302. doi: 10.1128/jvi.12.6.1293-1302.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Homyk T., Jr, Weil J. Deletion analysis of two nonessential regions of the T4 genome. Virology. 1974 Oct;61(2):505–523. doi: 10.1016/0042-6822(74)90286-4. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Bolle A., Epstein R. H. Regulation of the synthesis of bacteriophage T4 gene 32 protein. J Mol Biol. 1974 Sep 5;88(1):89–104. doi: 10.1016/0022-2836(74)90296-4. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Kutter E., Beug A., Sluss R., Jensen L., Bradley D. The production of undegraded cytosine-containing DNA by bacteriophage T4 in the absence of dCTPase and endonucleases II and IV, and its effects on T4-directed protein synthesis. J Mol Biol. 1975 Dec 25;99(4):591–607. doi: 10.1016/s0022-2836(75)80174-4. [DOI] [PubMed] [Google Scholar]
- North T. W., Stafford M. E., Mathews C. K. Biochemistry of DNA-defective mutants of bacteriophage T4. VI. Biological functions of gene 42. J Virol. 1976 Mar;17(3):973–982. doi: 10.1128/jvi.17.3.973-982.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy G. P., Singh A., Stafford M. E., Mathews C. K. Enzyme associations in T4 phage DNA precursor synthesis. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3152–3156. doi: 10.1073/pnas.74.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Riva S., Cascino A., Geiduschek E. P. Coupling of late transcription to viral replication in bacteriophage T4 development. J Mol Biol. 1970 Nov 28;54(1):85–102. doi: 10.1016/0022-2836(70)90447-x. [DOI] [PubMed] [Google Scholar]
- Russel M., Gold L., Morrissett H., O'Farrell P. Z. Translational, autogenous regulation of gene 32 expression during bacteriophage T4 infection. J Biol Chem. 1976 Nov 25;251(22):7263–7270. [PubMed] [Google Scholar]
- Short E. V., Jr, Warner H. R., Koerner J. F. The effect of cupric ions on the indole reaction for the determination of deoxyribonucleic acid. J Biol Chem. 1968 Jun 25;243(12):3342–3344. [PubMed] [Google Scholar]
- Snyder L., Gold L., Kutter E. A gene of bacteriophage T4 whose product prevents true late transcription on cytosine-containing T4 DNA. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3098–3102. doi: 10.1073/pnas.73.9.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford M. E., Reddy G. P., Mathews C. K. Further studies on bacteriophage T4 DNA synthesis in sucrose-plasmolyzed cells. J Virol. 1977 Jul;23(1):53–60. doi: 10.1128/jvi.23.1.53-60.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Wilson G. G., Tanyashin V. I., Murray N. E. Molecular cloning of fragments of bacteriophage T4 DNA. Mol Gen Genet. 1977 Nov 14;156(2):203–214. doi: 10.1007/BF00283493. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Wovcha M. G., Tomich P. K., Chiu C. S., Greenberg G. R. Direct participation of dCMP hydroxymethylase in synthesis of bacteriophage T4 DNA. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2196–2200. doi: 10.1073/pnas.70.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Geiduschek E. P. The role of replication proteins in the regulation of bacteriophage T4 transcription. I. Gene 45 and hydroxymethyl-C-containing DNA. J Mol Biol. 1975 Aug 25;96(4):513–538. doi: 10.1016/0022-2836(75)90137-0. [DOI] [PubMed] [Google Scholar]
- Wu R., Geiduschek E. P. The role of replication proteins in the regulation of bacteriophage T4 transcription. II. Gene 45 and late transcription uncoupled from replication. J Mol Biol. 1975 Aug 25;96(4):539–562. doi: 10.1016/0022-2836(75)90138-2. [DOI] [PubMed] [Google Scholar]



