SUMMARY
Background
Rodents use olfactory cues for species-specific behaviors. For example, mice emit odors to attract mates of the same species but not competitors of closely related species. This implies rapid evolution of olfactory signaling, although odors and chemosensory receptors involved are unknown.
Results
Here, we identify a mouse chemosignal, trimethylamine, and its olfactory receptor, trace amine-associated receptor 5 (TAAR5), to be involved in species-specific social communication. Abundant (>1,000-fold increased) and sex-dependent trimethylamine production arose de novo along the Mus lineage after divergence from Mus caroli. The two-step trimethylamine biosynthesis pathway involves synergy between commensal microflora and a sex-dependent liver enzyme, flavin-containing monooxygenase 3 (FMO3), which oxidizes trimethylamine. One key evolutionary alteration in this pathway is the recent acquisition in Mus of male-specific Fmo3 gene repression. Coincident with its evolving biosynthesis, trimethylamine evokes species-specific behaviors, attracting mice but repelling rats. Attraction to trimethylamine is abolished in TAAR5 knockout mice, and furthermore, attraction to mouse scent is impaired by enzymatic depletion of trimethylamine or TAAR5 knockout.
Conclusions
TAAR5 is an evolutionarily conserved olfactory receptor required for a species-specific behavior. Synchronized changes in odor biosynthesis pathways and odor-evoked behaviors could ensure species-appropriate social interactions.
INTRODUCTION
Many animals must effectively distinguish species-specific odors to ensure that mating and other social behaviors are properly directed. In some insect species, changes in pheromone blend compositions are sufficient to drive sexual isolation and speciation [1, 2]. Such evolutionary events require concurrent adaptations in neural systems that control pheromone-evoked behaviors [1].
In the mouse, signaling in the olfactory epithelium (OE) is essential for several odor-driven social behaviors, including mating, aggression, and nurturing of young [3-6]. Olfactory sensory neurons detect odors and pheromones using a large repertoire of chemosensory receptors, including ~1100 odorant receptors and 14 trace amine-associated receptors (TAARs) [7]. While receptors for natural mouse odors have been identified in the vomeronasal organ (VNO) [8, 9], TAAR5 is the only known OE receptor activated by a sexually dimorphic mouse odor [10]. TAAR5 detects male mouse odor with exquisite sensitivity, and this response is both sex- and age-dependent [10].
Pharmacological screens involving hundreds of chemically diverse odorants identified trimethylamine as a high affinity TAAR5 agonist [10]. Trimethylamine is a bacterial metabolite found in some animal odors, and to humans it is a repulsive odor associated with bad breath and spoiled food [11]. Furthermore, the human genetic disease trimethylaminuria is characterized by abnormally high excretion rates of trimethylamine and a resulting body odor that is profoundly aversive [11]. The molecular basis for this disease is mutation of flavin-containing monooxygenase 3 (FMO3) [12], an enzyme that removes endogenous trimethylamine odor by oxidation to form non-volatile trimethylamine oxide. Trimethylaminuria is a socially debilitating disease, and patients can ameliorate symptoms by reducing dietary intake of choline, a metabolic precursor of trimethylamine, or by taking a regimen of antibiotics [11]. In comparison with humans, mice normally release >1,000-fold more urinary trimethylamine, and do so in a sex-dependent manner. Furthermore, while TAAR5 sensitively detects mouse urine, it does not detect human urine at any concentration examined [10]. This suggests key evolutionary differences in the trimethylamine biosynthesis pathways of mouse and human.
Here, we identify specific evolutionary changes in this pathway that occurred along the Mus lineage. In mouse, the principal trimethylamine oxidase, FMO3, is expressed at >1,000-fold higher levels in female than male liver. In contrast, closely related rodent species produce low or undetectable levels of trimethylamine, and neither trimethylamine production nor Fmo3 gene expression is sex-dependent. Sexually dimorphic trimethylamine production is most distantly observed in Mus spretus, a species that recently diverged from Mus musculus. The recent and de novo evolution of sex-dependent Fmo3 gene control in mice provides a molecular basis for changes in scent odors between highly related mammalian species.
Furthermore, we provide evidence for coincident evolution of trimethylamine biosynthesis pathways and trimethylamine-evoked behavioral responses in rodents. Several TAAR agonists are aversive chemicals, such as 2-phenylethylamine, a carnivore odor that repels rodents [13, 14]. Trimethylamine is also aversive to humans, but behavioral responses of mice to this chemical have not been characterized. Since trimethylamine is abundant in mouse urine, a richly attractive odor source for mice, we reasoned that trimethylamine might evoke different behavioral responses in mice. Here, we find that the valence of mouse behavioral responses to trimethylamine is highly concentration-dependent, with attraction occurring at physiological concentrations found in urine. Knockout mice lacking TAAR5 do not display this attraction behavior, and rats, who retain a functional TAAR5, instead avoid trimethylamine at the same concentration. Enzymatic depletion of trimethylamine or disruption of TAAR5 by gene knockout decreases scent attraction in mice. Thus, we demonstrate that an odor response requires a particular OE receptor, and that this response is malleable across species. Synchronized changes in odor biosynthesis pathways and odor-evoked behaviors could ensure species-appropriate social interactions.
RESULTS
TAAR5 detects a species-dependent odor
Receptors in the olfactory epithelium that discriminate odors of closely related species are unknown. In the course of characterizing TAAR activators across species, we observed robust activation of TAAR5 by mouse odors but not by odors of related rodents.
TAAR5 responses were measured using an established reporter gene assay [10, 14]. HEK-293 cells were co-transfected with plasmids containing TAAR5 and a cAMP-dependent reporter gene (CRE-SEAP), incubated with titrations of test specimens, and assayed for reporter activity. Consistent with our previous findings [10], TAAR5 exhibited robust and sensitive responses to mouse urine (Figure 1A) that were sex-dependent (Figure 1B). Male mouse urine evoked threshold and half-maximal (EC50) TAAR5 responses when diluted 100,000-fold and 30,000-fold respectively.
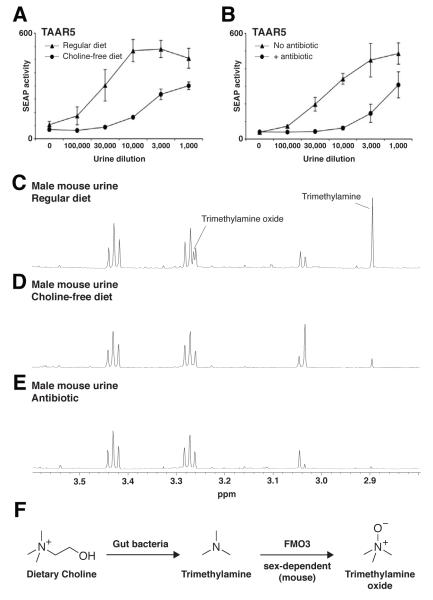
Figure 1. Species- and sex-dependent production of the TAAR5 activator, trimethylamine.
(A) Variations in production of the TAAR5 activator across mammalian species. HEK-293 cells were transfected with plasmids encoding CRE-SEAP and TAAR5 (black bars) or CRE-SEAP alone (white bars), incubated with serial dilutions of urine from six species indicated, and assayed for SEAP activity (triplicates ± s.d.). Rodent donors were males, while human donors were non-identifiable males and females. (B) Assays performed as above, except donors were male and female mice (triplicates ± s.e.). (C) A region (2.8 to 3.6 ppm) of the NMR spectra of male and female mouse urine (C57BL/6), trimethylamine, and trimethylamine oxide. Chemical assignments were based on published values [40] or analysis of spiked specimens. (D) qPCR analysis of Fmo3 gene expression in liver cDNA prepared from mice of sexes and ages indicated (triplicates ± s.e.). Copy numbers were calculated in cDNA derived from 10 ng of liver RNA by comparison with PCR reactions involving titrations of an Fmo3-containing plasmid.
We examined TAAR5 responses to urine from 5 other mammalian species: rat, gerbil, hamster, guinea pig, and human. In contrast to the sensitive responses observed to mouse urine, TAAR5 did not detect rat, guinea pig, or human specimens at any dilution tested (up to 100-fold, Figure S1). Hamster and gerbil urine elicited threshold responses at 300-fold and 1,000-fold dilutions respectively, and thus contained significantly lower levels of the TAAR5 activator than mouse urine. Furthermore, TAAR5 responses were not observed to urine from either male or female rats. Variations in production of the TAAR5 activator were thus greater between species (>1,000-fold between mouse and rat) than between mouse sexes (~10- to 30-fold).Since multiple rodents do not similarly produce the TAAR5 agonist, a parsimonious interpretation would be that its abundant and sex-dependent production in mouse is a recent gain-of-function adaptation.
Identification of species- and sex-dependent mouse odors by NMR spectroscopy
To probe how TAAR5 distinguishes mouse and rat urine, as well as male and female mouse urine, we characterized abundant species-specific and sex-specific urinary odors by nuclear magnetic resonance (NMR) spectroscopy (Figure 1C). The largest peak in the NMR spectrum of male mouse urine corresponded to trimethylamine (5 mM), and production of trimethylamine was sex-dependent, with ~20-fold enrichment in male compared to female urine. This is consistent with data indicating TAAR5 to be a trimethylamine-activated olfactory receptor that discriminates male and female mouse odors [10]. The abundance of trimethylamine is sufficient to explain the striking sensitivity of TAAR5 for male mouse urine. Furthermore, trimethylamine was not detected in male rat urine by NMR spectroscopy (Figure S1), consistent with the inability of rat specimens to activate TAAR5 (Figure 1A).
Comparative NMR spectroscopy of male and female mouse urine also identified a second related chemical produced with opposing sex dependence. Based on analysis of spiked specimens, this female-enriched chemical was trimethylamine oxide, a chemical in the same biosynthetic pathway as trimethylamine [15]. The levels of other major metabolites, including creatine and taurine, were similar between males and females. The reciprocal production of trimethylamine and trimethylamine oxide by males and females suggested trimethylamine oxidation to be a sex-dependent chemical reaction in mice.
Sex-specific expression of trimethylamine oxidase in the mouse
FMO3 is the principal trimethylamine oxidase as mutation of the Fmo3 gene in humans causes trimethylaminuria [12]. We asked whether differences in FMO3 expression between male and female mice could explain sex-dependent trimethylamine production. It was previously reported that FMO3 expression in mouse liver, its primary site of expression, is regulated by a testosterone-dependent mechanism, although a function for this regulation was not clear [16].
Here, we performed a quantitative analysis of Fmo3 gene expression in liver cDNA derived from male and female mice of different ages (Figure 1D). Consistent with previous results [16], we observed a striking >1,000-fold elevation of Fmo3 gene expression in adult females compared to adult males. Male-specific silencing of Fmo3 gene expression is discernible between 4 and 5 weeks of age, matching the time course of trimethylamine appearance in male urine [10]. For comparison, expression of Gapdh was similar across all specimens (Figure S1). Expression levels of Fmo3 in liver are thus inversely correlated with levels of trimethylamine in urine of male, female, and prepubescent mice.
A two-step trimethylamine biosynthesis pathway in mouse involves microbial metabolism and FMO3 oxidation
The trimethylamine biosynthesis pathway is well studied in humans [17], but exhibits several key differences in mice where urinary trimethylamine release is sex-dependent and elevated >1,000-fold. Trimethylamine is initially derived by bacterial metabolism of dietary choline [18], and trimethylaminuria is ameliorated by decreasing choline intake or by antibiotic treatment [11]. Here, we asked whether the normal production of trimethylamine in mouse urine is similarly controlled. Urine was collected from mice fed a choline/methionine-free diet or treated with the antibiotic neomycin sulfate (2 mM in drinking water) for one week, and verified to have similar concentrations of several common metabolites by NMR spectroscopy (see below).
Specimens were then analyzed for the ability to activate TAAR5 using the reporter gene assay (Figures 2A, 2B). As described above, TAAR5 exhibited robust and sensitive responses to urine of mice fed a standard diet. In contrast, TAAR5 had ~10-fold reduced sensitivity for the urine of sex-matched mice fed a choline/methionine-free diet or placed on a regimen of antibiotics for one week (Figures 2A, 2B).
Figure 2. Trimethylamine biosynthesis in mouse involves microbial metabolism and a sex-dependent oxidation reaction.
Urine was collected from male animals provided with normal chow, choline/methionine-free diet, or antibiotics for 1 week. Trimethylamine levels in these specimens were determined by the TAAR5-mediated reporter gene assay (A, B, triplicates ± s.d.) and by NMR spectroscopy (C, D, E). These data are consistent with a two-step pathway regulating trimethylamine production in mouse (F).
Next, we performed NMR spectroscopy on urine from diet-altered and normal-fed mice to determine whether shifts in TAAR5 activation curves could be explained by reductions in trimethylamine levels (Figures 2C, 2D). Diet alteration did not impact urinary levels of taurine and creatine, but reduced levels of both trimethylamine and trimethylamine oxide. Trimethylamine levels were 5 mM in urine from normal-fed males, and 400 μM in diet-altered males, a ~12-fold difference that matched observed changes in TAAR5 activation curves (Figure 2A). Antibiotic treatment resulted in a similar reduction in urinary trimethylamine (Figure 2E). The antibiotic treatment and diet modification eliminated most urinary trimethylamine (~90%), but not all. Residual trimethylamine in mouse urine could be derived from metabolism of other nitrogen-containing nutrients or from the endogenous reservoir of phosphatidylcholine lipid. Nevertheless, these data indicate that a major step in the trimethylamine biosynthesis pathway of mice involves the metabolism of dietary choline by endogenous microbes.
Together, these data are consistent with a two-step biosynthetic pathway for trimethylamine in mice: first, trimethylamine is derived largely via metabolism of dietary choline by gut flora, and second, trimethylamine odor is reduced in females but not males by a sex-dependent oxidation reaction involving FMO3 (Figure 2F).
Evolution of a sexually dimorphic biosynthesis pathway for a mouse odor
We next sought to understand the evolutionary origins of abundant and sex-dependent trimethylamine biosynthesis along the Mus lineage (Figure 3A). We obtained urine from 5 highly related Mus species: Mus musculus, Mus caroli, Mus spicilegus, Mus spretus, and Mus domesticus. Specimens were collected from age- and sex-matched animals housed under similar environmental conditions and fed standard diets with identical levels of choline. Fresh collections were used to prevent odor evaporation or bacterial growth, and samples were verified to have similar levels of creatinine (Figure S2). TAAR5 responses were then measured to serial dilutions of each specimen over a large concentration range using our cellular reporter gene assay (Figure 3B).
Figure 3. Evolution of sex-dependent trimethylamine biosynthesis and Fmo3 gene repression along the Mus lineage.
(A) The phylogenetic relationship of Mus species, with Rattus norvegicus as an outgroup. The figure is adapted from published data [41]. Mus musculus sub-species are shown in the grey box, and bracketed letters indicate species providing specimens for subsequent panels. (B) Urine from males (grey bars) and females (black bars) of species indicated were collected and analyzed using the TAAR5 reporter gene assay. (C, D) Fmo3 gene expression in liver and kidney of various rodents. Liver and kidney cDNA was prepared from species indicated, as coded by bracketed letters in the phylogenetic tree (A), and analyzed for Fmo3 gene expression by qPCR analysis (triplicates ± s.e.). Fmo3 expression levels in male animals (grey bars) and female animals (black bars) were compared. The Y-axis indicates copy number in cDNA derived from 10 ng RNA, and was calculated by comparison with PCR reactions involving titrations of an Fmo3-containing plasmid.
Mus caroli specimens, as with Rattus norvegicus specimens, did not activate TAAR5 at any concentration tested (up to 100-fold, Figure S1). Based on these data, Mus musculus males emit >1,000-fold higher levels of trimethylamine than either of these species. In contrast, TAAR5 did detect urine from Mus spretus and Mus spicilegus, but did so with reduced sensitivity. These species produce lower amounts of trimethylamine than Mus musculus but higher amounts than Rattus norvegicus (Figure S2), and could represent evolutionary intermediates in this biosynthesis pathway. Sexually dimorphic trimethylamine production is most distantly observed in Mus spretus, and maintained in both Mus domesticus and Mus musculus. Lower levels of sexual dimorphism are observed in Mus spicilegus; males and females have slight differences in the production ratio of trimethylamine to creatinine (Figure S2), but the extent of sexual dimorphism is reduced in this species. Together, these data indicate that abundant and sex-dependent production of trimethylamine arose recently along the Mus lineage, and likely within the Eurasian clade of mice.
FMO3 expression is an evolutionary node in the trimethylamine biosynthesis pathway
Since FMO3 is a key regulator of trimethylamine production [12], we asked whether FMO3 expression varied across Mus species in accordance with changes in trimethylamine production. We prepared liver and kidney cDNA from male and female animals of several species, including Rattus norvegicus, Mus caroli, Mus spicilegus, Mus spretus, Mus domesticus, and four strains of Mus musculus. Levels of Gapdh mRNA were similar in all of these specimens (Figure S2). We then determined Fmo3 gene expression levels by qPCR using primers that recognized a highly conserved region of Fmo3 coding sequence.
We found that Fmo3 gene expression levels in liver were sexually dimorphic in some but not all Mus species (Figure 3C). Differences in Fmo3 gene expression between species were primarily due to differences in male-specific Fmo3 gene silencing. Moreover, there was a strong correlation between sex-dependent Fmo3 gene repression and sex-dependent trimethylamine production. Fmo3 gene expression was sexually dimorphic, with a male to female expression ratio of >1,000 in 4 strains of Mus musculus (Figure S2), and >100 in Mus domesticus, a closely related sub-species. Sexually dimorphic Fmo3 gene expression is most distantly observed in Mus spretus, and is not similarly observed in Mus caroli or Rattus norvegicus. These data indicate that Fmo3 gene repression in males is a recent alteration that occurs in those Mus species with sexually dimorphic trimethylamine production.
In addition, we found that Fmo3 expression in the kidney also varied between related rodent species (Figure 3D). Interestingly, Fmo3 gene expression in kidney was not sex-specific in any species, and was ~100-fold lower in Mus musculus than Rattus norvegicus. This second evolutionary change in Fmo3 expression likely explains why trimethylamine levels differ between female mice and female rats, despite similar levels of liver Fmo3.
Trimethylamine attracts mice at concentrations normally found in mouse urine
We present evidence that trimethylamine is abundant in mouse urine, and that multiple genetic changes have occurred in the trimethylamine biosynthesis pathway during Mus evolution. These data suggest that trimethylamine could be a species-specific chemosignal for mice, although associated behavioral responses have not been investigated. We reasoned that trimethylamine could be either attractive to mice, like mouse urine that contains chemicals important for social behaviors, or aversive to mice, like trimethylamine to humans or other TAAR agonists, 2-phenylethylamine and isoamylamine, to rodents [11, 13, 19].
We measured the valence of trimethylamine-evoked behavioral responses in mice using a two-choice compartment assay based on previously reported paradigms [13, 19]. Briefly, mice were introduced into a two-compartment test arena (Figure 4A), with one compartment containing test stimuli (400 μl) in a volatile odor applicator. The two compartments were separated by a curtain and odor plumes controlled to prevent mixing across compartments. Time spent on each side of the arena was recorded during a 2 minute test period, and differences in compartment occupancy scored as attraction or aversion. This experimental design was validated with control odors (Figure 4B), as opposite sex urine, a powerful attractant for mice, increased test compartment occupancy (+43%), while a fox-derived predator odor, 2,5-dihydro-2,4,5-trimethylthiazole (TMT) [20], decreased test compartment occupancy (−45%). Furthermore, water and eugenol, a novel odor, neither increased nor decreased test compartment occupancy significantly in this paradigm (water −1.5%; 10 mM eugenol +2.3%, 4.2 M eugenol +2.7%).
Figure 4. Biphasic valence of mouse behavioral responses to trimethylamine.
(A) A cartoon depiction of the mouse behavioral assay. (B) Side preferences of mice in a two-compartment arena containing test odors and water were measured as differential occupancy. Increases in test odor side occupancy were scored as attraction, while decreases were scored as aversion. (*p < 0.05, **p < 0.01, ± s.e.). All experiments involved equal numbers of males and females, except as noted in Experimental Procedures.
Trimethylamine evoked biphasic and concentration-dependent behavioral responses in mice, with aversion occurring to high trimethylamine concentrations and attraction occurring to lower, physiologically relevant trimethylamine concentrations (Figure 4B). Decreased test compartment occupancy was observed to trimethylamine at 4.2 M (−28%, n=20, p<.001) and 1 M (−19%, n=16, p=.03), but increased test compartment occupancy was observed to trimethylamine at 100 mM (+26.4%, n=20, p<.001), 10 mM (+20.7%, n=32, p=.001), and 1 mM (+11.7%, n=20, p=.07). At all concentrations, trimethylamine responses were indistinguishable in male and female animals (Figure S3). Thus, trimethylamine, in the absence of other urinary cues, attracts mice at concentrations normally found in mouse urine. We also examined a role for trimethylamine in other behavioral paradigms, but observed partial or no effect in the resident-intruder aggression paradigm and various odor discrimination tasks (Figure S3).
Trimethylamine is aversive to rats
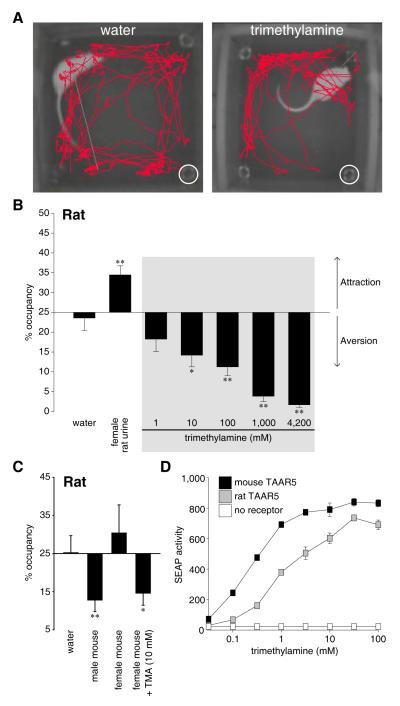
While trimethylamine is attractive to mice, it is highly aversive to humans. Furthermore, trimethylamine production is elevated >1,000-fold in mice than rats, so we reasoned that rats and mice could display distinct behavioral responses to this chemical. We previously published an open field quadrant assay to measure the valence of odor responses in rats, and used this assay to demonstrate avoidance of predator odors, including the TAAR4 agonist 2-phenylethylamine [13, 21]. As with the mouse paradigm, we observed similar responses to control stimuli, including neutral responses to water (24% quadrant occupancy or qo) and attraction responses to opposite sex urine (35% qo).
Unlike mice, rats were not attracted to any concentration of trimethylamine tested (Figures 5A, 5B). Rats exhibited decreases in odor quadrant occupancy when trimethylamine was used as a stimulus at 4.2 M (1.7% qo), 1 M (4.3% qo), 100 mM (11.2% qo), and 10 mM (14.2% qo). We next investigated behavioral responses of rats to mouse urine, an abundant source of trimethylamine. Rats are predators of mice, so we reasoned that mouse urine, as prey odor, could contain either attractive kairomones intercepted by rats or aversive allomones released by mice for defensive behavior. Using the open field quadrant assay, we observed that male mouse urine was aversive to rats and that female urine became aversive when male levels of trimethylamine were added (Figure 5C). These data suggest a function for trimethylamine as an aversive allomone and demonstrate that trimethylamine evokes responses of different valence in mice and rats.
Figure 5. Rats avoid a trimethylamine odor source.
(A) Motion tracks (red) of individual male rats behaving in the open field paradigm. White circles indicate the location of odor sources (water, 4.2 M trimethylamine). (B, C) Occupancy of quadrants, defined as 25% of the arena, containing odors indicated (n=9-12, *p < 0.05, **p < 0.01, ± s.e.). (D) Dose-dependent trimethylamine responses of HEK-293 cells transfected with reporter gene alone (white squares), reporter gene and mouse TAAR5 (black squares), or reporter gene and rat TAAR5 (grey squares) were measured using the cellular reporter gene assay (triplicates ± s.e.).
We next asked whether pharmacological differences in TAAR5 orthologs from mouse and rat could explain variations in the valence of trimethylamine responses. However, using the reporter gene assay (Figure 5D), we observed that both rat and mouse TAAR5 detected trimethylamine with similar sensitivity (mouse EC50 = 300 nM; rat EC50 = 1 μM). Furthermore, single neuron imaging and post-hoc expression analysis determined that mouse TAAR5, in the context of the olfactory sensory neuron, also detected trimethylamine at 300 nM but not isoamylamine or 2-phenylethylamine, agonists for TAAR3 and TAAR4 (Figure S4). Trimethylamine thresholds that evoke behavioral responses are understandably different from those that activate TAAR5 in heterologous cells as our behavioral assays require investigation of a trimethylamine odor source from a distance. It is possible that these species-particular odor responses involve differences in olfactory circuits downstream of receptor activation. Alternatively, trimethylamine may activate different repertoires of receptors in mouse and rat, and receptors other than TAAR5 could mediate either mouse attraction or rat aversion.
TAAR5 is required for mouse attraction to trimethylamine
While several TAAR agonists are aversive odors, trimethylamine evokes a more complex and distinct response in mice. We next asked whether TAAR5 was required for the avoidance response to high levels of trimethylamine or the attraction response to physiological levels of trimethylamine. We obtained TAAR5 knockout mice (Taar5−/−) from the Knockout Mouse Project (KOMP) repository [22], and Taar5 expression was undetectable in olfactory epithelium derived from homozygous animals by in situ hybridization (Figure 6A). Next, we measured the valence of behavioral responses to trimethylamine in Taar5−/− animals using the odor compartment assay (Figure 6B). We observed that Taar5−/− animals displayed decreased attraction to trimethylamine at all concentrations tested. At high concentrations (4.2M), avoidance behavior was more pronounced as occupancy of Taar5−/− animals in the odor compartment was lower (−61.5%, n=12) than wild type animals (−28%, n=20). Furthermore, at concentrations that attracted wild type mice, trimethylamine evoked neither attraction nor aversion responses in Taar5−/− animals, including at 100 mM (−11.1%, n=8) and 10 mM (−2.2%, n=14). These responses were statistically indistinguishable from neutral responses to control odors. Thus, TAAR5 is a single mouse OE receptor required for attraction to trimethylamine.
Figure 6. TAAR5 and trimethylamine are required for mouse scent attraction.
(A) Expression of Taar5 and lacZ in olfactory sensory neurons of Taar5−/−, Taar5+/−, and Taar5+/+ mice visualized by two-color in situ hybridization. Scale bar = 50 μm. (B) Responses of Taar5+/+ and Taar5−/− mice to trimethylamine were measured using the two-choice odor compartment assay (mean ± s.e.). Experiments involved equal numbers of males and females, except as noted in Experimental Procedures. (C) Male urine was depleted of trimethylamine by incubation with FMO3-containing microsomes. Effective depletion was shown by an inability of TMA-depleted male urine to activate TAAR5 in HEK-293 cells at dilutions indicated (triplicates ± s.e.). (D) Behavioral responses of mice to stimuli indicated (40 μl) using the two-choice compartment assay (mean ± s.e.). (E) Behavioral responses of female mice (n=8-10, ± s.e.) of genotypes indicated to mock-depleted male urine (40 μl). (*p < 0.05, **p < 0.01)
Trimethylamine and TAAR5 function in scent attraction
Next, we asked whether the ligand-receptor pair of trimethylamine and TAAR5 is required for attraction to mouse urine. We developed a strategy to deplete trimethylamine from mouse urine based on its biosynthesis pathway. Male mouse urine was incubated with or without FMO3-containing microsomes to create ‘TMA-depleted male urine’ and ‘mock-depleted male urine’. Trimethylamine levels were decreased >300-fold in TMA-depleted male urine, as determined by an inability to activate TAAR5 across a range of concentrations (Figure 6C). While FMO3 has high substrate specificity for trimethylamine [23], we reasoned that FMO3 could potentially oxidize other urinary chemicals under these reaction conditions. To control for this, we created an additional test specimen (‘TMA-respiked male urine’) in which trimethylamine was restored to normal levels following removal of FMO3 microsomes by centrifugation.
Next, we examined whether TMA-depleted, mock-depleted, and TMA-respiked male urine (40 μl) were similarly attractive to mice using the two-choice compartment assay (Figure 6D). Mock-depleted urine was a highly attractive stimulus (+37.8%, n=18), similar to what we observed for intact urine (Figure 4). In contrast, mice exhibited decreased attraction towards TMA-depleted male urine (+17.8%, n=20), and this decrease persisted over several urine doses tested (Figure S5). Furthermore, attraction was restored to TMA-respiked male urine (+30.8%, n=20). Simultaneous presentation of mock-depleted and TMA-depleted urine in the two-choice arena also indicated a preference for mock-depleted urine (Figure S5). Finally, Taar5−/− animals showed reduced attraction to mock-depleted male urine (+17.1%, n=10) that was not seen in sex- and age-matched wild type controls (Figure 6E). These data indicate a role for trimethylamine and TAAR5 in attraction to mouse scent, and also indicate that there are other attractive olfactory cues in male mouse urine. Thus, mouse scent is an attractive odor blend containing multiple chemicals, among them trimethylamine, a species-dependent odor with a rapidly evolving biosynthesis pathway.
DISCUSSION
Animals have evolved elaborate and diverse sex traits important for species-specific behaviors. Rodents use olfactory cues as such traits, and both odor production pathways and odor-evoked behavioral responses are under strong evolutionary pressure for diversification between related species. We previously discovered an olfactory receptor, TAAR5, that detects an abundant mouse odor, trimethylamine [10].Here, we show that both trimethylamine biosynthesis pathways and trimethylamine-evoked behavioral responses vary considerably between related rodents.
Stepwise evolution of the trimethylamine biosynthesis pathway
Mechanisms by which volatile scent odor biosynthesis pathways have evolved in rodents to include sex-specific and species-specific chemical reaction steps are unknown. In invertebrates, alterations to odor biosynthesis pathways that include changes in the function or expression pattern of specific biosynthetic enzymes can drive the evolution of new species [1, 2, 24]. In European corn borer moths, altered substrate recognition of a fatty-acyl reductase changes the predominant lipid acetate isomer in a pheromone blend [2]. This change in enzyme function can cause reproductive isolation, a first step in speciation. In Drosophila, rapid changes in the expression patterns of desaturases correlate with species-particular differences in the production of long-chain cuticular hydrocarbons that function as sex pheromones [24].
Here, we identify FMO3-catalyzed oxidation to be an evolutionary node in the trimethylamine biosynthesis pathway of rodents. In humans, rats, and other species, FMO3 normally clears trimethylamine odor by conversion to non-volatile trimethylamine oxide [12, 17]. Mice have evolved specific mechanisms for decreasing the efficiency of this oxidation reaction and enhancing trimethylamine release >1,000-fold compared to rats. The trimethylamine pathway has undergone stepwise alteration in the Mus genus, with Mus spicilegus and Mus spretus representing intermediates. Furthermore, abundant and sex-specific trimethylamine production arose de novo after divergence from Mus caroli.
How could sex-dependent FMO3 expression arise de novo along the Mus lineage? FMO3 is highly expressed in the liver, and sex-dependent liver gene expression predominantly occurs through a growth hormone-mediated mechanism [25]. In mice, androgen signaling modulates the release dynamics of growth hormone from the pituitary, as males release growth hormone in rhythmic bursts while females release growth hormone at lower, constant levels. Pulsatile growth hormone release in males enables activation and de-activation cycles of growth hormone-mediated signaling cascades not observed in females. One critical growth hormone-activated transcription factor in liver is Stat5b, which is required for sex-dependent induction of some male-enriched proteins, such as major urinary proteins (MUPs) that function as mouse pheromones, and repression of other female-enriched proteins [26]. Mechanisms by which Stat5b activates some genes but silences others are not understood [25], and could involve transcriptional co-activators and co-repressors that are not yet identified. Sequence analysis of the Fmo3 promoter across species (Supplemental Methods) identified several regions upstream of the Fmo3 transcription start site (TSS) that encoded transcription factor binding sites and varied across rodents in accordance with sex-dependent trimethylamine production and Fmo3 gene repression. One site that arose de novo is a consensus binding site for Stat5b, while other mutations created binding sites for transcription factors with no known and direct function in sex-dependent liver gene expression. The identification of novel transcription factor binding sites in the Fmo3 gene promoter that arose concurrently with sex-dependent Fmo3 gene repression is potentially interesting for future study since Stat5b co-repressors that mediate male-specific gene silencing in liver are unknown.
Bacteria and scent odors
These studies highlight an important role for endogenous microflora in generating odor cues that influence mouse scent composition. In humans, bacterial metabolites influence the odor quality of axillary sweat and other secretions [27]. In rodents, bacterial and dietary metabolites could provide a mechanism for distinguishing urinary odors of genetically identical animals [28, 29], and could be dynamic in wild mice, varying with ecological niche. In laboratory mice, under homogenous conditions, levels of trimethylamine production were not highly variable between adult male animals.
In contrast to odors generated by endogenous bacteria, odors generated by invasive bacteria can provide repulsive signals that indicate infection or illness [30]. Formyl peptide receptor-related sequences function as sensory receptors in the vomeronasal organ, and are related to immune system receptors that recognize formylated peptides released at sites of tissue damage and bacterial infection [31, 32]. Furthermore, solitary chemosensory cells in the mouse respiratory epithelium contain bitter taste receptors that detect acyl-homoserine lactones involved in bacterial quorum sensing [33]. Together, mice have evolved numerous chemosensory mechanisms for recognizing endogenous, invasive, or environmental bacteria.
Mouse attraction to a trimethylamine odor source requires TAAR5
Trimethylamine is abundant in mouse urine, highly volatile, produced with sex-, age-, and species-dependence, and activates a mouse olfactory receptor with high affinity. We also find that trimethylamine (10 mM) is an attractive cue to mice, and mouse urine depleted of trimethylamine evokes decreased attraction responses. Trimethylamine is one of several attractive components of mouse urine, consistent with previous imaging and behavioral studies indicating that mouse odor recognition involves detection of a blend of constituents [34, 35]. Other attractive components in mouse urine include volatiles such as methylthio-methylthiol, 2-sec-butyl-dihydrothiazole, dehydro-exo-brevicomin, and farnesenes, as well as a non-volatile MUP termed darcin [34-37].
In our paradigm, male and female mice display similar levels of attraction to trimethylamine (Figure S3). It is possible that trimethylamine evokes a sex-specific behavioral response that we have not identified, and in future studies, it would be interesting to examine a role for trimethylamine and TAAR5 in influencing sexual receptivity of females. Alternatively, trimethylamine could provide a general attraction signal, with selective pressure for enriched production by males arising because scent marking is a stereotyped territorial behavior typically displayed by male animals.
Mouse attraction responses to trimethylamine require a particular OE receptor, TAAR5. There are only a few examples where alterations of individual olfactory receptors in mammals change odor-evoked behaviors. In mice, genetic deletion of a cluster of VNO receptors influences some social behaviors [38], while deletion of a single VNO receptor effects a specific mating response to the male lacrimal pheromone ESP1 [8]. Furthermore, polymorphisms in a human odorant receptor for androstenone account for changes in human odor perception [39]. Here, we show that knockout of a specific OE receptor in mice alters behavioral responses to the volatile mouse odor, trimethylamine.
Trimethylamine evokes different behavioral responses in mice and rats
Rat and mouse TAAR5 similarly detect trimethylamine, yet TAAR5-mediated attraction behavior is only observed in mice. Since rats avoid trimethylamine, it is possible that trimethylamine functions in a defensive capacity as an allomone that reduces attraction of predators or competing species to mouse scents. Consistent with this notion, rats avoid male mouse urine, an abundant source of trimethylamine.
How do the mouse and rat olfactory systems mediate distinct trimethylamine responses? Since several TAAR ligands are aversive to rodents, and trimethylamine is aversive to both humans and rats, a parsimonious interpretation would be that TAAR5-mediated attraction is a recent adaptation in mouse. Adaptive changes in TAAR5-activated olfactory circuits could occur downstream of receptor activation, and involve either developmental alterations to hard-wired neural circuits or learning. A role for learning in adaptive behavioral responses simplifies how a species-specific odor response might evolve, as a single change in an odor biosynthesis pathway could be sufficient to drive rapid evolution of olfactory communication. Alternatively, TAAR5 could couple to similar circuits in all species, and rats and humans might have an additional trimethylamine-activated olfactory receptor not found in mice that mediates dominant aversion responses. Consistent with the existence of additional mechanisms for trimethylamine sensing, Taar5−/− animals do retain avoidance of high trimethylamine concentrations.
Here, we identify an olfactory receptor that is both evolutionarily conserved and required for a species-specific attraction response. The ligand for this olfactory receptor is the product of a rapidly evolving biosynthesis pathway. Fmo3 gene control provides a key evolutionary node in this pathway, and a molecular basis for changes in odor production between highly related mammalian species. Synchronous evolution of biosynthesis pathways and evoked responses could help ensure that social behaviors are properly targeted to animals of the same species.
EXPERIMENTAL PROCEDURES
A description of reagents and methods for TAAR5 functional assays, qPCR, NMR spectrocopy, mouse behavior, rat behavior, odor depletion, single neuron calcium imaging, single neuron expression analysis, in situ hybridization, specimen collection, animal husbandry, and statistical analysis are detailed in the Supplemental Information.
Supplementary Material
HIGHLIGHTS.
Evolution of a two-step biosynthesis pathway for the mouse odor trimethylamine.
Male-specific repression of FMO3, a key biosynthetic enzyme, arose de novo in Mus.
Trimethylamine evokes species-specific behaviors, attracting mice but repelling rats.
Knockout of TAAR5 or trimethylamine depletion impairs scent attraction responses.
ACKNOWLEDGMENTS
We thank Sandeep Robert Datta for helpful advice and use of his mouse behavioral suite. We also thank Tom Rapoport, Mark Albers, and Sandeep Robert Datta for valuable comments on the manuscript. We thank the Nikon Imaging Center and NMR Facility at Harvard Medical School. This work was supported by a grant from the National Institute On Deafness And Other Communicative Disorders (SDL, Award Number R01DC010155). DMF is supported by a Boehringer Ingelheim Fonds PhD Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Smadja C, Butlin RK. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity (Edinb) 2009;102:77–97. doi: 10.1038/hdy.2008.55. [DOI] [PubMed] [Google Scholar]
- 2.Lassance JM, Groot AT, Lienard MA, Antony B, Borgwardt C, Andersson F, Hedenstrom E, Heckel DG, Lofstedt C. Allelic variation in a fatty-acyl reductase gene causes divergence in moth sex pheromones. Nature. 2010;466:486–489. doi: 10.1038/nature09058. [DOI] [PubMed] [Google Scholar]
- 3.Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 4.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nature neuroscience. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Balet Sindreu C, Li V, Nudelman A, Chan GC, Storm DR. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci. 2006;26:7375–7379. doi: 10.1523/JNEUROSCI.1967-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Storm DR. Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase. Neuropsychopharmacology. 2011;36:772–781. doi: 10.1038/npp.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nei M, Niimura Y, Nozawa M. The evolution of animal chemosensory receptor gene repertoires: roles of chance and necessity. Nature reviews. 2008;9:951–963. doi: 10.1038/nrg2480. [DOI] [PubMed] [Google Scholar]
- 8.Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- 9.Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberles SD, Buck LB. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell SC, Smith RL. Trimethylaminuria: the fish malodor syndrome. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:517–521. [PubMed] [Google Scholar]
- 12.Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Missense mutation in flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- 13.Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, Spehr M, Fendt M, Liberles SD. Detection and avoidance of a carnivore odor by prey. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11235–11240. doi: 10.1073/pnas.1103317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrero DM, Wacker D, Roque MA, Baldwin MW, Stevens RC, Liberles SD. Agonists for 13 trace amine-associated receptors provide insight into the molecular basis of odor selectivity. ACS Chem Biol. 2012;7:1184–1189. doi: 10.1021/cb300111e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falls JG, Ryu DY, Cao Y, Levi PE, Hodgson E. Regulation of mouse liver flavin-containing monooxygenases 1 and 3 by sex steroids. Archives of biochemistry and biophysics. 1997;342:212–223. doi: 10.1006/abbi.1997.9965. [DOI] [PubMed] [Google Scholar]
- 17.Bain MA, Fornasini G, Evans AM. Trimethylamine: metabolic, pharmacokinetic and safety aspects. Curr Drug Metab. 2005;6:227–240. doi: 10.2174/1389200054021807. [DOI] [PubMed] [Google Scholar]
- 18.al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41:135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 19.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 20.Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neuroscience and biobehavioral reviews. 2005;29:1145–1156. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Fendt M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. Journal of chemical ecology. 2006;32:2617–2627. doi: 10.1007/s10886-006-9186-9. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MA, Tsai L, Roy DS, Valenzuela DH, Mosley C, Magklara A, Lomvardas S, Liberles SD, Barnea G. Neurons expressing trace amine-associated receptors project to discrete glomeruli and constitute an olfactory subsystem. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13410–13415. doi: 10.1073/pnas.1206724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacology & therapeutics. 2005;106:357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirangi TR, Dufour HD, Williams TM, Carroll SB. Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol. 2009;7:e1000168. doi: 10.1371/journal.pbio.1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol. 2006;20:1333–1351. doi: 10.1210/me.2005-0489. [DOI] [PubMed] [Google Scholar]
- 27.Gower DB, Holland KT, Mallet AI, Rennie PJ, Watkins WJ. Comparison of 16-androstene steroid concentrations in sterile apocrine sweat and axillary secretions: interconversions of 16-androstenes by the axillary microflora--a mechanism for axillary odour production in man? The Journal of steroid biochemistry and molecular biology. 1994;48:409–418. doi: 10.1016/0960-0760(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 28.Schellinck HM, West AM, Brown RE. Rats can discriminate between the urine odors of genetically identical mice maintained on different diets. Physiology & behavior. 1992;51:1079–1082. doi: 10.1016/0031-9384(92)90096-k. [DOI] [PubMed] [Google Scholar]
- 29.Zomer S, Dixon SJ, Xu Y, Jensen SP, Wang H, Lanyon CV, O’Donnell AG, Clare AS, Gosling LM, Penn DJ, et al. Consensus multivariate methods in gas chromatography mass spectrometry and denaturing gradient gel electrophoresis: MHC-congenic and other strains of mice can be classified according to the profiles of volatiles and microflora in their scent-marks. Analyst. 2009;134:114–123. doi: 10.1039/b807061j. [DOI] [PubMed] [Google Scholar]
- 30.Zala SM, Potts WK, Penn DJ. Scent-marking displays provide honest signals of health and infection. Behavioral Ecology. 2004;15:338–344. [Google Scholar]
- 31.Liberles SD, Horowitz LF, Kuang D, Contos JJ, Wilson KL, Siltberg-Liberles J, Liberles DA, Buck LB. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9842–9847. doi: 10.1073/pnas.0904464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riviere S, Challet L, Fluegge D, Spehr M, Rodriguez I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature. 2009;459:574–577. doi: 10.1038/nature08029. [DOI] [PubMed] [Google Scholar]
- 33.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jemiolo B, Alberts J, Sochinski-Wiggins S, Novotny M. Behavioural and endocrine responses of female mice to synthetic analogues of volatile compounds in male urine. Anim. Behav. 1985;33:1114–1118. [Google Scholar]
- 35.Lin DY, Zhang SZ, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 36.Jemiolo B, Xie TM, Novotny M. Socio-sexual olfactory preference in female mice: attractiveness of synthetic chemosignals. Physiology & behavior. 1991;50:1119–1122. doi: 10.1016/0031-9384(91)90570-e. [DOI] [PubMed] [Google Scholar]
- 37.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Del Punta K, Leinders-Zufall T, Rodriguez I, Jukam D, Wysocki CJ, Ogawa S, Zufall F, Mombaerts P. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 39.Keller A, Zhuang H, Chi Q, Vosshall LB, Matsunami H. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- 40.Gavaghan McKee CL, Wilson ID, Nicholson JK. Metabolic phenotyping of nude and normal (Alpk:ApfCD, C57BL10J) mice. Journal of proteome research. 2006;5:378–384. doi: 10.1021/pr050255h. [DOI] [PubMed] [Google Scholar]
- 41.Runck AM, Moriyama H, Storz JF. Evolution of duplicated beta-globin genes and the structural basis of hemoglobin isoform differentiation in Mus. Molecular biology and evolution. 2009;26:2521–2532. doi: 10.1093/molbev/msp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.