Abstract
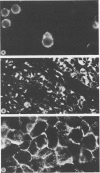
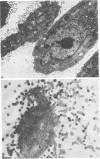
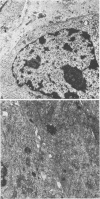
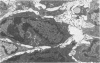
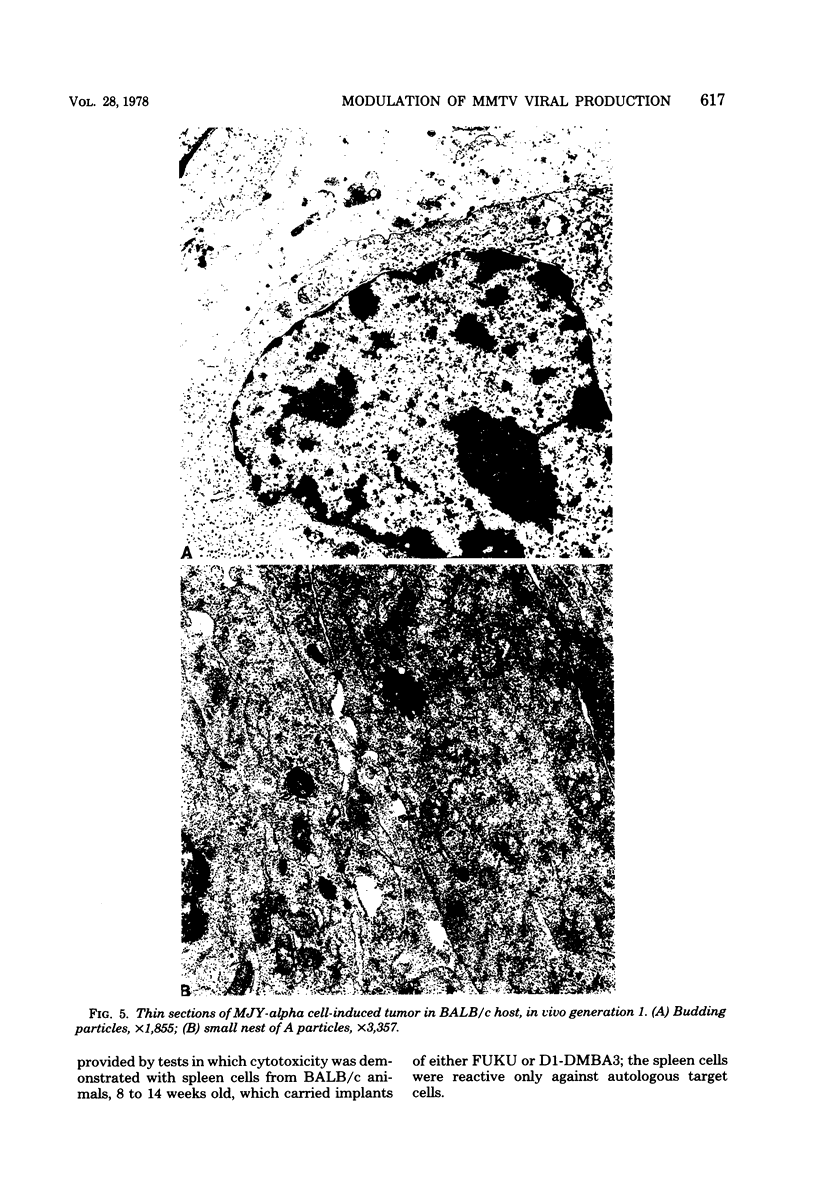
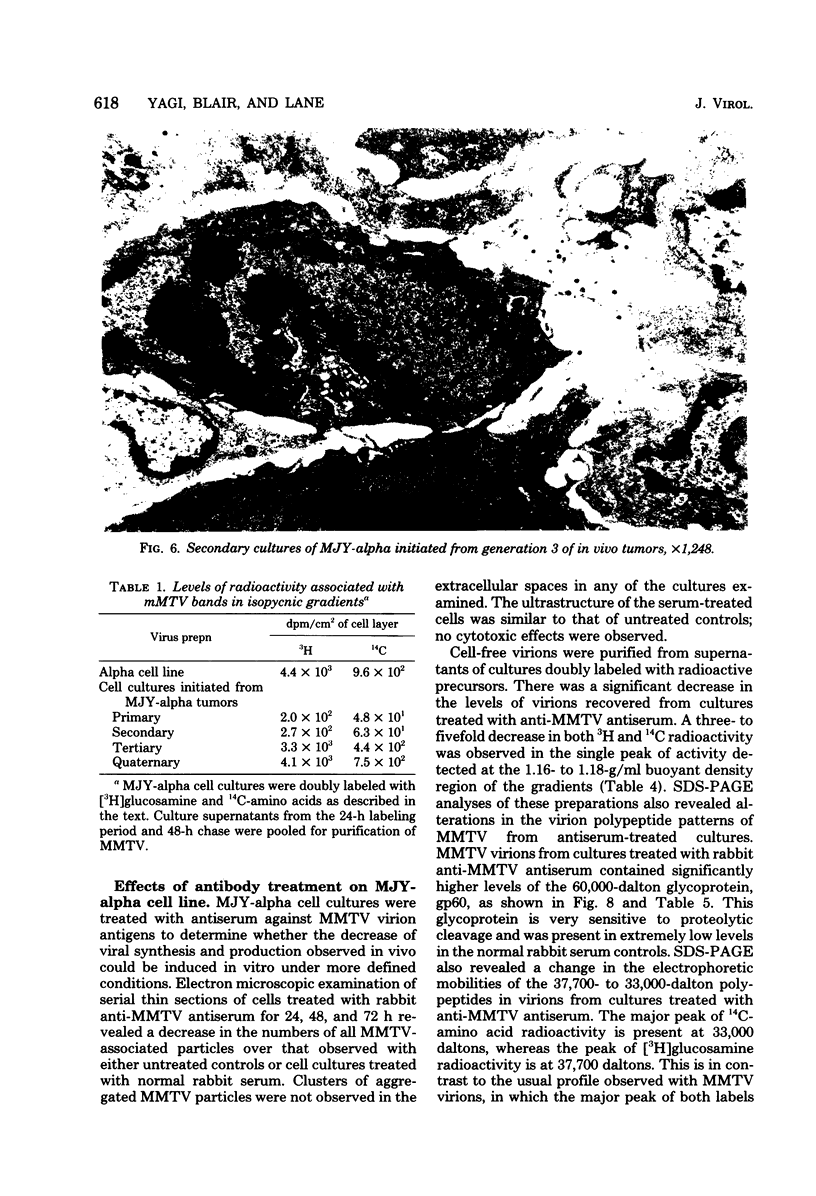
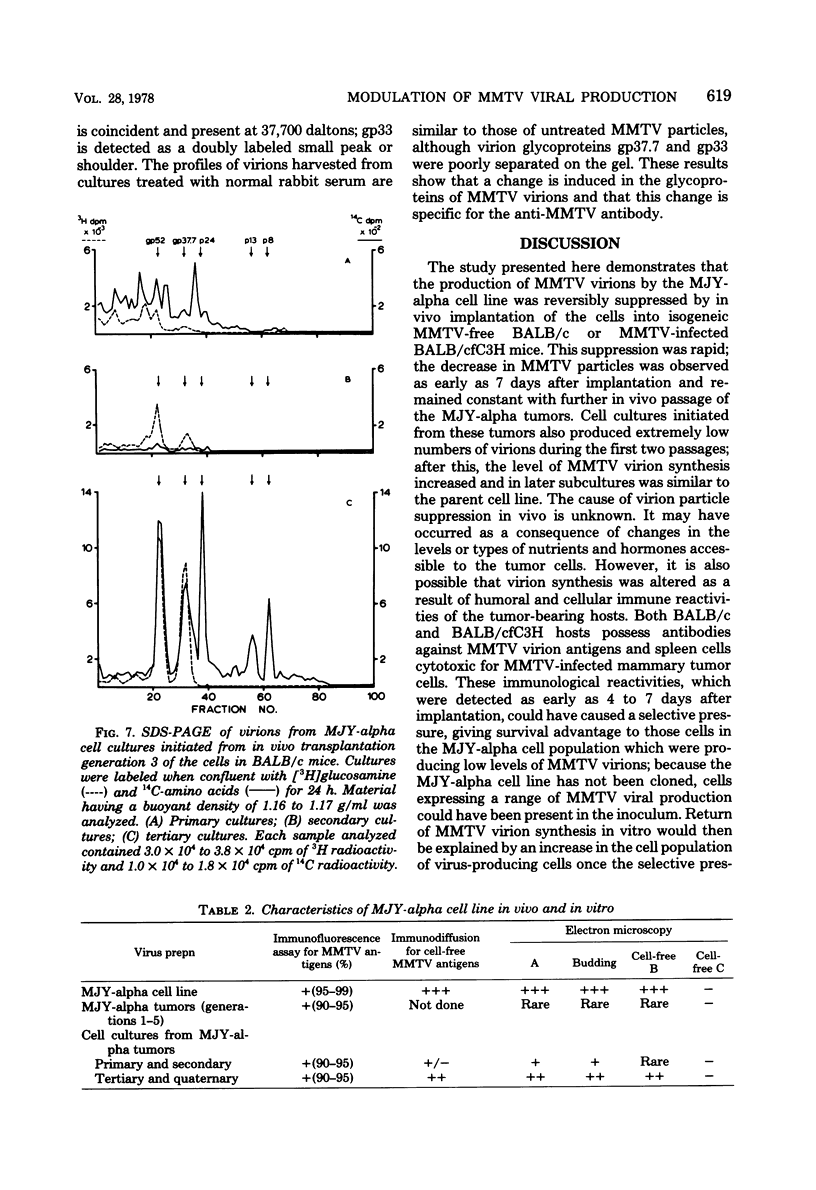
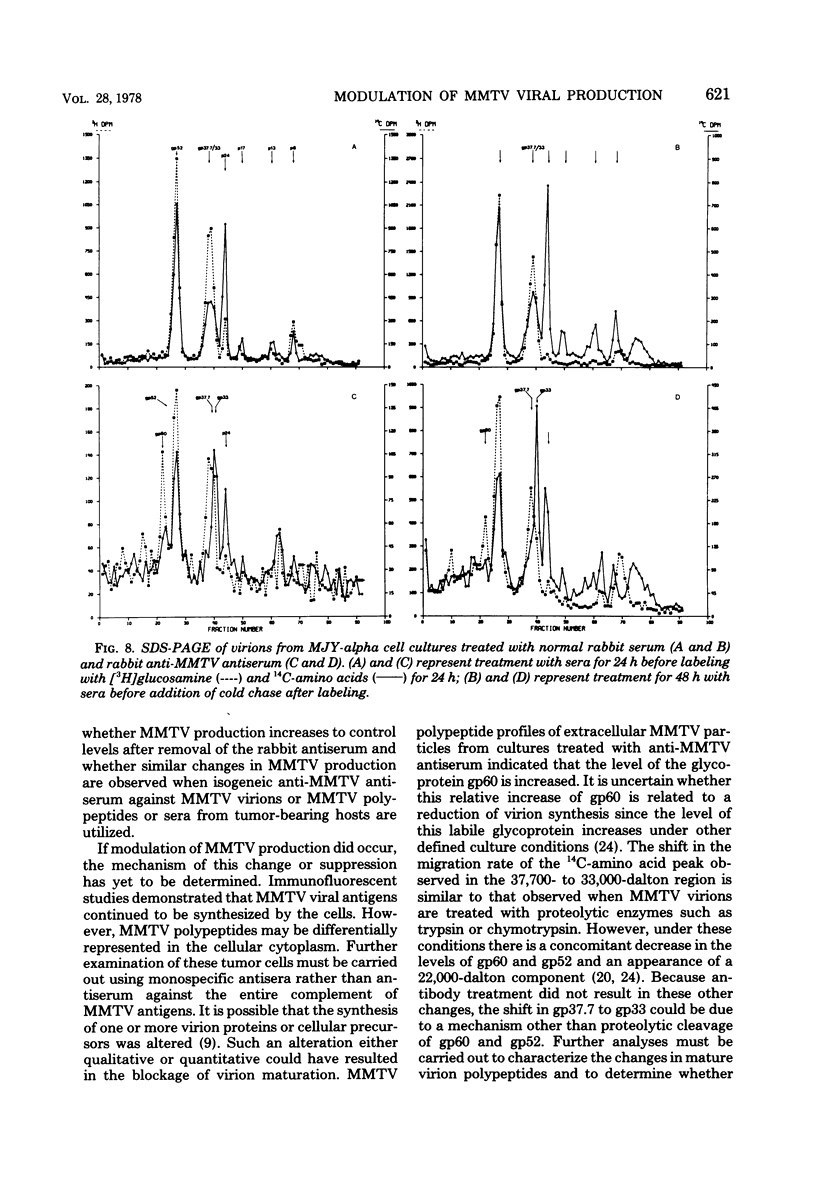
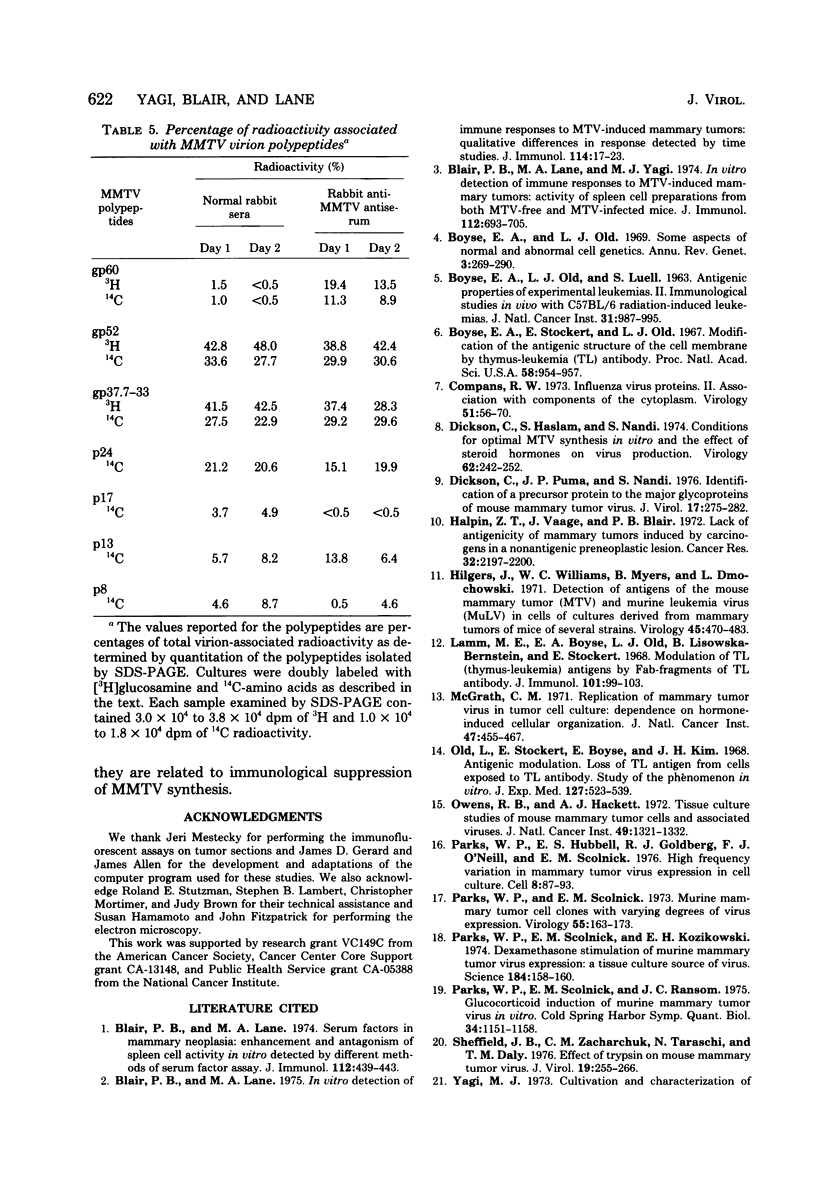
Implantation of the mouse mammary tumor virus (MMTV)-producing mammary tumor cell line MJY-alpha into isogeneic mice elicited both humoral and T-cell responses against MMTV virion antigens. The carcinosarcomas which developed from the implanted cells showed a significant decrease in MMTV synthesis, compared with cells remaining in culture, which was detectable as early as 7 days after implantation and for five transplant generations. Electron microscopic examination of thin sections of the tumors revealed that intracytoplasmic A particles, budding particles, and cell-free MMTV B particles were all affected. However, immunofluorescence assays of tumor sections demonstrated the presence of MMTV viral antigens in the cells. Cell cultures initiated from first-, third-, and fourth-generation tumors were morphologically identical to the original in vitro cell line, although virus production was barely detectable. Analysis of the cultures by electron microscopy revealed a significant increase in MMTV virions after in vitro passage 3. Polypeptide profiles obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of virions purified from these cultures were identical to MMTV. Immunodiffusion demonstrated the cross-reactivity between these virions and MMTV particles obtained from mouse milk. In vitro treatment of MJY-alpha cell cultures with rabbit anti-MMTV antiserum resulted in a reduction of extracellular MMTV virions, as well as alterations in their sodium dodecyl sulfate-polyacrylamide gel electrophoretic polypeptide patterns.
Full text
PDF

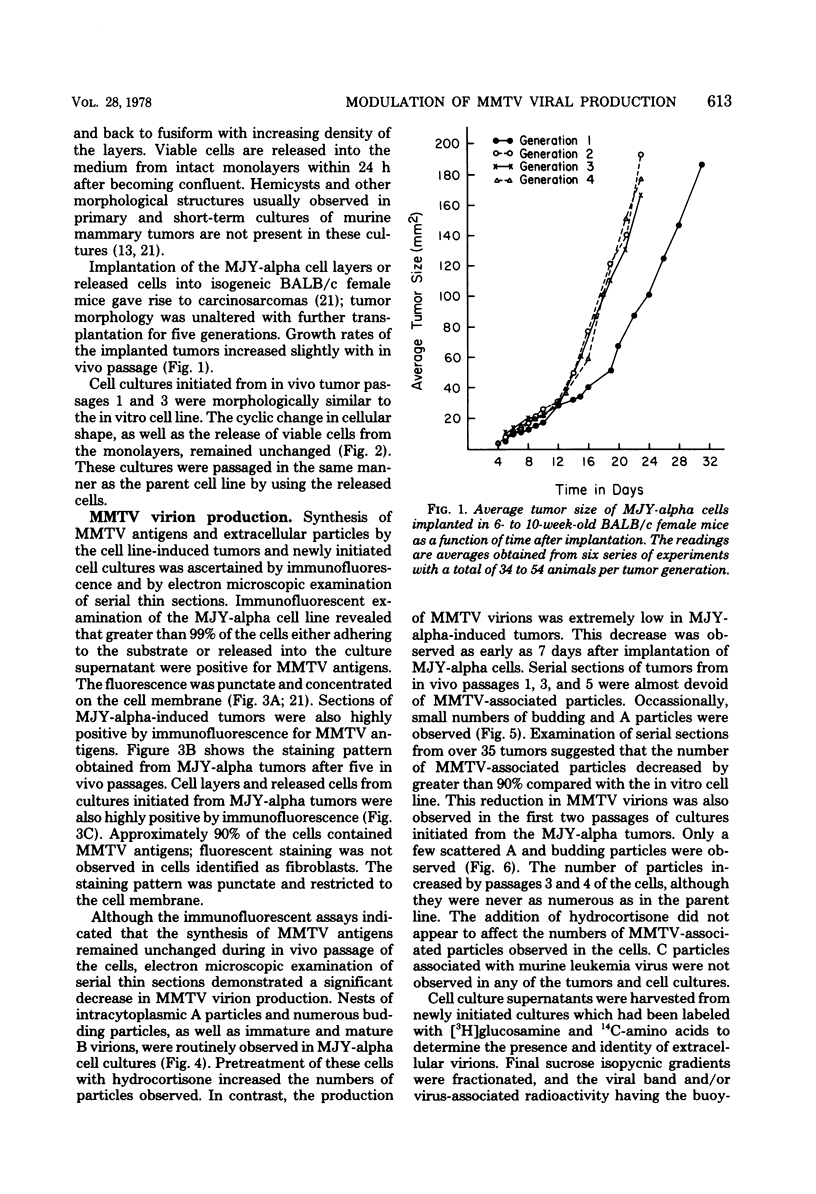
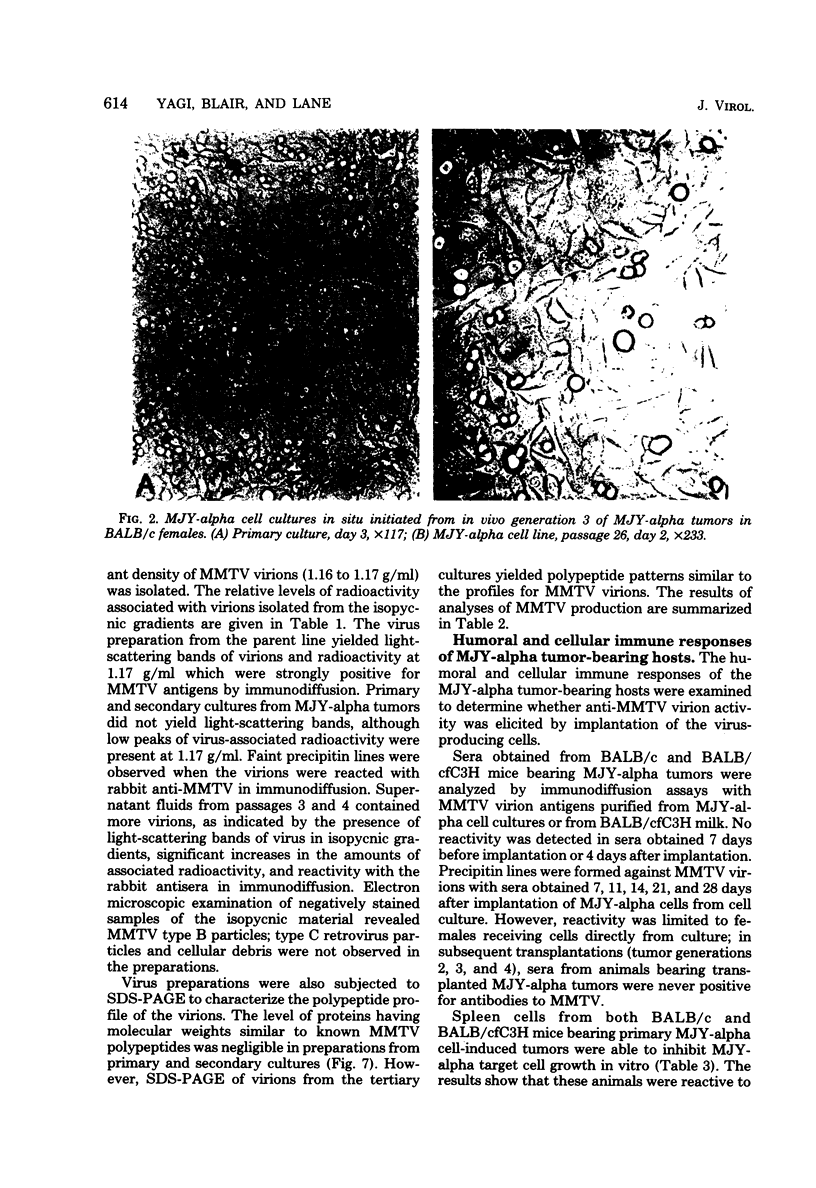
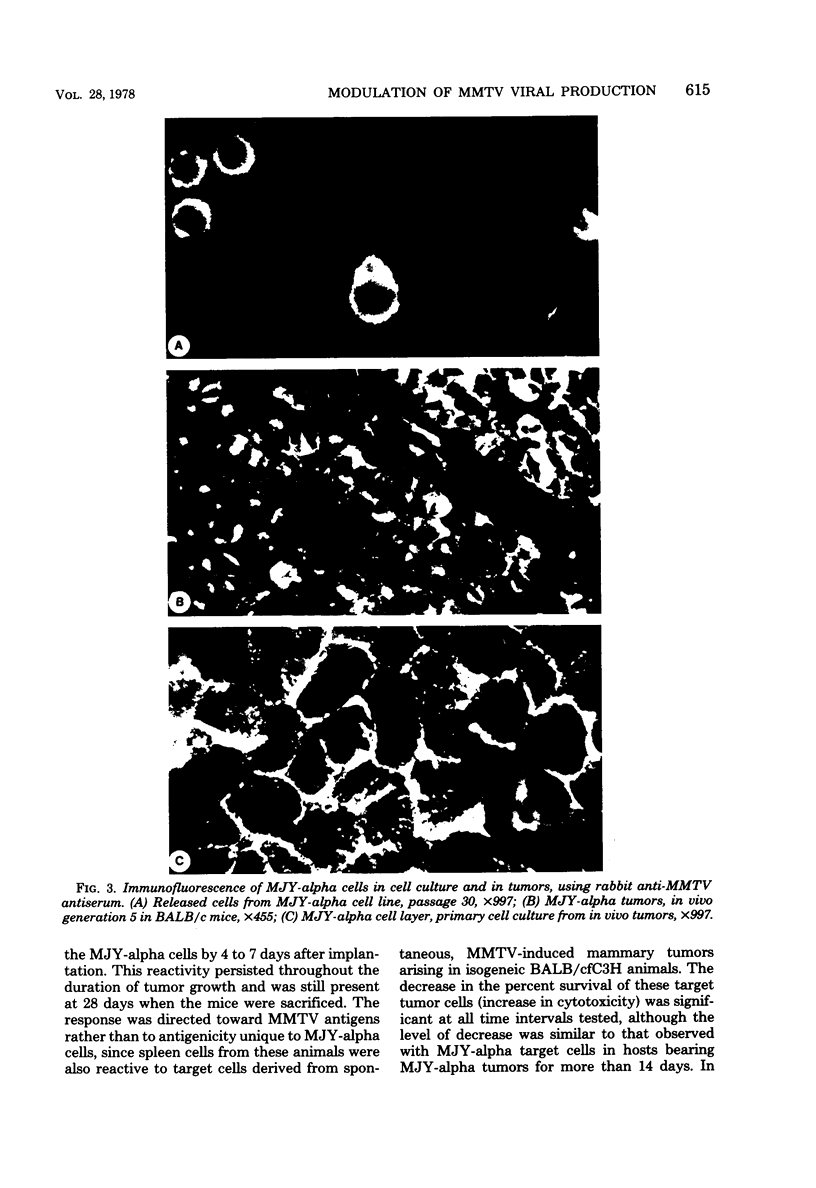
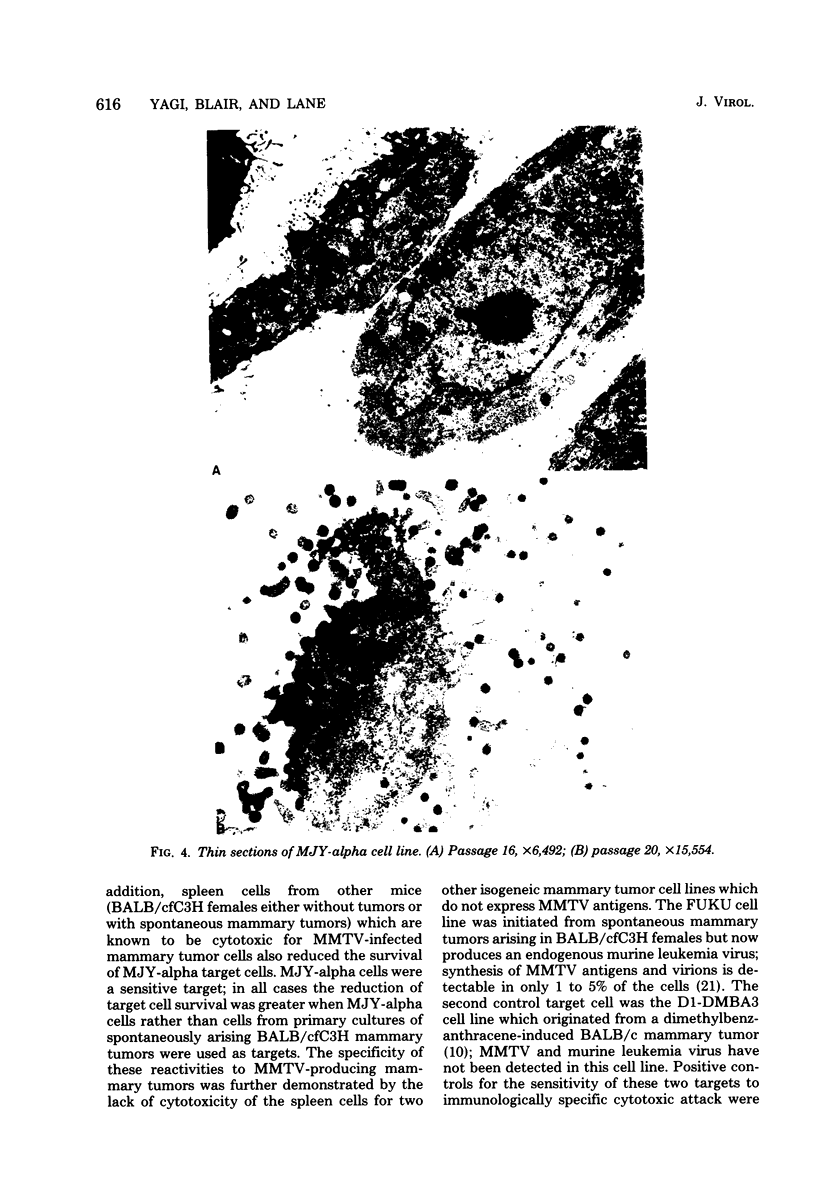

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair P. B., Lane M. A. In vitro detection of immune responses to MTV-induced mammary tumors: qualitative differences in response detected by time studies. J Immunol. 1975 Jan;114(1 Pt 1):17–23. [PubMed] [Google Scholar]
- Blair P. B., Lane M. A. Serum factors in mammary neoplasia: enhancement and antagonism of spleen cell activity in vitro detected by different methods of serum factor assay. J Immunol. 1974 Feb;112(2):439–453. [PubMed] [Google Scholar]
- Blair P. B., Lane M. A., Yagi M. J. In vitro detection of immune responses to MTV-induced mammary tumors: activity of spleen cell preparations from both MTV-free and MTV-infected mice. J Immunol. 1974 Feb;112(2):693–705. [PubMed] [Google Scholar]
- Boyse E. A., Stockert E., Old L. J. Modification of the antigenic structure of the cell membrane by thymus-leukemia (TL) antibody. Proc Natl Acad Sci U S A. 1967 Sep;58(3):954–957. doi: 10.1073/pnas.58.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W. Influenza virus proteins. II. Association with components of the cytoplasm. Virology. 1973 Jan;51(1):56–70. doi: 10.1016/0042-6822(73)90365-6. [DOI] [PubMed] [Google Scholar]
- Dickson C., Haslam S., Nandi S. Conditions for optimal MTV synthesis in vitro and the effect of steroid hormones on virus production. Virology. 1974 Nov;62(1):242–252. doi: 10.1016/0042-6822(74)90319-5. [DOI] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Identification of a precursor protein to the major glycoproteins of mouse mammary tumor virus. J Virol. 1975 Jan;17(1):275–282. doi: 10.1128/jvi.17.1.275-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin Z. T., Vaage J., Blair P. B. Lack of antigenicity of mammary tumors induced by carcinogens in a nonantigenic preneoplastic lesion. Cancer Res. 1972 Oct;32(10):2197–2200. [PubMed] [Google Scholar]
- Hilgers J., Williams W. C., Myers B., Dmochowski L. Detection of antigens of the mouse mammary tumor (MTV) and murine leukemia virus (MuLV) in cells of cultures derived from mammary tumors of mice of several strains. Virology. 1971 Aug;45(2):470–483. doi: 10.1016/0042-6822(71)90347-3. [DOI] [PubMed] [Google Scholar]
- Lamm M. E., Boyse E. A., Old L. J., Lisowska-Bernstein B., Stockert E. Modulation of TL (thymus-leukemia) antigens by Fab-fragments of TL antibody. J Immunol. 1968 Jul;101(1):99–103. [PubMed] [Google Scholar]
- McGrath C. M. Replication of mammary tumor virus in tumor cell cultures: dependence on hormone-induced cellular organization. J Natl Cancer Inst. 1971 Aug;47(2):455–467. [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. ANTIGENIC PROPERTIES OF EXPERIMENTAL LEUKEMIAS. I. SEROLOGICAL STUDIES IN VITRO WITH SPONTANEOUS AND RADIATION-INDUCED LEUKEMIAS. J Natl Cancer Inst. 1963 Oct;31:977–995. [PubMed] [Google Scholar]
- Old L. J., Stockert E., Boyse E. A., Kim J. H. Antigenic modulation. Loss of TL antigen from cells exposed to TL antibody. Study of the phenomenon in vitro. J Exp Med. 1968 Mar 1;127(3):523–539. doi: 10.1084/jem.127.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- Parks W. P., Hubbell E. S., Goldberg R. J., O'Neill F. J., Scolnick E. M. High frequency variation in mammary tumor virus expression in cell culture. Cell. 1976 May;8(1):87–93. doi: 10.1016/0092-8674(76)90189-6. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Murine mammary tumor cell clones with varying degrees of virus expression. Virology. 1973 Sep;55(1):163–173. doi: 10.1016/s0042-6822(73)81018-9. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Ransom J. C. Glucocorticoid induction of murine mammary tumor virus in vitro. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1151–1158. doi: 10.1101/sqb.1974.039.01.132. [DOI] [PubMed] [Google Scholar]
- Sheffield J. B., Zacharchuk C. M., Taraschi N., Daly T. M. Effect of trypsin on mouse mammary tumor virus. J Virol. 1976 Jul;19(1):255–266. doi: 10.1128/jvi.19.1.255-266.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M. J. Characteristics of mammary tumor cultures from four mouse strains infected with mammary tumor virus. Cancer Res. 1975 Feb;35(2):370–373. [PubMed] [Google Scholar]
- Yagi M. J., Compans R. W. Structural components of mouse mammary tumor virus. I. Polypeptides of the virion. Virology. 1977 Feb;76(2):751–766. doi: 10.1016/0042-6822(77)90256-2. [DOI] [PubMed] [Google Scholar]
- Yagi M. J. Cultivation and characterization of BALB-cfC3H mammary tumor cell lines. J Natl Cancer Inst. 1973 Dec;51(6):1849–1860. doi: 10.1093/jnci/51.6.1849. [DOI] [PubMed] [Google Scholar]
- Yagi M. J. Further observations on the production of oncornaviruses by MJY-alpha cell line. J Natl Cancer Inst. 1974 Nov;53(5):1383–1385. doi: 10.1093/jnci/53.5.1383. [DOI] [PubMed] [Google Scholar]
- Yagi M. J., Stutzman R. E., Robertson B. H., Compans R. W. Structural components of mouse mammary tumor virus. II. Isolation and purification of virion polypeptides. J Virol. 1978 May;26(2):448–456. doi: 10.1128/jvi.26.2.448-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]