Abstract
Suppressor of cytokine signaling 2 (SOCS2) is known as a feedback inhibitor of cytokine signaling and is highly expressed in primary bone marrow (BM) cells from patients with chronic myeloid leukemia (CML). However, it has not been established whether SOCS2 is involved in CML, caused by the BCR/ABL1 fusion gene, or important for normal hematopoietic stem cell (HSC) function. In this study, we demonstrate that although Socs2 was found to be preferentially expressed in long-term HSCs, Socs2-deficient HSCs were indistinguishable from wild-type HSCs when challenged in competitive BM transplantation experiments. Furthermore, by using a retroviral BCR/ABL1-induced mouse model of CML, we demonstrate that SOCS2 is dispensable for the induction and propagation of the disease, suggesting that the SOCS2-mediated feedback regulation of the JAK/STAT pathway is deficient in BCR/ABL1-induced CML.
Keywords: CML, BCR/ABL1, SOCS2, HSC, STAT5 phosphorylation
Introduction
Chronic myeloid leukemia (CML) arises from hematopoietic stem cells (HSCs) that have acquired a reciprocal t(9;22) translocation, creating the BCR/ABL1 fusion gene. The P210 BCR/ABL1 fusion protein has been described to initiate signaling through several downstream pathways such as STAT5, PI3K, AKT, RAS and WNT.1 However, only a few downstream mediators have so far been thoroughly studied in vivo in the context of BCR/ABL1-mediated induction of CML, using mice deficient for the specific genes. Such studies have demonstrated that interleukin 3 (Il3), granulocyte macrophage colony stimulating factor (Csf2) and Cbl are dispensable for a BCR/ABL1-induced CML-like disorder.2, 3 In contrast, Alox5 was reported to be a critical regulator of leukemic stem cells in BCR/ABL1-induced CML.4, 5 Moreover, cells deficient for both Stat5a and Stat5b lack the capacity to develop disease and JAK/STAT signaling has emerged as a pivotal pathway by which BCR/ABL1 elicits its transforming effects.6, 7, 8, 9
The STAT5-induced suppressor of cytokine signaling 2 (SOCS2), a known feedback inhibitor of the JAK/STAT pathway, has previously been found upregulated in the advanced stages of CML and to become downregulated following imatinib treatment in both CML cell lines and primary cells.10, 11, 12, 13, 14, 15 In addition, SOCS2 was recently identified as one of the core genes in gene expression signatures shared between normal HSC and acute myeloid leukemia leukemic stem cells, with both signatures being associated with worse clinical outcome in acute myeloid leukemia.16 Collectively, these findings suggest that SOCS2 may be important for both normal HSC function and BCR/ABL1-induced leukemia. SOCS2 is one of eight members (CIS and SOCS1–7) of the SOCS gene family which all have both SH2 and SOCS box domains in common.17 This family of proteins is normally expressed upon stimulation with various cytokines and has mainly been characterized as feedback inhibitors of cytokine-induced signaling.18, 19, 20 However, their function is more complex, as activating properties also have been suggested for SOCS2.21, 22, 23, 24 SOCS2 is present in several tissues and involved in the regulation of several cytokines such as GH, IGF1, IL2, IL3 and EPO, with both inhibitory and enhancing properties through other SOCS proteins and receptor-JAK complexes.23, 24, 25 The most striking feature of Socs2-deficient mice is gigantism caused by an STAT5B-dependent increase of sensitivity to GH signaling.26, 27 However, the steady-state hematopoiesis of Socs2-deficient mice appears normal.26
In this study, we found that although Socs2 was preferentially expressed in the long-term HSC (LT-HSC) population, it was dispensable for normal HSC function as determined by competitive stem cell transplantation. To clarify the role of SOCS2 upregulation in CML, we evaluated BCR/ABL1-induced CML-like disease from Socs2−/− versus Socs2+/+ primitive bone marrow (BM) cells. Both groups developed indistinguishable CML-like disorders with similar survival curves, spleen weights, white blood cell counts and levels of STAT5 phosphorylation. Collectively, our data demonstrate that SOCS2 is dispensable for normal HSC function and for BCR/ABL1-mediated induction of CML.
Materials and methods
Mice and genotyping
All animal experiments were approved by the local ethics committee in Lund, Sweden. Generation of the Socs2−/− mice in C57BL/6 background has been described previously.26 The primers used for genotyping were 5′-CGAGCTCAGTCAAACAGGTAGG-3′ and 5′-GCTTTCAGATGTAGGGTGCTTTCC-3′ to detect Socs2, and 5′-GCAGACGATGGTGCAGGATATCC-3′ and 5′-GGATCGACAGATTTGATCCAGC-3′ to detect the β-galactosidase gene replacing Socs2.28 B6SJL mice and F1 crossings of C57BL/6xB6SJL were used as recipients and for competitor/support cells in the transplantations.
Analysis of Socs2 expression from global gene expression data
To study the gene expression pattern of Socs2 in various sub-populations of normal mouse hematopoietic cells, the raw CEL files for the data set GSE14833 were downloaded from gene expression omnibus (http://www.ncbi.nlm.nih.gov/geo/). Gene expression values were normalized using GCRMA.29 The mean expression value for the probe sets 1449109_at and 1418507_s_at was used to determine Socs2 expression.
Flow cytometry analysis and sorting
Fluorescence-activated cell sorting (FACS) analysis was performed with either FACS Canto II or FACS Aria (Becton Dickinson, Franklin Lakes, NJ, USA). All cells sorted for HSC analysis were freshly extracted from Socs2+/+ or Socs2−/− mice and stained with several antibodies, to identify various sub-populations. The stem cell population used for expression analysis of Socs2 was defined as lineage negative for B220, CD3, Mac1, Gr-1, Ter.119 and, in addition, IL7Rα−c-kit+Sca1+CD34−flt3−. The common myeloid progenitors were defined as c-kit+Sca-1−CD34+CD16/32mid. The HSCs used for transplantation were sorted as above but with the additional SLAM markers CD150+CD48−. Antibodies used were purchased either from Biolegend (San Diego, CA, USA) or eBioscience (San Diego, CA, USA).
For multicolor analysis of lineage distribution, we used antibodies against CD19 and CD4 (eBioscience); TER.119, CD8a, CD11b, CD45.1 and CD45.2 (Biolegend); CD45R/B220, Ly-6G/Ly-6C and Gr-1 (Becton Dickinson).
HSC transplantation
B6SJL recipient mice were lethally irradiated (900 cGy) 18 h before transplantation and Ciprofloxacin was supplemented with the drinking water following transplantation. BM cells were harvested and pooled from five age and sex matched Socs2−/− and Socs2+/+ donors, respectively. For each recipient, 200 lineage−Sca-1+c-kit+CD34−flt3−CD150+CD48− cells (LT-HSC) were sorted and injected intravenously together with 300 000 C57BL/6xB6SJL competitor cells. Repopulation was calculated as (proportion CD45.2+ cells)/(proportion CD45.2+ cells+proportion CD45.1.2+ cells). Lineage distribution was analyzed within the donor population, determined by CD45.2 expression. Approximately 3 × 106 BM cells from each primary recipient were transplanted into two lethally irradiated secondary recipients.
P210 transduction and transplantation model
The retroviral construct MSCV-BCR/ABL1-IRES-GFP was kindly provided by Prof. Connie Eaves together with a control vector expressing only GFP (MIG).30 Ecotropic viral particles were produced by transient transfection into 293T cells. The transductions and transplantation experiments were performed essentially as described by Li et al.31 In brief, matched Socs2+/+ and Socs2−/− donors were killed and c-kit+ cells were isolated using midi MACS and anti-CD117 micro beads (Miltenyi Biotech, Bergisch Gladbach, Germany). A total of 400 000 cells per recipient were prestimulated in StemSpan serum-free expansion medium (Stemcell Technologies, Grenoble, France) supplemented with 50 ng/ml murine SCF, 10 ng/ml murine IL-3, 50 ng/ml human IL-6 (Peprotech, Rocky Hill, NJ, USA), for 48 h. Transductions were performed by preloading viral particles using Retronectin (Takara bio Inc., Otsu, Japan). Cells for transplantation were harvested ∼18 h after transduction, washed, and injected in bulk via the tail vein along with 100 000 freshly isolated supporting cells from whole BM. The transduction efficiency obtained was between 10 and 15% of the transplanted cells, as determined by FACS analysis of GFP on in-vitro cultured cells, 2 days after the transduction. Recipient mice were irradiated as described above and transplanted mice received Ciprofloxacin with the drinking water throughout experiment.
Blood analysis and histopathology
Untreated mice were bled from tail veins to determine steady-state blood cell counts in Socs2−/− mice, and mice transplanted with transduced cells were bled around 15 days after transplantation. Blood cell counts were analyzed on ABX Micros 60 (Horiba ABX, Montpellier, France). Femur and spleen from diseased mice were conserved in 4% formaldehyde, paraffin embedded and sectioned before staining with hematoxylin and eosin as previously described.32
Taqman PCR and western blot
Whole BM cells were extracted from transplanted mice after euthanasia and the BM cells were viably frozen. Freshly isolated HSCs and common myeloid progenitors were sorted as described above and cryopreserved, viable and GFP-expressing cells were sorted from diseased mice (from MIG control mice all myeloid cells were sorted due to lack of GFP expression). RNA was extracted from the sorted cells using the RNeasy micro kit (Qiagen, Hilden, Germany). Gene expression was analyzed by real-time quantitative reverse-transcriptase PCR (RT-QPCR) using predesigned TaqMan probes for all known members of the Socs gene family and murine Actb or Gapdh as endogenous controls (Applied Biosystems, Foster City, CA, USA). RT-QPCR was performed on an ABI Prism 7500 analyzer (Applied Biosystems) and ddCT values were calculated as described.33 Western blot analysis to detect STAT5A/B proteins and STAT5 tyrosine phosphorylation was performed as described, using 150 000 sorted PI−GFP+Mac1+ cells.32 The membrane was stripped after P-STAT5 detection and relabeled to detect the STAT5 protein.
Intracellular flow cytometric analysis
Cells were harvested 48 h after a P210 transduction was made, as described above. Fc receptors were blocked with unlabeled CD16/CD32 antibodies (Becton Dickinson). Fixation and permeabilization of cells were performed using the Cytofix/cytoperm kit (Becton Dickinson) and the cells were stained with antibodies detecting phosphorylation of STAT5 or STAT3 (Becton Dickinson).
Results
Socs2 expression is high in HSCs
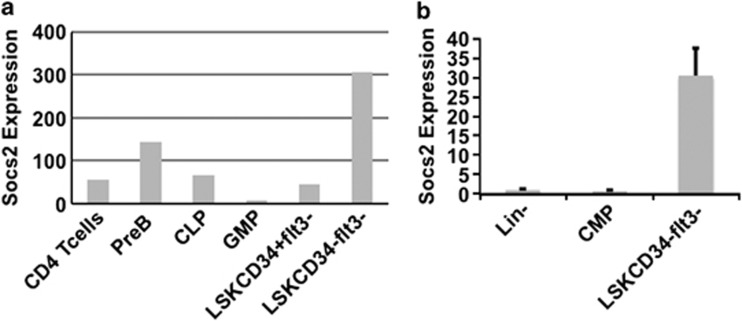
To study the role of SOCS2 in normal hematopoiesis, we first investigated the gene expression pattern of Socs2 in various hematopoietic lineages using public gene expression data. Interestingly, Socs2 was found highly expressed in normal LT-HSC relative to other hematopoietic lineages (Figure 1a). Validations using RT-QPCR showed that the Socs2 expression was about 30 times higher in HSCs compared with Lin− cells and common myeloid progenitors (Figure 1b), implying that SOCS2 may have a role in HSC function.
Figure 1.
(a) Comparison of Socs2 expression in various FACS sorted hematopoietic BM populations, analyzed by Affymetrix microarray. Expression data were acquired from the Gene Expression Omnibus data set GSE 14833 and normalized using the GCRMA method (see Materials and Methods). The expression values provided represent relative Socs2 expression values for the different cell populations. (b) Validation by RT-QPCR confirmed that Socs2 is expressed at high levels in HSCs compared with other hematopoietic cell populations. Socs2 expression is presented as fold change compared with the Socs2 expression in lin− cells, with Gapdh as endogenous control.
Socs2-deficient mice display normal HSC function
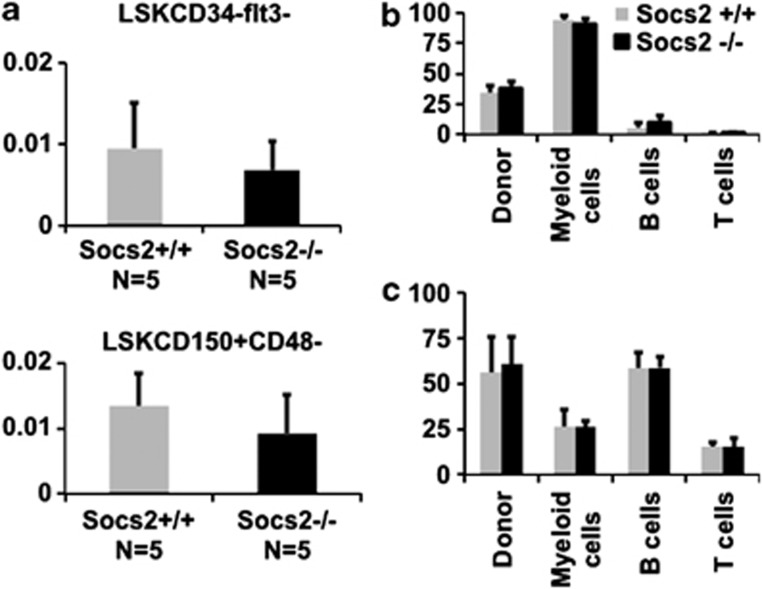
In accordance with previous findings demonstrating that mice lacking Socs2 have normal steady-state hematopoiesis,26 the Socs2-deficient mice had normal peripheral blood cell counts. Although steady-state hematopoiesis was unaffected in Socs2-deficient mice, it could still be envisioned that SOCS2, due to its role in cytokine signaling, could have an important role in regulating HSC function under conditions when the hematopoietic system is stressed. Thus, to investigate whether loss of SOCS2 affects the function of HSCs, we performed competitive BM transplantations, in which Socs2-deficient BM cells were challenged with wild-type competitor cells. At steady state, no significant difference in stem cell frequency as determined by LSKCD150+CD48− or LSKCD34−flt3− was found between Socs2−/− and Socs2+/+ BM cells (Figure 2a). Furthermore, we observed no difference in repopulation between Socs2+/+ and Socs2−/− cells in either primary or secondary recipients, suggesting that SOCS2, also under conditions of hematopoietic stress, is dispensable for both short- and long-term HSC function (Figures 2b and c; Supplementary Figure 1).
Figure 2.
(a) Frequency of HSCs (in percent) determined by FACS phenotyping of BM cells from Socs2+/+ and Socs2−/− mice. The LT-HSC population was defined by the markers LSKCD34−flt3− (top panel) or by LSK and the alternative SLAM markers CD150+CD48− (lower panel). (b, c) BM reconstitution in peripheral blood at 4 (a) and 18 weeks (b) after competitive BM transplantation. Repopulation is shown as the percentage of donor cells compared with the total amount of donor and competitor cells. Bars indicating lineage distribution show the donor cell population.
SOCS2 is dispensable for BCR/ABL1-induced CML-like disease in mice
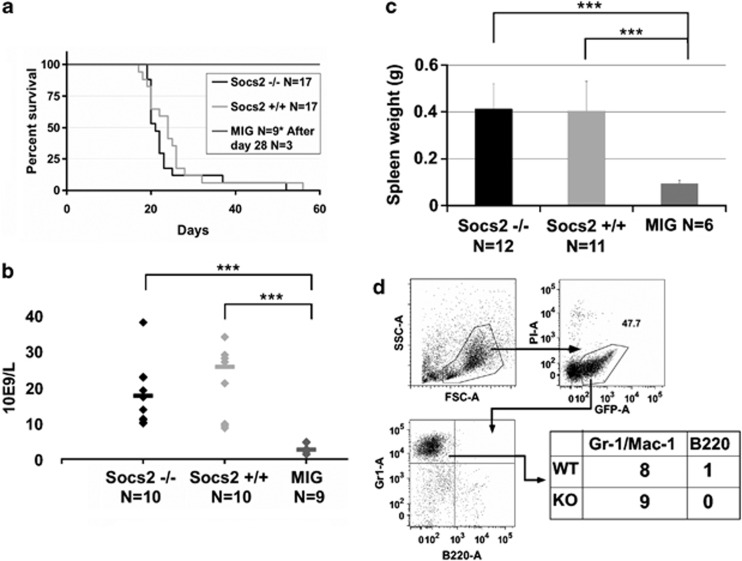
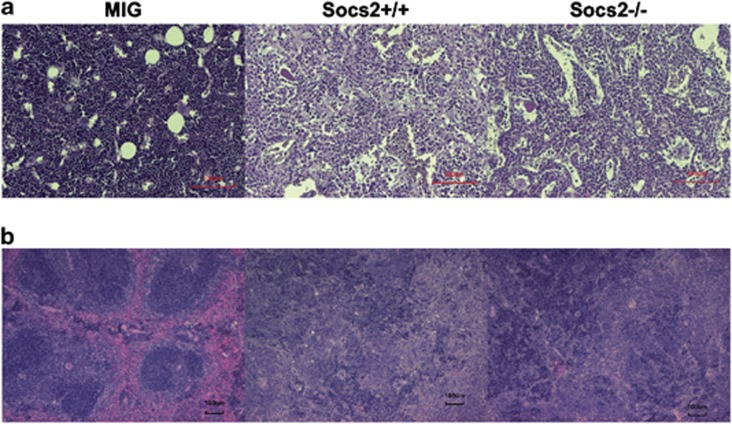
As a regulator of JAK/STAT mediated signaling, SOCS2 has been suggested to have a role in both BCR/ABL1 and JAK2 V617F-mediated myeloid leukemogenesis.11, 13, 34 The findings that loss of SOCS2 did not have any dramatic effects on steady-state hematopoiesis or HSC function, allowed us to examine the role of BCR/ABL1-mediated CML-like disease in Socs2−/− cells without confounding factors attributed to effects of Socs2 deficiency on normal HSC function. By using the BCR/ABL1 retroviral transduction and transplantation model previously described,31 c-kit+ BM cells were transduced and transplanted into lethally irradiated mice, to examine disease progression in vivo. About 3 weeks after transplantation, most mice receiving BCR/ABL1-expressing cells developed CML-like symptoms similar to previous reports,4, 31 and had to be euthanized shortly thereafter (Figure 3a). No difference in disease latency between mice receiving either Socs2+/+ or Socs2−/− cells was observed. Furthermore, when peripheral blood was analyzed 14–16 days after transplantation, both Socs2+/+ and Socs2−/− mice had elevated white blood cell and platelet counts compared with controls (Figure 3b and data not shown). When euthanized due to disease symptoms, mice from both groups suffered from severe splenomegaly (Figure 3c) and FACS analysis showed that most had an expansion of myeloid GFP+ cells in the BM (Figure 3d). To further address whether the Socs2+/+ and Socs2−/− cells caused different leukemic phenotypes, histopathologic examinations were performed on femurs and spleens from diseased mice. In the BM, BCR/ABL1 caused similar phenotypes in Socs2+/+ and Socs2−/− mice with foci of histocytes, enlarged sinusoids, and reduced erythropoiesis compared with the MIG control (Figure 4a and data not shown). All mice with disease had markedly enlarged spleens with infiltration of granulocytes in various maturation stages (Figure 4b and data not shown). In a number of enlarged spleens, we also found focal regions with blasts in both Socs2+/+ and Socs−/− transplanted recipients (data not shown). In summary, the histopathological features were clearly pathologic but recipient mice, transplanted with BCR/ABL1 transduced Socs2+/+ or Socs2−/− cells, were similar.
Figure 3.
(a) Survival of mice after transplantation of BCR/ABL1-expressing cells. Six out of nine mice transplanted with empty MIG vector were killed at day 28 to extract organs for analysis. (b) White blood cell counts in peripheral blood, measured 14–16 days after transplantation. (c) Spleen weight of leukemic mice at euthanasia. Statistical significance with a P value <0.001 is indicated by ***. (d) Representative FACS analysis of BM cells from a mouse with BCR/ABL1-induced disease. The table summarizes the dominating lineage commitment of GFP+-expressing cells in BM. Gr-1 or Mac-1 was used as myeloid markers and B220 to detect B cells.
Figure 4.
Histopathologic staining of BM and spleen after disease onset. (a) Hematoxylin eosin staining of BM sections showing increased granulopoiesis and enlarged sinusoids both in mice transplanted with Socs2+/+ and Socs2−/− cells compared with MIG. (b) Hematoxylin eosin staining of spleen with marked pathology, including severe infiltration of hematopoietic cells at various maturation stages, after Socs2+/+ and Socs2−/− transplants.
Expression analysis of all Socs family members does not suggest compensatory expression of other Socs genes
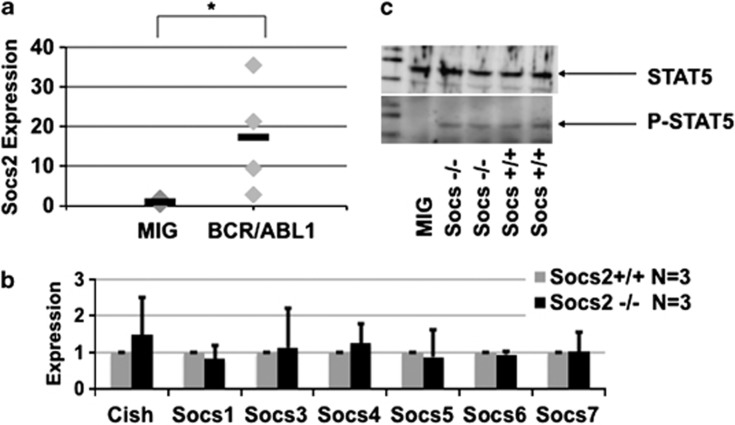
In BCRABL1-expressing cells from diseased mice, the Socs2 expression was determined to be about 10-fold increased (Figure 5a). Since different SOCS proteins can interact in both enhancing and inhibitory ways and in part have overlapping functions, it is possible that compensatory upregulation of other Socs genes might compensate for the loss of SOCS2 in BCR/ABL1 transformed cells.21, 35 To test this hypothesis, we sorted GFP+ cells from diseased mice transplanted with Socs2+/+ or Socs2−/− cells, as well as fresh common myeloid progenitors from Socs2+/+ and Socs2−/− mice, and measured the expression of individual members of the Socs gene family. In this analysis, no increase in gene expression was observed throughout the Socs gene family in Socs2-deficient cells (Figure 5b; Supplementary Figure 2), suggesting that other SOCS family members do not compensate for the lack of SOCS2. SOCS2 is known to act as a feedback inhibitor of STAT5 signaling, suggesting that a BCR/ABL1-expressing cell with functional SOCS2 should have lower STAT5 phosphorylation compared with Socs2−/− cells. However, similar levels of STAT5 phosphorylation were detected in both Socs2+/+ and Socs2−/− cells from leukemic mice, indicating that the effect of BCR/ABL1 bypasses SOCS2-mediated feedback inhibition of STAT5 (Figure 5c). Because also STAT3 has been suggested to be phosphorylated by BCR/ABL1,36 we measured STAT3 and STAT5 phosphorylation in BCR/ABL1 transduced Socs2+/+ and Socs2−/− cells by intracellular FACS. Both the Socs2+/+ and Socs2−/− cells had equally increased STAT5 phosphorylation compared with MIG control, while no increase in STAT3 phosphorylation was observed (Supplementary Figure 3).
Figure 5.
Gene expression analysis and western blot of STAT5 phosphorylation of BM cells from diseased mice. The cells were sorted before analysis, selecting GFP+Mac1+ leukemic Socs2+/+ or Socs2−/− cells and Mac1+ MIG control cells. (a) Fold difference in expression of Socs2 in leukemic Socs2+/+ cells relative to empty MIG control, with Actb as endogenous control. Transduced cells show increased expression of Socs2 in BCR/ABL1-expressing cells. Statistical significance with a P value <0.05 is indicated by *. (b) Expression of individual members of the Socs gene family in Socs2+/+ versus Socs2−/− cells. The expression of each Socs family member in Socs2−/− cells is presented as fold change relative to Socs expression in the Socs2+/+ cells using Actb as endogenous control. (c) Western blot analysis of total STAT5 (upper panel) and phosphorylated STAT5 (lower panel) in sorted GFP+ bone marrow cells from one control MIG mouse and two Socs2−/− and Socs2+/+ mice, respectively.
Discussion
As a modulator of the JAK/STAT signaling pathway, a pathway with a demonstrated role in CML, the strong upregulation of SOCS2 in CML has raised the question whether SOCS2 is involved in BCR/ABL1-induced transformation or whether it is part of an inadequate feedback loop.6, 11, 12, 13, 14, 15 SOCS2 has been suggested to have a role as feedback inhibitor in certain types of cancers, but its role may be more complex, as it is variably reported as having both enhancing and inhibitory functions in normal cytokine-induced signaling.21, 22, 23, 24, 37 As we observed a strong upregulation of Socs2 in mouse LT-HSC, in accordance with recent findings in human candidate HSC,16 we first explored the role of SOCS2 in normal HSCs using competitive BM transplantation experiments. Although it could be speculated that high expression of Socs2 in LT-HSCs would serve as a brake, keeping this cell population quiescent due to insensitivity to stimulating growth factors, Socs2−/− HSCs showed equal repopulation properties both short-term and long-term, as wild-type HSCs. Although we cannot fully exclude that Socs2 deficiency may have a subtle effect on HSCs, our findings strongly suggest that Socs2 is not critical for normal HSC function.
To evaluate the potential role of SOCS2 in CML, we used the previously established retroviral BCR/ABL1 transduction and transplantation model,31 comparing disease manifestation derived from Socs2+/+ versus Socs2−/− cells. Mice transplanted with either donor types showed similar disease onset, displayed similar symptoms such as elevated white blood cell counts, splenomegaly and expansion of myeloid cells, and had similar survival curves. These findings demonstrate that SOCS2 is not crucial for disease initiation and propagation in this model of CML. However, the rapid disease course and the short survival times clearly pose a potential problem as weak phenotypes may be overlooked. In an attempt to investigate if a disease model with longer disease latency could be established, we also transduced and transplanted fewer cells. However, transplantation of fewer MIG-BCR/ABL1 transduced cells resulted in similar disease latency, but with a reduced penetrance with only a few recipients developing a CML-like disease (data not shown). Thus, using this well-established model of murine CML-like disease, it is difficult to entirely exclude subtle effects of SOCS2 in the initiation and progression of CML.
It is also well possible that functional redundancy could exist between different members of the Socs gene family, which could explain the lack of phenotype in this model. However, examining the expression levels of other Socs family members in Socs2-deficient cells, we did not find any compensatory upregulation of the other Socs family members. This indicates that functional redundancy involving other Socs family members is an unlikely cause of the unaltered disease course in mice receiving BCR/ABL1-transduced Socs2−/− BM cells. However, both CISH and SOCS3 have previously been reported to be upregulated in CML patients.13 Thus, future studies combining silencing of additional Socs members will be needed to ultimately clarify this issue.
BCR/ABL1 is well known to activate several downstream signaling mediators such as STAT5.6, 38 The transcription of Socs2 is normally induced by STAT5 activation, and the SOCS2 protein typically acts as a feedback inhibitor upstream of STAT5 by targeting the interaction between JAK and cell surface receptors such as the GH receptor.10, 27, 34, 39 In this study, we demonstrate that SOCS2 is dispensable for BCR/ABL1-mediated induction of CML, despite Socs2 being highly upregulated by BCR/ABL1. Our finding that cells expressing BCR/ABL1 have equal STAT5 phosphorylation independently of Socs2 deficiency supports a hypothesis that SOCS2 fails to act as a feedback inhibitor of the JAK2/STAT5 pathway in the context of BCR/ABL1 signaling. Because BCR/ABL1 has been shown to activate both JAK2 and STAT5 directly,8, 9, 36 it seems reasonable that the upstream inhibition of this pathway provided by SOCS2 would have minimal effects on BCR/ABL1-induced signaling, which is consistent with our results.
In conclusion, we have demonstrated that SOCS2 is dispensable for both normal HSC function and CML-like disease. These findings support the hypothesis that Socs2 upregulation by BCR/ABL1 is caused by an inadequate or non-functional feedback loop induced by BCR/ABL1.
Acknowledgments
We wish to thank Dr. Jonas Larsson and Dr. Johan Richter for valuable discussions and Dr. Tor Olofsson for support and advice on cell sorting. Dr. Kavitha Siva is thanked for sharing her experiences of the CML mouse model. We are also grateful for the technical assistance from Carin Lassen. This work was supported by the Swedish Cancer Society, the Swedish Children′s Cancer Foundation, the Inga-Britt and Arne Lundberg Foundation, the Gunnar Nilsson Cancer Foundation, the Medical Faculty of Lund University, and the Swedish Research Council (personal project grant to TF; Hemato-Linné and BioCare strategic program grants), a Program Grant (461219), Fellowship and Independent Research Institutes Infrastructure Support Scheme Grant from the Australian National Health and Medical Research Council, a Victorian State Government Operational Infrastructure Support grant (to WSA) and the NIHR Biomedical Research Center (UK) funding scheme.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- Li S, Gillessen S, Tomasson MH, Dranoff G, Gilliland DG, Van Etten RA. Interleukin 3 and granulocyte-macrophage colony-stimulating factor are not required for induction of chronic myeloid leukemia-like myeloproliferative disease in mice by BCR/ABL. Blood. 2001;97:1442–1450. doi: 10.1182/blood.v97.5.1442. [DOI] [PubMed] [Google Scholar]
- Dinulescu DM, Wood LJ, Shen L, Loriaux M, Corless CL, Gross AW, et al. c-CBL is not required for leukemia induction by Bcr-Abl in mice. Oncogene. 2003;22:8852–8860. doi: 10.1038/sj.onc.1206892. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009;41:783–792. doi: 10.1038/ng.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Li D, Li S. The Alox5 gene is a novel therapeutic target in cancer stem cells of chronic myeloid leukemia. Cell Cycle. 2009;8:3488–3492. doi: 10.4161/cc.8.21.9852. [DOI] [PubMed] [Google Scholar]
- Hoelbl A, Kovacic B, Kerenyi MA, Simma O, Warsch W, Cui Y, et al. Clarifying the role of Stat5 in lymphoid development and Abelson-induced transformation. Blood. 2006;107:4898–4906. doi: 10.1182/blood-2005-09-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, et al. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421–3429. doi: 10.1182/blood-2009-11-255232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Wang Y, Liu J, Sun T, Wilson MB, Smithgall TE, et al. Involvement of Jak2 tyrosine phosphorylation in Bcr-Abl transformation. Oncogene. 2001;20:6188–6195. doi: 10.1038/sj.onc.1204834. [DOI] [PubMed] [Google Scholar]
- Samanta A, Perazzona B, Chakraborty S, Sun X, Modi H, Bhatia R, et al. Janus kinase 2 regulates Bcr-Abl signaling in chronic myeloid leukemia. Leukemia. 2011;25:463–472. doi: 10.1038/leu.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey HW, McLachlan MJ, Wilkins RJ, Hilton DJ, Adams TE. STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver. Mol Cell Endocrinol. 1999;158:111–116. doi: 10.1016/s0303-7207(99)00175-6. [DOI] [PubMed] [Google Scholar]
- Schultheis B, Carapeti-Marootian M, Hochhaus A, Weisser A, Goldman JM, Melo JV. Overexpression of SOCS-2 in advanced stages of chronic myeloid leukemia: possible inadequacy of a negative feedback mechanism. Blood. 2002;99:1766–1775. doi: 10.1182/blood.v99.5.1766. [DOI] [PubMed] [Google Scholar]
- Zheng C, Li L, Haak M, Brors B, Frank O, Giehl M, et al. Gene expression profiling of CD34+ cells identifies a molecular signature of chronic myeloid leukemia blast crisis. Leukemia. 2006;20:1028–1034. doi: 10.1038/sj.leu.2404227. [DOI] [PubMed] [Google Scholar]
- Håkansson P, Nilsson B, Andersson A, Lassen C, Gullberg U, Fioretos T. Gene expression analysis of BCR/ABL1-dependent transcriptional response reveals enrichment for genes involved in negative feedback regulation. Genes Chromosomes Cancer. 2008;47:267–275. doi: 10.1002/gcc.20528. [DOI] [PubMed] [Google Scholar]
- Järås M, Johnels P, Ågerstam H, Lassen C, Rissler M, Edén P, et al. Expression of P190 and P210 BCR/ABL1 in normal human CD34+ cells induces similar gene expression profiles and results in a STAT5-dependent expansion of the erythroid lineage. Exp Hematol. 2009;37:367–375. doi: 10.1016/j.exphem.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Radich JP, Dai H, Mao M, Oehler V, Schelter J, Druker B, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc Natl Acad Sci USA. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- Hilton DJ, Richardson RT, Alexander WS, Viney EM, Willson TA, Sprigg NS, et al. Twenty proteins containing a C-terminal SOCS box form five structural classes. Proc Natl Acad Sci USA. 1998;95:114–119. doi: 10.1073/pnas.95.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Tannahill GM, Elliott J, Barry AC, Hibbert L, Cacalano NA, Johnston JA. SOCS2 can enhance interleukin-2 (IL-2) and IL-3 signaling by accelerating SOCS3 degradation. Mol Cell Biol. 2005;25:9115–9126. doi: 10.1128/MCB.25.20.9115-9126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiu H, Greenhalgh CJ, Thaus A, Hilton DJ, Nicola NA, Alexander WS, et al. Regulation of multiple cytokine signalling pathways by SOCS3 is independent of SOCS2. Growth Factors. 2009;27:384–393. doi: 10.3109/08977190903210954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico-Bautista E, Flores-Morales A, Fernández-Pérez L. Suppressor of cytokine signaling (SOCS) 2, a protein with multiple functions. Cytokine Growth Factor Rev. 2006;17:431–439. doi: 10.1016/j.cytogfr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Piessevaux J, Lavens D, Montoye T, Wauman J, Catteeuw D, Vandekerckhove J, et al. Functional cross-modulation between SOCS proteins can stimulate cytokine signaling. J Biol Chem. 2006;281:32953–32966. doi: 10.1074/jbc.M600776200. [DOI] [PubMed] [Google Scholar]
- Piessevaux J, Lavens D, Peelman F, Tavernier J. The many faces of the SOCS box. Cytokine Growth Factor Rev. 2008;19:371–381. doi: 10.1016/j.cytogfr.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Greenhalgh CJ, Viney E, Willson TA, Starr R, Nicola NA, et al. Gigantism in mice lacking suppressor of cytokine signalling-2. Nature. 2000;405:1069–1073. doi: 10.1038/35016611. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Bertolino P, Asa SL, Metcalf D, Corbin JE, Adams TE, et al. Growth enhancement in suppressor of cytokine signaling 2 (SOCS-2)-deficient mice is dependent on signal transducer and activator of transcription 5b (STAT5b) Mol Endocrinol. 2002;16:1394–1406. doi: 10.1210/mend.16.6.0845. [DOI] [PubMed] [Google Scholar]
- Michaylira CZ, Simmons JG, Ramocki NM, Scull BP, McNaughton KK, Fuller CR, et al. Suppressor of cytokine signaling-2 limits intestinal growth and enterotrophic actions of IGF-I in vivo. Am J Physiol Gastrointest Liver Physiol. 2006;291:G472–G481. doi: 10.1152/ajpgi.00218.2005. [DOI] [PubMed] [Google Scholar]
- Wu Z, Irizarry RA, Gentleman R, Martinez-Murillo F, Spencer F. A model-based background adjustment for oligonucleotide expression arrays. J Am Stat Assoc. 2004;99:909–917. [Google Scholar]
- Hawley RG, Lieu FH, Fong AZ, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Therapy. 1994;1:136–138. [PubMed] [Google Scholar]
- Li S, Ilaria RLJ, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agerstam H, Järås M, Andersson A, Johnels P, Hansen N, Lassen C, et al. Modeling the human 8p11-myeloproliferative syndrome in immunodeficient mice. Blood. 2010;116:2103–2111. doi: 10.1182/blood-2009-05-217182. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Quentmeier H, Geffers R, Jost E, Macleod RA, Nagel S, Rohrs S, et al. SOCS2: inhibitor of JAK2V617F-mediated signal transduction. Leukemia. 2008;22:2169–2175. doi: 10.1038/leu.2008.226. [DOI] [PubMed] [Google Scholar]
- Jegalian AG, Wu H. Differential roles of SOCS family members in EpoR signal transduction. J Interferon Cytokine Res. 2002;22:853–860. doi: 10.1089/107999002760274863. [DOI] [PubMed] [Google Scholar]
- Ilaria RL, Van Etten RA. P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- Greenhalgh CJ, Metcalf D, Thaus AL, Corbin JE, Uren R, Morgan PO, et al. Biological evidence that SOCS-2 can act either as an enhancer or suppressor of growth hormone signaling. J Biol Chem. 2002;277:40181–40184. doi: 10.1074/jbc.C200450200. [DOI] [PubMed] [Google Scholar]
- Shuai K, Halpern J, ten Hoeve J, Rao X, Sawyers CL. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–254. [PubMed] [Google Scholar]
- Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.